Efficient Fiducial Point Detection of ECG QRS Complex Based on Polygonal Approximation
Abstract
1. Introduction
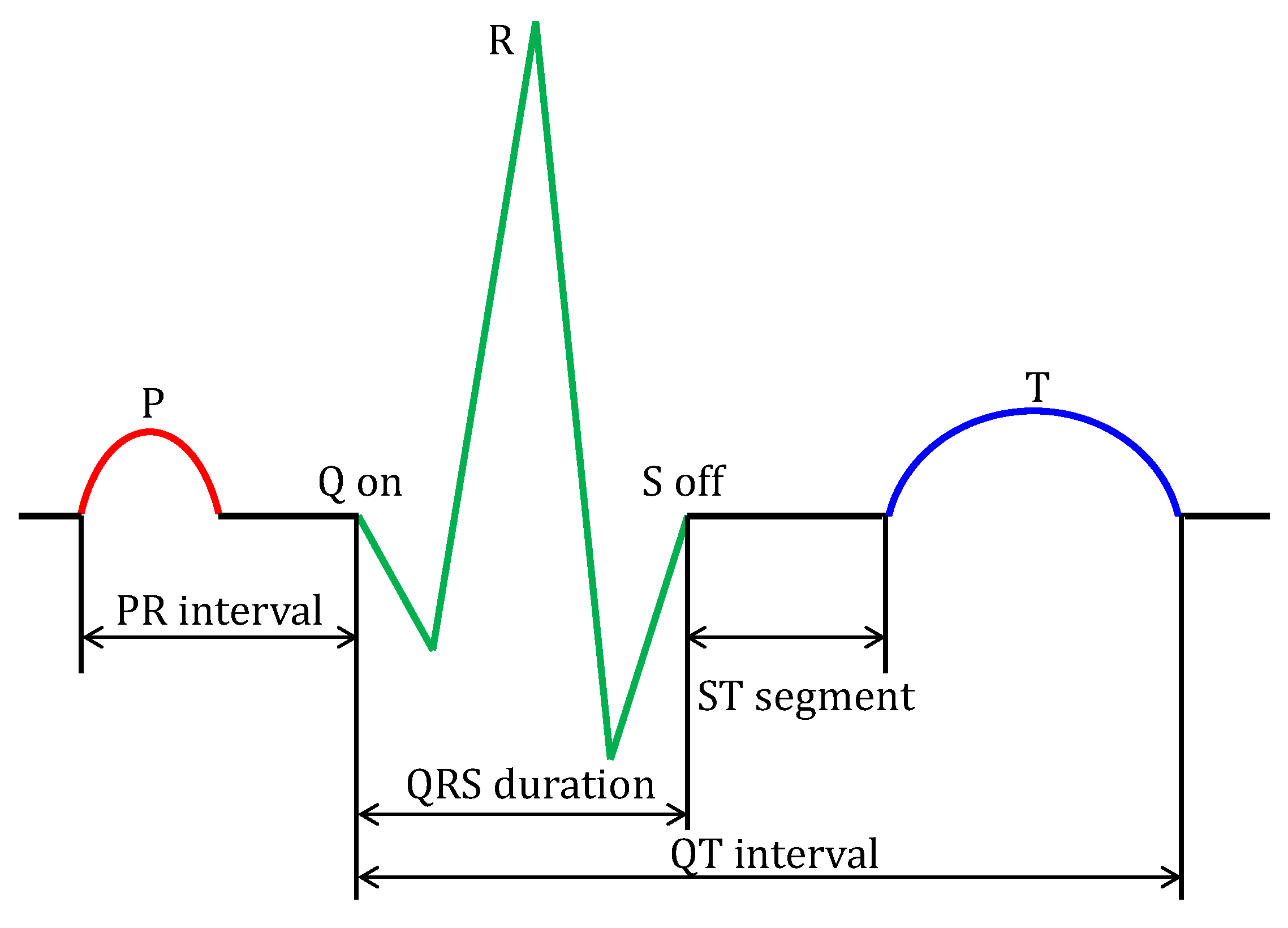
2. Composition of ECG Signal
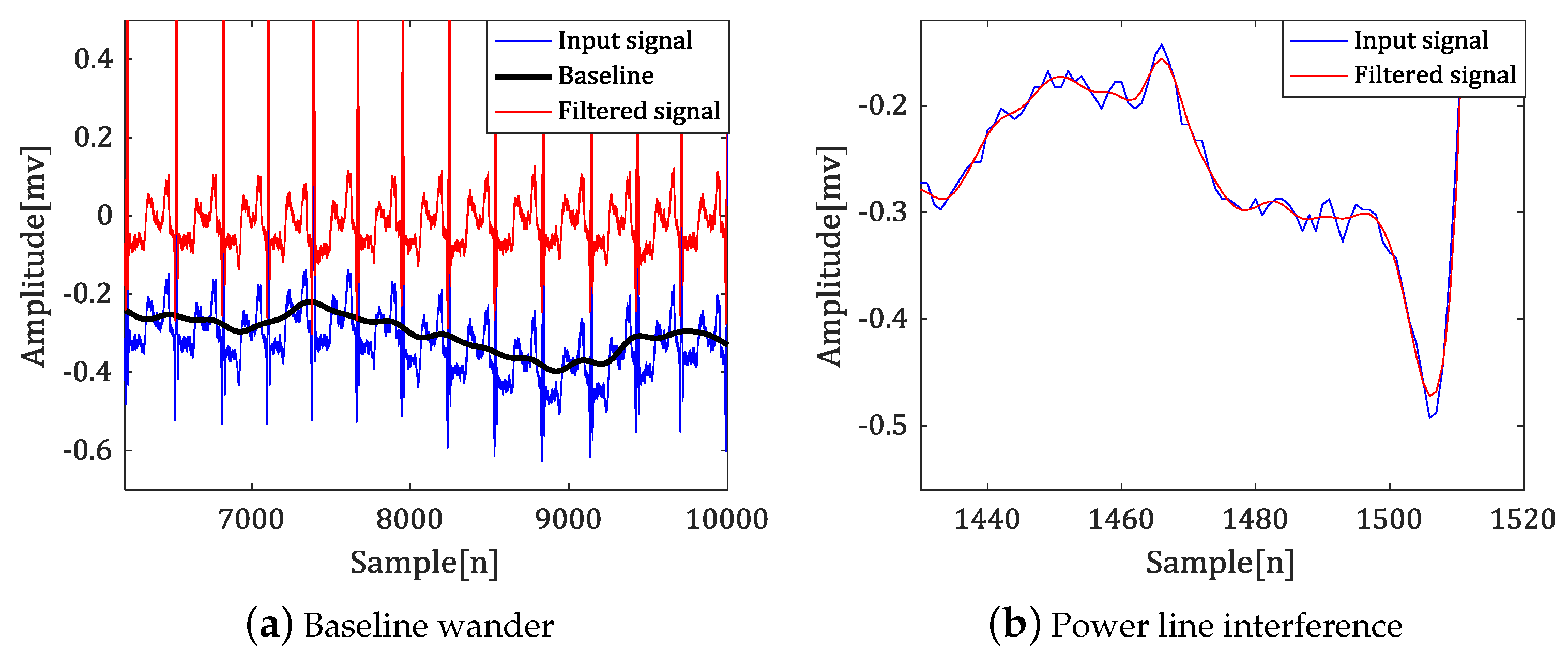
- Power line interference: various high frequency noise according to country.
- Baseline wander: a low-frequency noise (0.15 up to 0.3 Hz). This noise results from the patient breathing and leads to a baseline shift in the signals.
- Electrode contract noise, electrode motion artifacts, muscle contractions, electrosurgical noise, instrumentation noise, and so on.
