Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
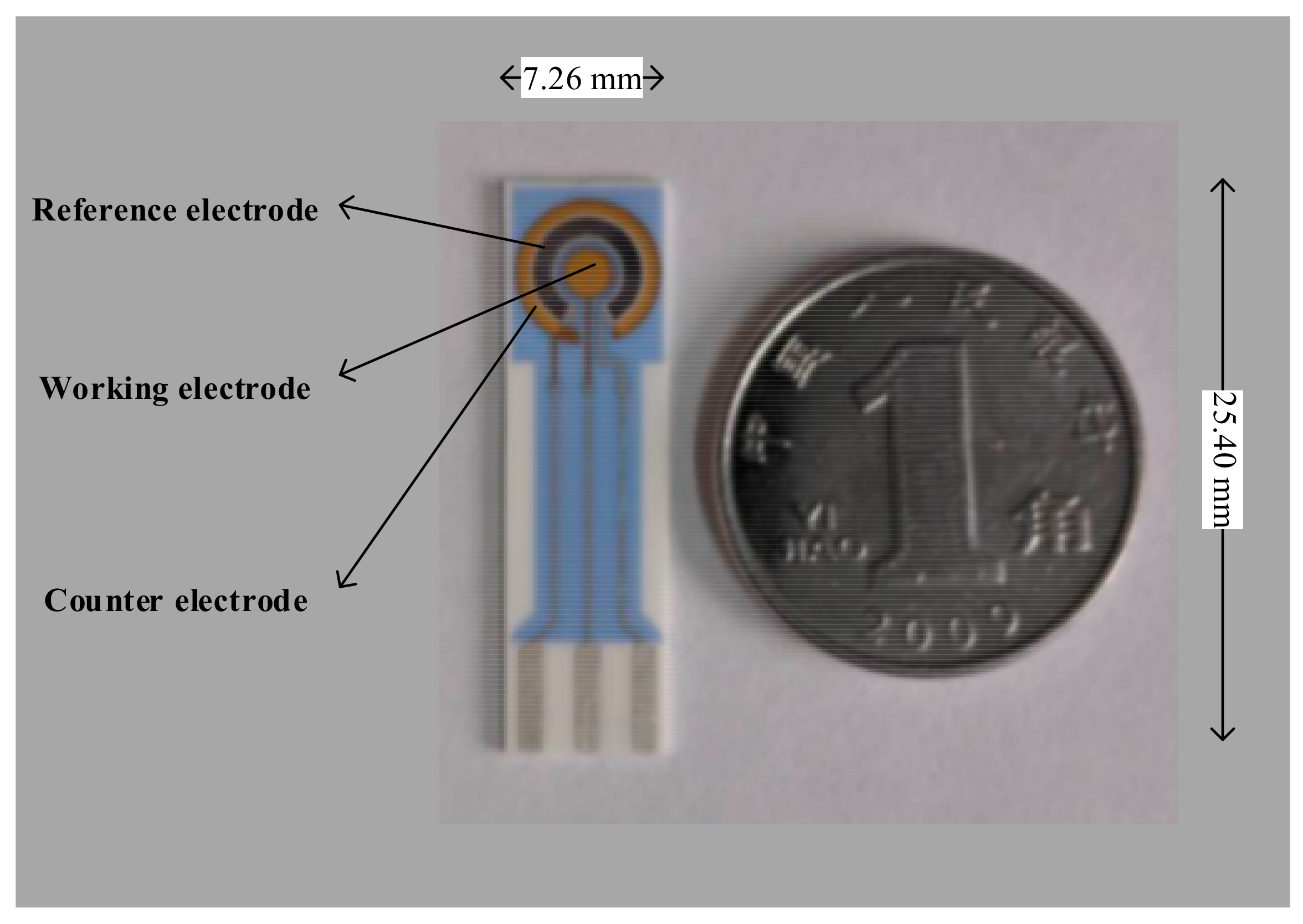
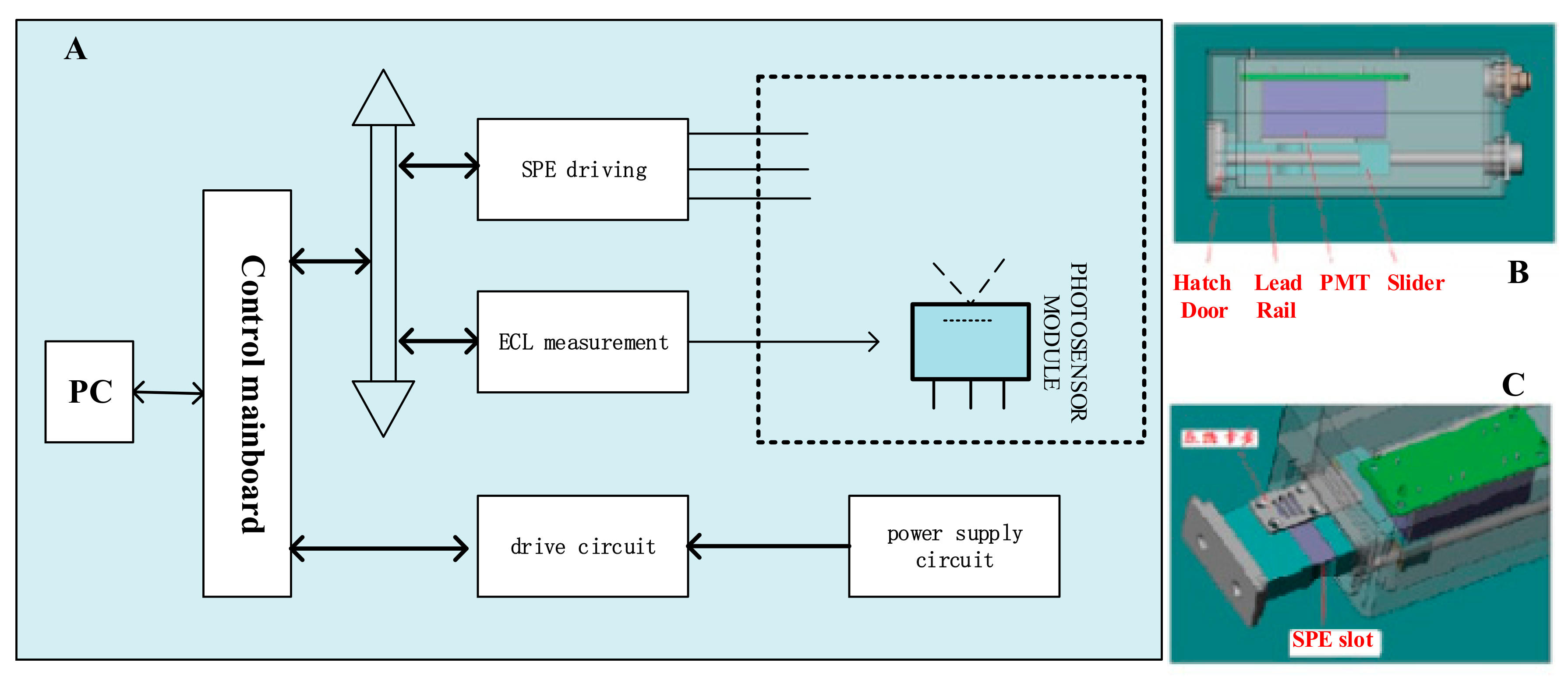
2.2. Design and Development of Portable ECL Sensing Platform
2.3. Experimental Method
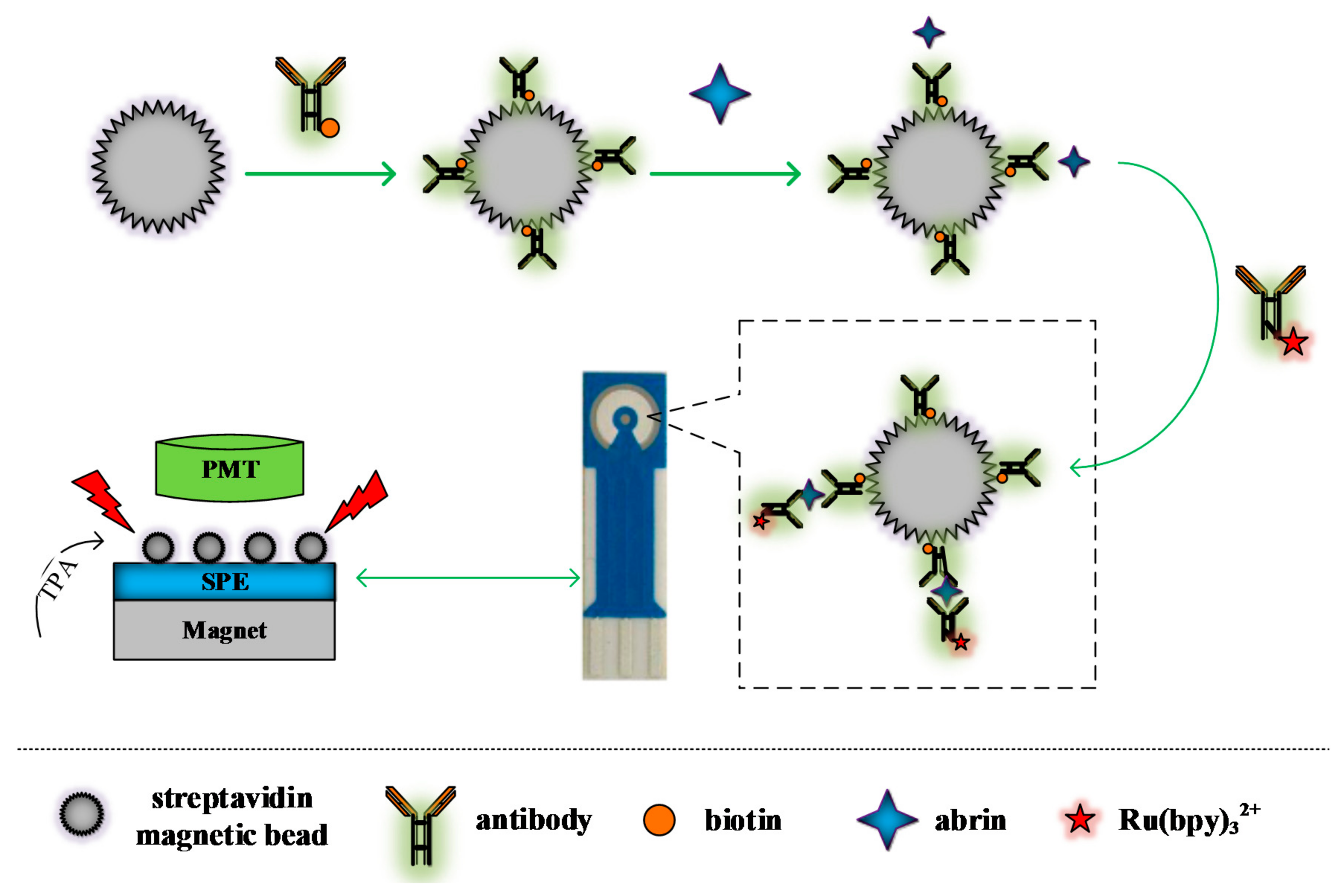
2.3.1. Preparation of Capture Probe
2.3.2. Preparation of ECL Labeled Probe
2.3.3. Determination of Abrin
3. Results and Discussion
3.1. Parameter Optimization of the Sensor
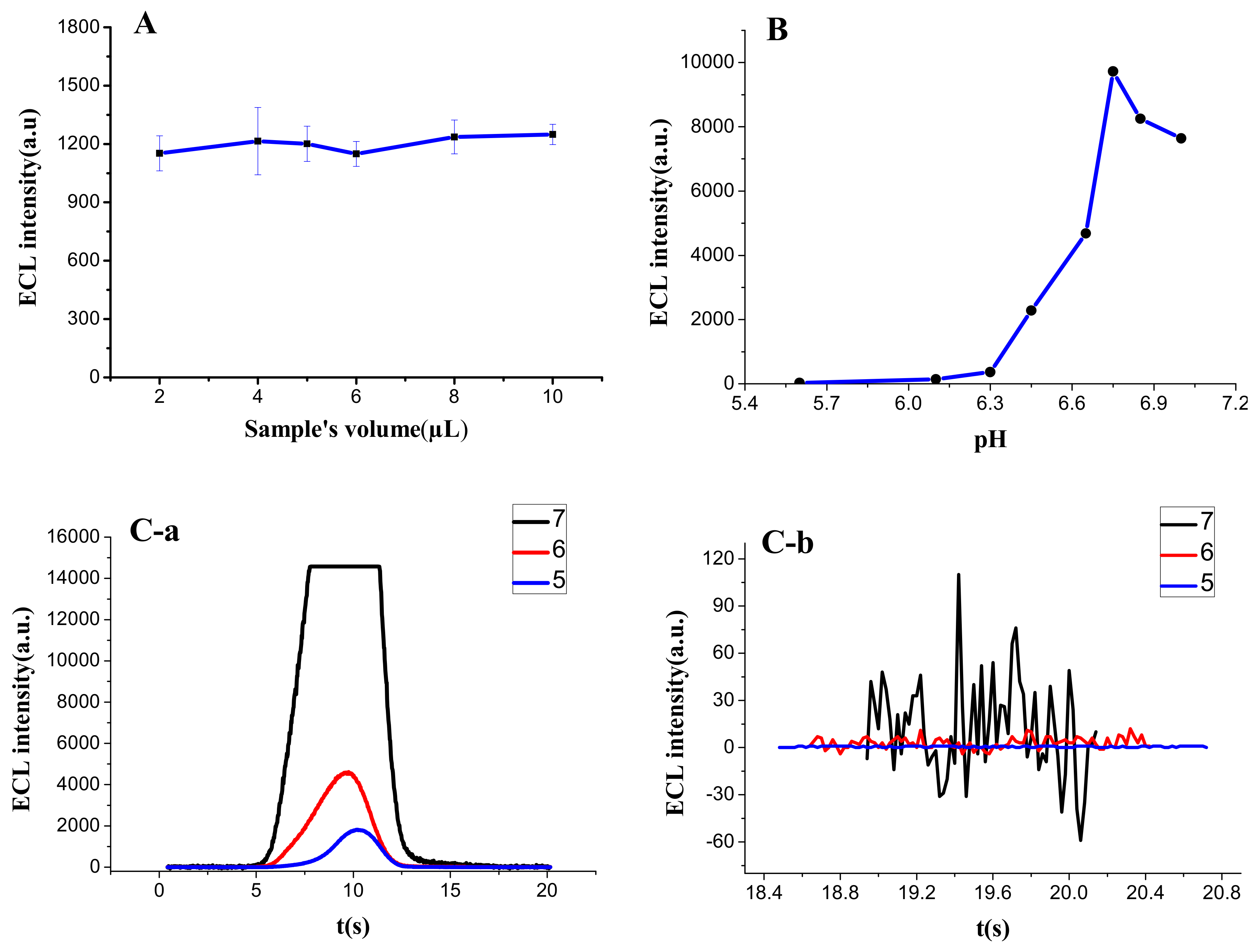
3.1.1. Additive Amount of Sample
3.1.2. pH of Sample
3.1.3. Multiplier Series of PMT
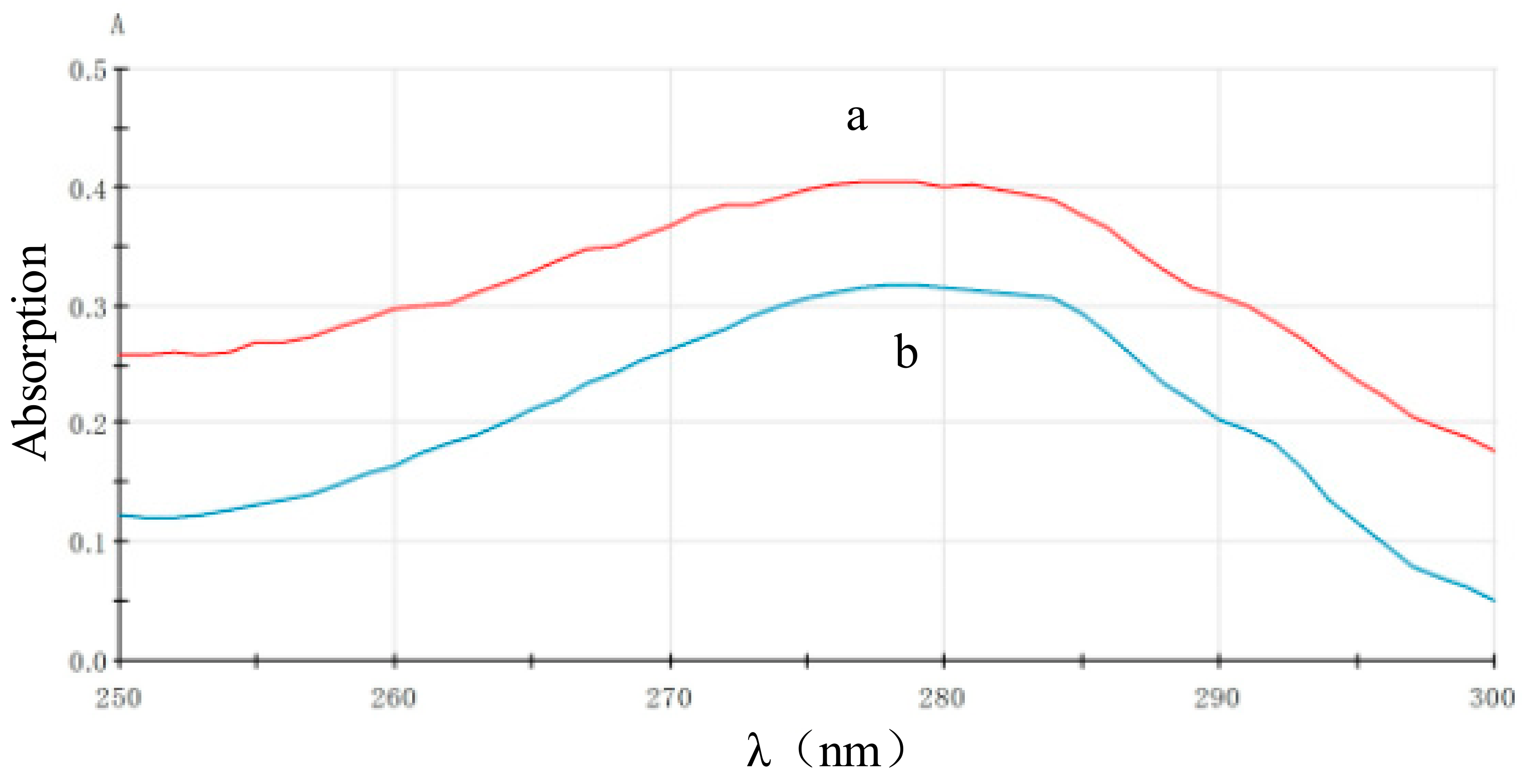
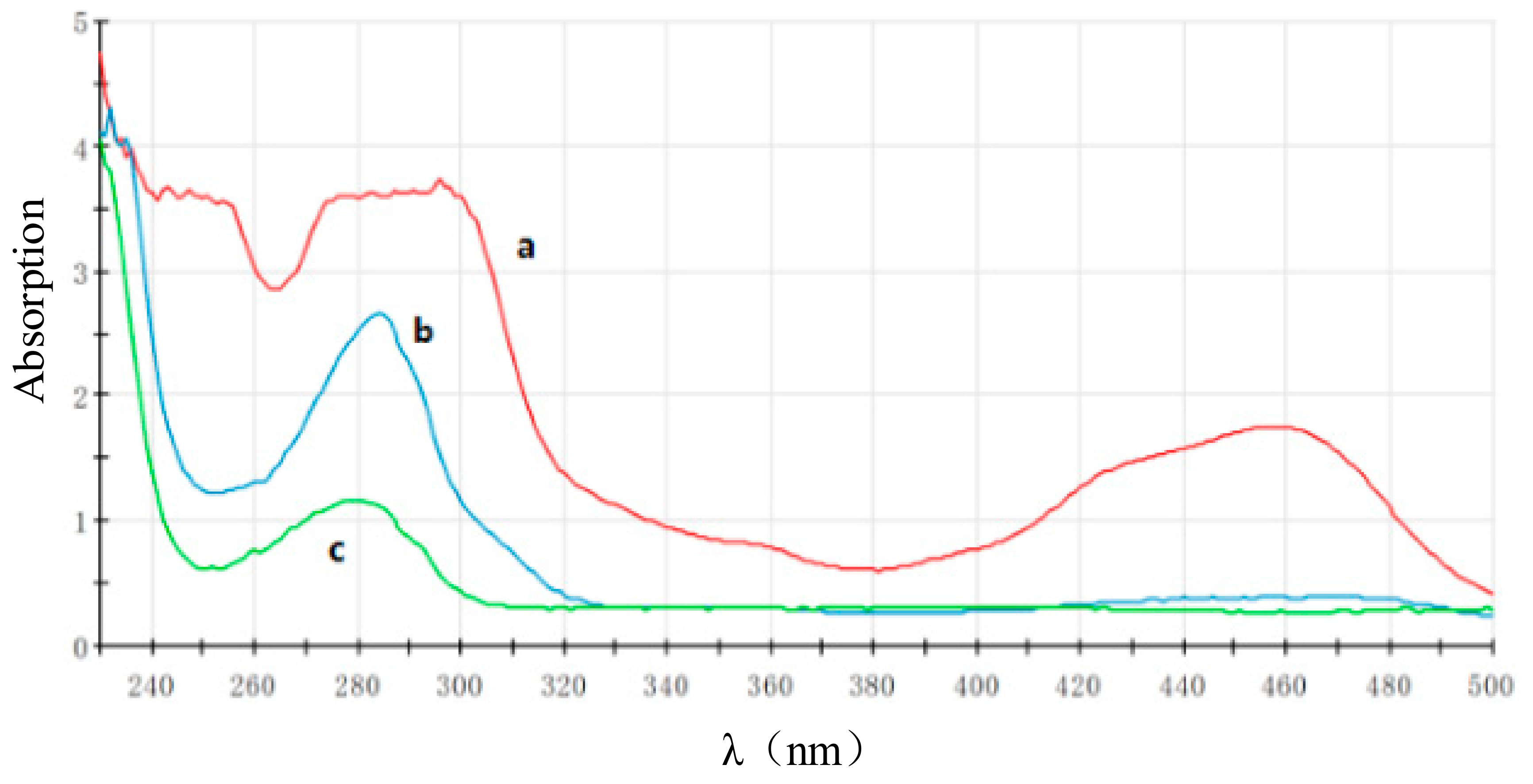
3.2. The Characterization of ECL Capture Probe
3.3. The Characterization of ECL Labeled Probe
3.3.1. Characterized by the UV-Vis Absorption Spectrum
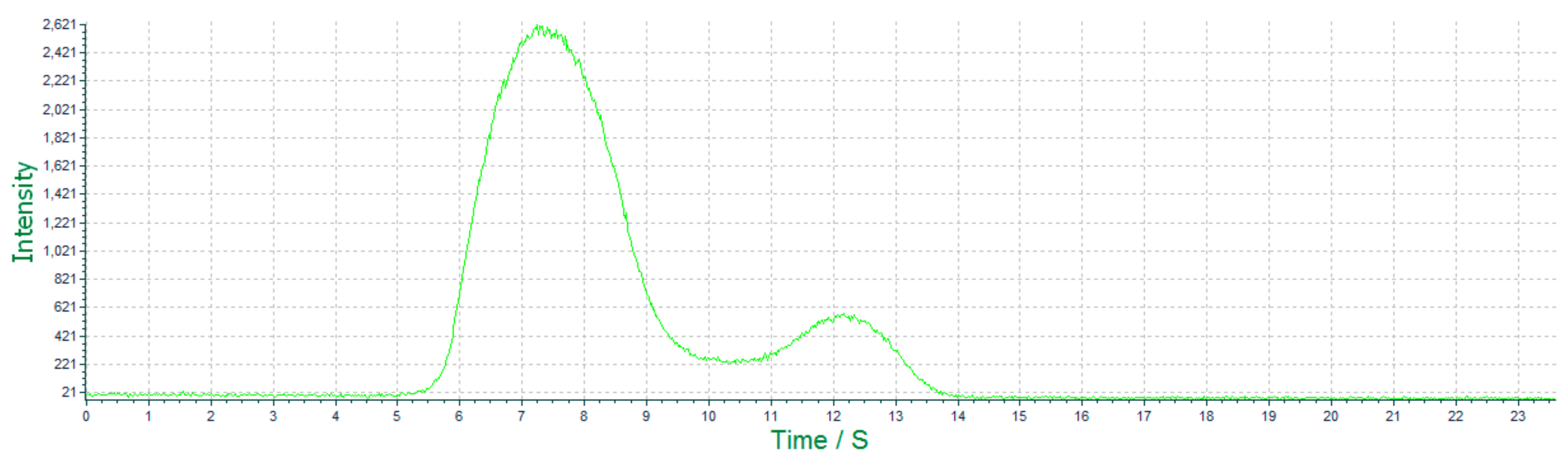
3.3.2. Characterized by ECL Scanning
3.4. The Performance of the Portable ECL Sensor
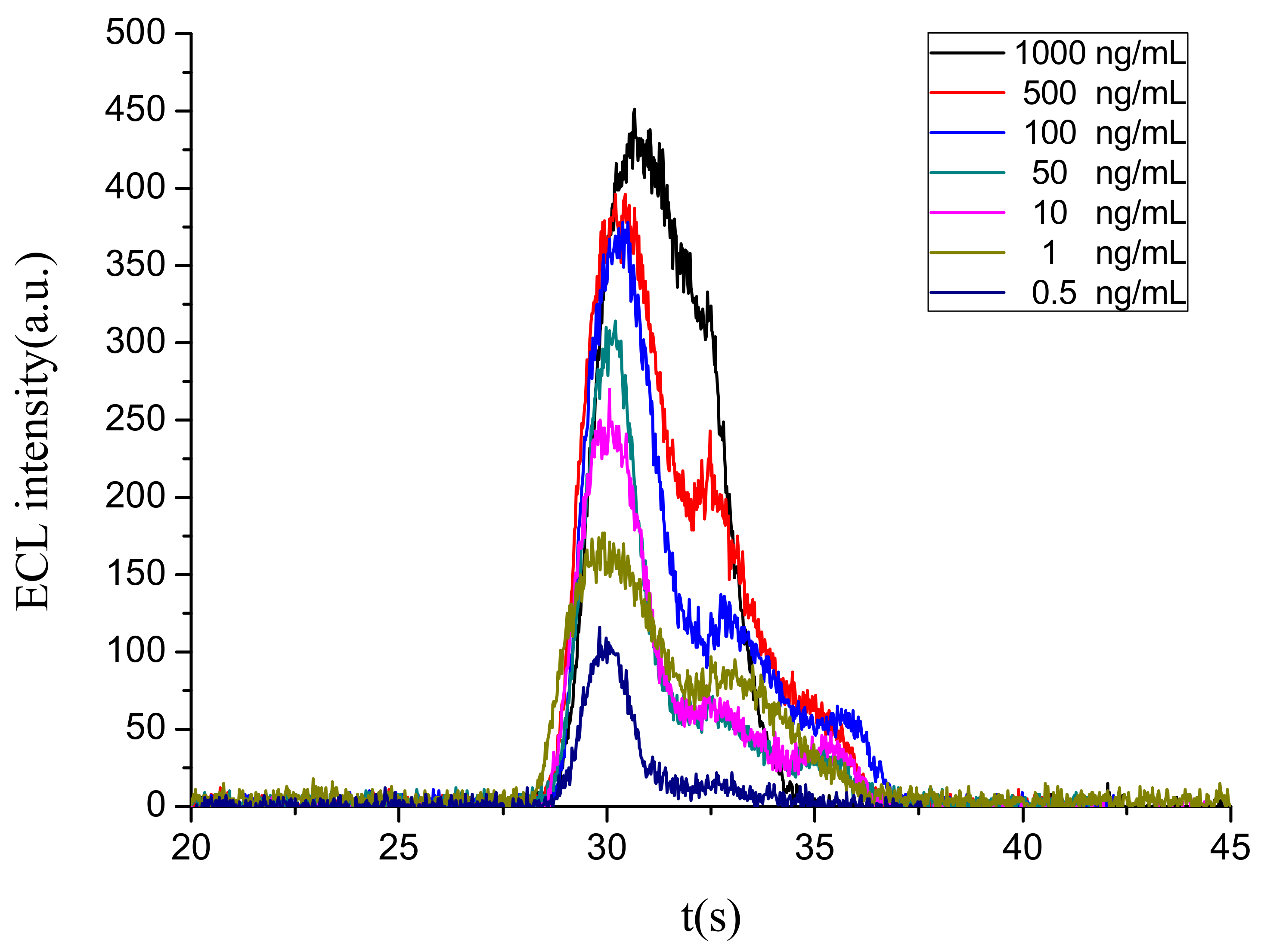
3.4.1. Limit of Detection (LOD) and Quantitative Range
3.4.2. Reproducibility
3.4.3. Specificity