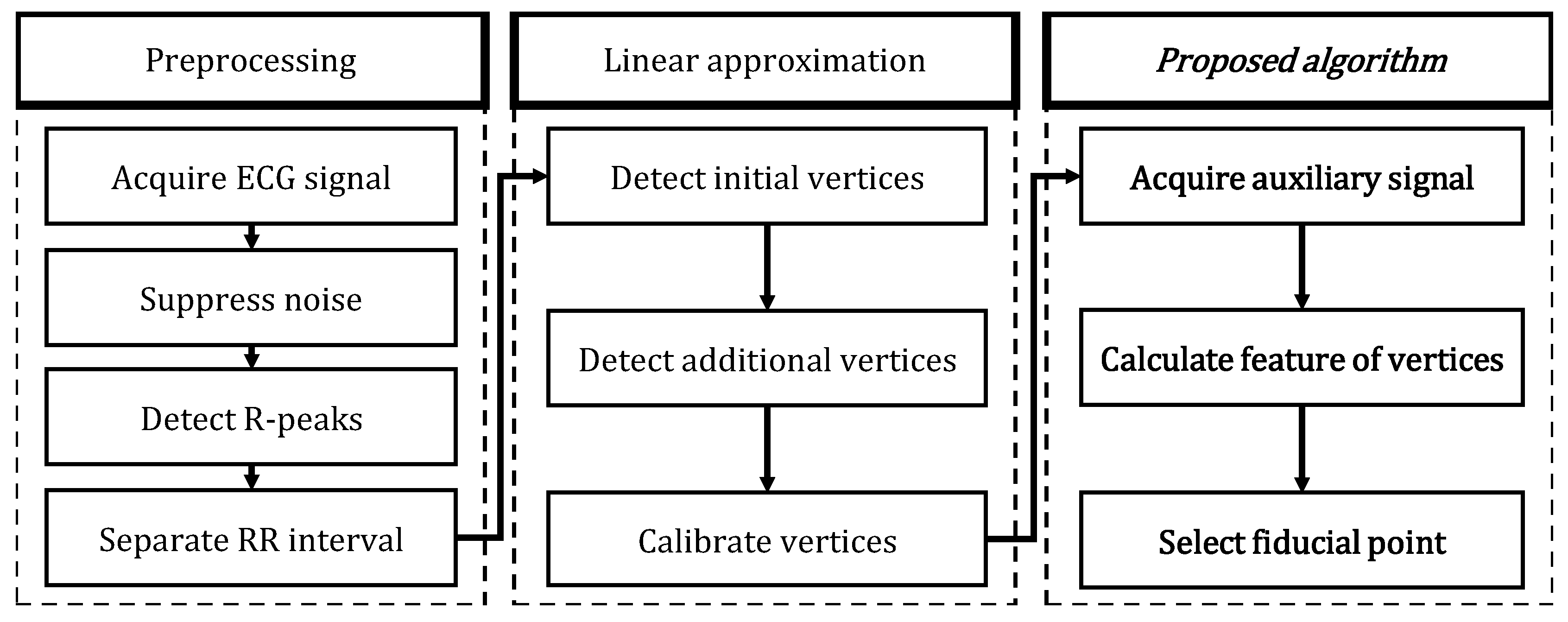
3. Polygonal Approximation of ECG Signal
- Separate the R-R section of the input signal. In this paper, we detect the R-peak by Pan’s method.
- After calculating the curvature for the separated R-R section, the curvature-based polygonal approximation technique is applied to select the initial vertices. Equation (1) represents the set of initial vertices.
- We apply the sequential polygonal approximation method to the interval between each initial vertex to select additional vertices. Equation (2) represents a set of additional vertices between the i-th initial vertex and the -th initial vertex, and both end vertices coincide with the two initial vertices.
- Dynamic programming is applied to the additional vertices to optimize their position. Equation (3) is a set of corrected vertices for the additional vertex set .
- Repeat steps 2–4 to proceed with polygonal approximation for the entire input signal. Equation (4) represents the set of vertices as the result of vertex selection.
4. Fiducial Point Detection Based on Polygonal Approximation
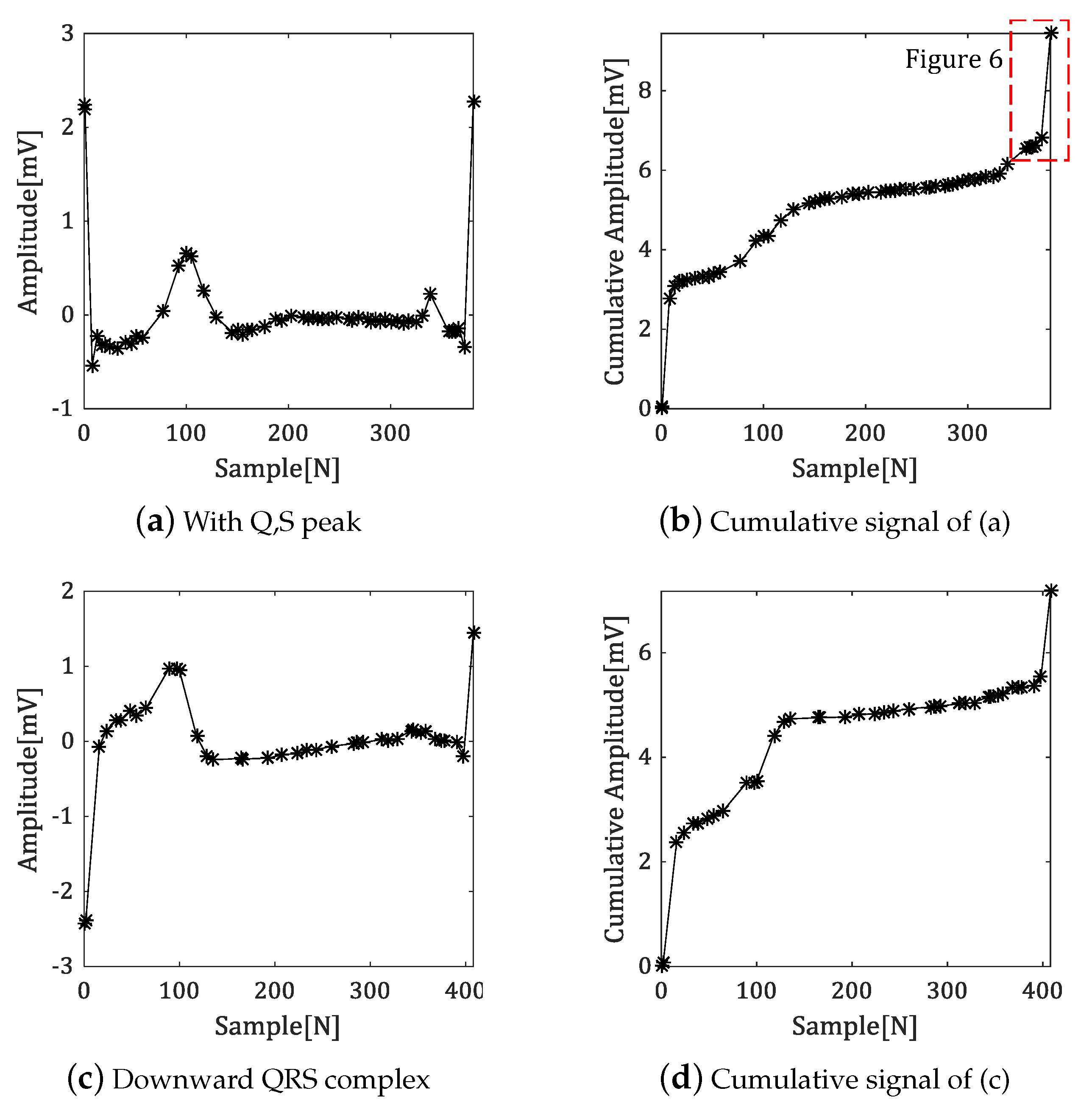
4.1. Generate the Cumulative Signal