3.4.4. Detection of Spiked Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olsnes, S.; Sandvig, K.; Eiklid, K.; Pihl, A. Properties and action mechanism of the toxic lectin modeccin: Interaction with cell lines resistant to modeccin, abrin, and ricin. J. Supramol. Struct. 1978, 9, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning: A comprehensive review. JAMA 2005, 294, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.J. Threats in bioterrorism. II: CDC category B and C agents. Emerg. Med. Clin. N. Am. 2002, 20, 311–330. [Google Scholar] [CrossRef]
- Garber, E.A. Toxicity and detection of ricin and abrin in beverages. J. Food Prot. 2008, 71, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Garber, E.A.; Walker, J.L.; O’Brien, T.W. Detection of abrin in food using enzyme-linked immunosorbent assay and electrochemiluminescence technologies. J. Food Prot. 2008, 71, 1868–1874. [Google Scholar] [CrossRef]
- Godal, A.; Olsnes, S.; Pihl, A. Radioimmunoassays of abrin and ricin in blood. J. Toxicol. Environ. Health 1981, 8, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Nie, C.; Wang, J.H.; Wang, J.; Kang, L.; Zhou, Y.; Wang, J.L. Colloidal gold-based immunochromatographic test strip for rapid detection of abrin in food samples. J. Food Prot. 2012, 75, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhou, Z.; Tong, Z.; Liu, B.; Hao, L. Detection of abrin by piezoelectric immunosensor based on biotin-avidin system. Chin. J. Anal. Chem. 2009, 37, 1499–1502. [Google Scholar]
- Felder, E.; Mossbrugger, I.; Lange, M.; Wölfel, R. Simultaneous detection of ricin and abrin DNA by real-time PCR (qPCR). Toxins 2012, 4, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, M.; Shan, G.; Chen, S.; Kang, L.; Wang, J.H.; Xin, W.; Zhang, T.; You, Z.; An, Y.; et al. Capillary-driven surface-enhanced Raman scattering (SERS)-based microfluidic chip for abrin detection. Nanoscale Res. Lett. 2014, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tong, Z.Y.; Liu, B. Electrochemiluminescence biosensor based on screening printed electrode. Transducer Microsyst. Technol. 2017, 36, 1–5. [Google Scholar]