4.2. Algorithm of Fiducial Point Detection
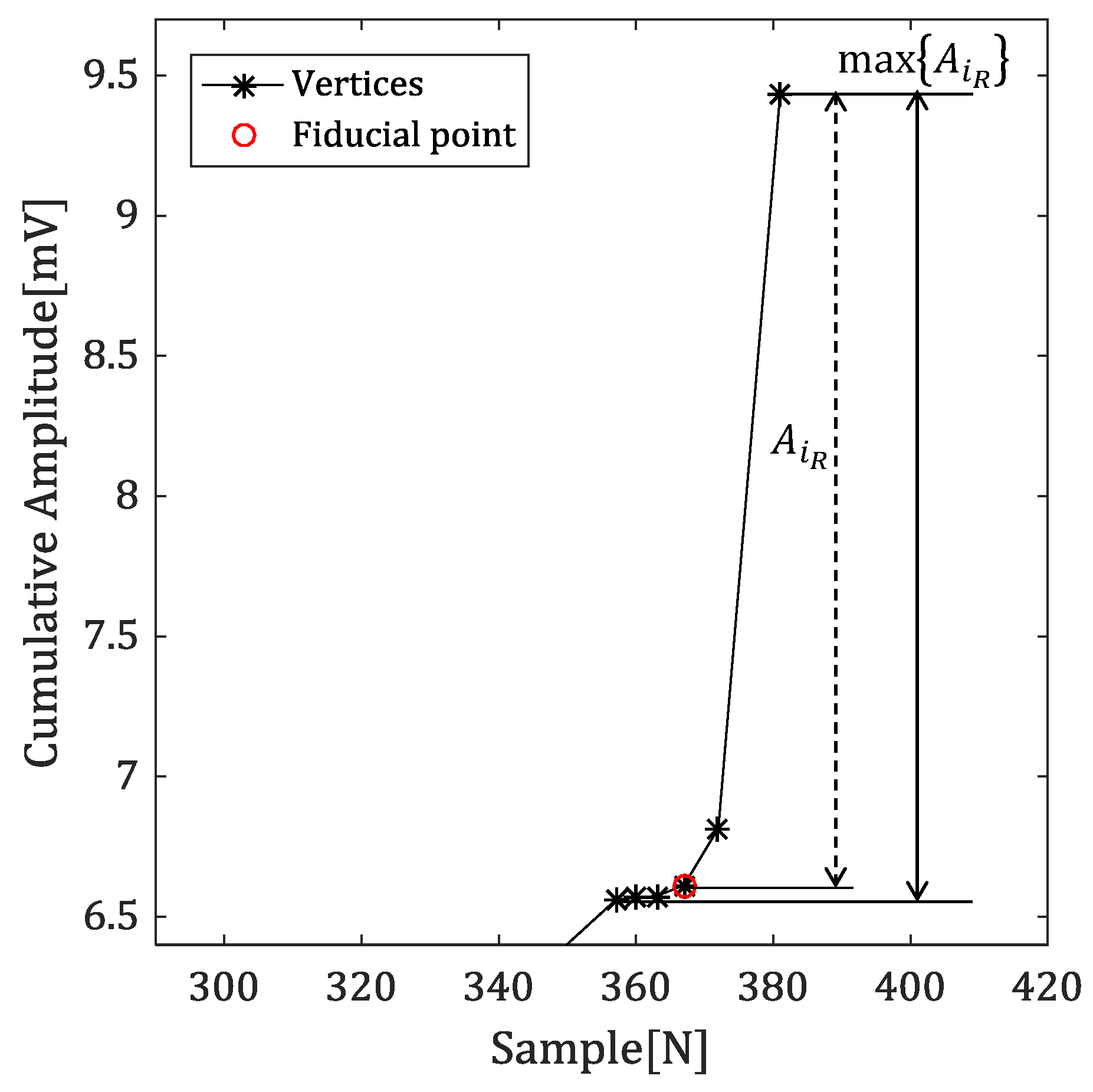
4.2.1. Amplitude Difference between R-Peak and Vertex
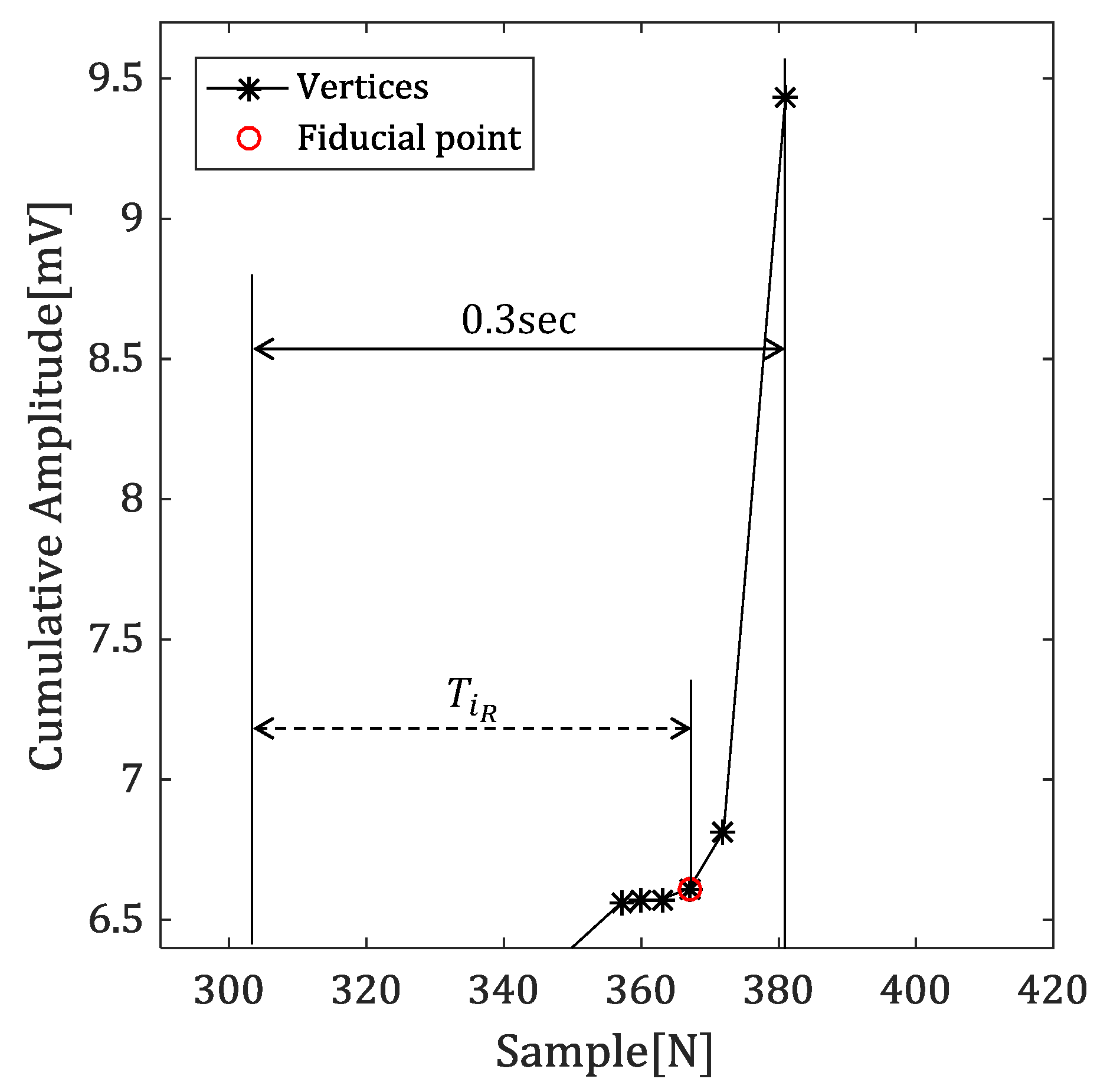
4.2.2. Time Difference between Reference Point and Vertex