- Luo, F.; Xiang, G.; Pu, X.; Yu, J.; Chen, M.; Chen, G. A novel ultrasensitive ECL sensor for DNA detection based on nicking endonuclease-assisted target recycling amplification, rolling circle amplification and hemin/g-quadruplex. Sensors 2015, 15, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Martínezolmos, A.; Ballestaclaver, J.; Palma, A.J.; Valenciamirón, M.D.C.; Capitánvallvey, L.F. A portable luminometer with a disposable electrochemiluminescent biosensor for lactate determination. Sensors 2009, 9, 7694–7710. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.H.; Yan, J.L.; Tu, Y.F. Study on a luminol-based electrochemiluminescent sensor for label-free DNA sensing. Sensors 2010, 10, 9481–9492. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gan, N.; Zhang, H.; Yan, Q.; Li, T.; Cao, Y.; Hu, F.; Yu, H.; Jiang, Q. A novel “dual-potential” electrochemiluminescence aptasensor array using CdS quantum dots and luminol-gold nanoparticles as labels for simultaneous detection of malachite green and chloramphenicol. Biosens. Bioelectron. 2015, 74, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Taokaenchan, N.; Tangkuaram, T.; Pookmanee, P.; Phaisansuthichol, S.; Kuimalee, S.; Satienperakul, S. Enhanced electrogenerated chemiluminescence of tris(2,2′-bipyridyl)ruthenium(II) system by l-cysteine-capped CdTe quantum dots and its application for the determination of nitrofuran antibiotics. Biosens. Bioelectron. 2015, 66, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Spehar-Délèze, A.M.; Julich, S.; Gransee, R.; Tomaso, H.; Dulay, S.B.; O’Sullivan, C.K. Electrochemiluminescence (ECL) immunosensor for detection of Francisella tularensis on screen-printed gold electrode array. Anal. Bioanal. Chem. 2016, 408, 7147–7153. [Google Scholar] [CrossRef] [PubMed]
- Ballesta-Claver, J.; Rodríguez-Gómez, R.; Capitán-Vallvey, L.F. Disposable biosensor based on cathodic electrochemiluminescence of tris(2,2-bipyridine)ruthenium(II) for uric acid determination. Anal. Chim. Acta 2013, 770, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Saqib, M.; Qi, L.; Zhang, W.; Xu, G. Recent advances in electrochemiluminescence devices for point-of-care testing. Curr. Opin. Electrochem. 2017, 3, 4–10. [Google Scholar] [CrossRef]
- Muzyka, K. Current trends in the development of the electrochemiluminescent immunosensors. Biosens. Bioelectron. 2014, 54, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Miao, H.E.; Shi, H.C.; Cai, Q. An amperometric immunosensor for microcystin-(leucine-arginine) based on screen-printed carbon electrode. Chin. J. Anal. Chem. 2011, 39, 443–448. [Google Scholar]
- Zhang, J.G.; Kang, T.F.; Xue, R.; Sun, X. An immunosensor for microcystins based on Fe3O4 @Au magnetic nanoparticle modified screen-printed electrode. Chin. J. Anal. Chem. 2013, 41, 1353–1358. [Google Scholar] [CrossRef]
- Lotierzo, M.; Abuknesha, R.; Davis, F.; Tothill, I.E. A membrane-based ELISA assay and electrochemical immunosensor for microcystin-LR in water samples. Environ. Sci. Technol. 2012, 46, 5504–5510. [Google Scholar] [CrossRef] [PubMed]
- Renedo, O.D.; Alonsolomillo, M.A.; Martínez, M.J. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Zahra, T.; Alireza, K.; Mohammad, M.A. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar]
- Liu, B.; Tong, Z.; Hao, L.; Mu, X.; Liu, W.; Huang, Q. Research on abrin detection by electrochemiluminescence immunosensor. Transducer Microsyst. Technol. 2013, 32, 28–30. [Google Scholar]
- Mu, X.; Tong, Z.; Huang, Q.; Liu, B.; Liu, Z.; Hao, L.; Dong, H.; Zhang, J.; Gao, C. An electrochemiluminescence immunosensor based on gold-magnetic nanoparticles and phage displayed antibodies. Sensors 2016, 16, 308. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dai, W.; Ge, L.; Yan, M.; Yu, J.; Song, X.; Ge, S.; Huang, J. Rechargeable battery-triggered electrochemiluminescence detection on microfluidic origami immunodevice based on two electrodes. Chem. Commun. 2012, 48, 9971–9973. [Google Scholar] [CrossRef] [PubMed]

| Substrate | conc. (ng/mL) | ECL Intensity | RSD (%) | Deviation (%) |
|---|---|---|---|---|
| BSA | 1000 | 63.7 | 9.6 | 1.6 |
| ricin | 1000 | 67.7 | 6.7 | 8.0 |
| Hu-IgG | 1000 | 65 | 10.1 | 3.7 |
| PBS(blank) | - | 62.7 | 10.3 | - |
| Sample | Added (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| milk | 100 | 97.7 | 97.7 | 12.7 |
| honey | 100 | 100.65 | 100.7 | 10.1 |
| soil | 100 | 89.1 | 89.1 | 9.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tong, Z.; Mu, X.; Liu, B.; Du, B.; Liu, Z.; Gao, C. Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode. Sensors 2018, 18, 357. https://doi.org/10.3390/s18020357
Liu S, Tong Z, Mu X, Liu B, Du B, Liu Z, Gao C. Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode. Sensors. 2018; 18(2):357. https://doi.org/10.3390/s18020357
Chicago/Turabian StyleLiu, Shuai, Zhaoyang Tong, Xihui Mu, Bing Liu, Bin Du, Zhiwei Liu, and Chuan Gao. 2018. "Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode" Sensors 18, no. 2: 357. https://doi.org/10.3390/s18020357
APA StyleLiu, S., Tong, Z., Mu, X., Liu, B., Du, B., Liu, Z., & Gao, C. (2018). Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode. Sensors, 18(2), 357. https://doi.org/10.3390/s18020357