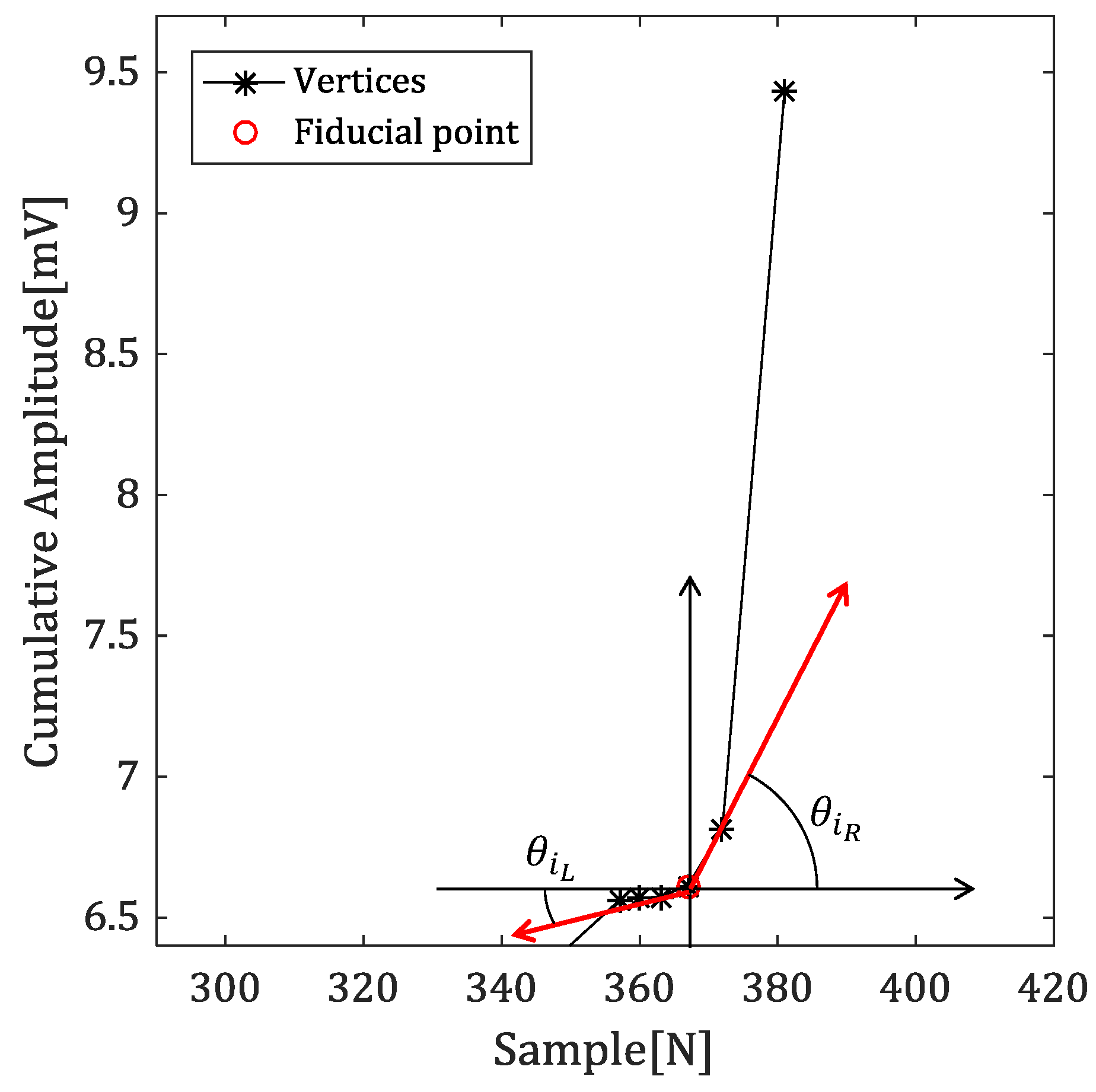
4.2.3. Angles with Neighbor Vertices
4.2.4. Detecting the Fiducial Point
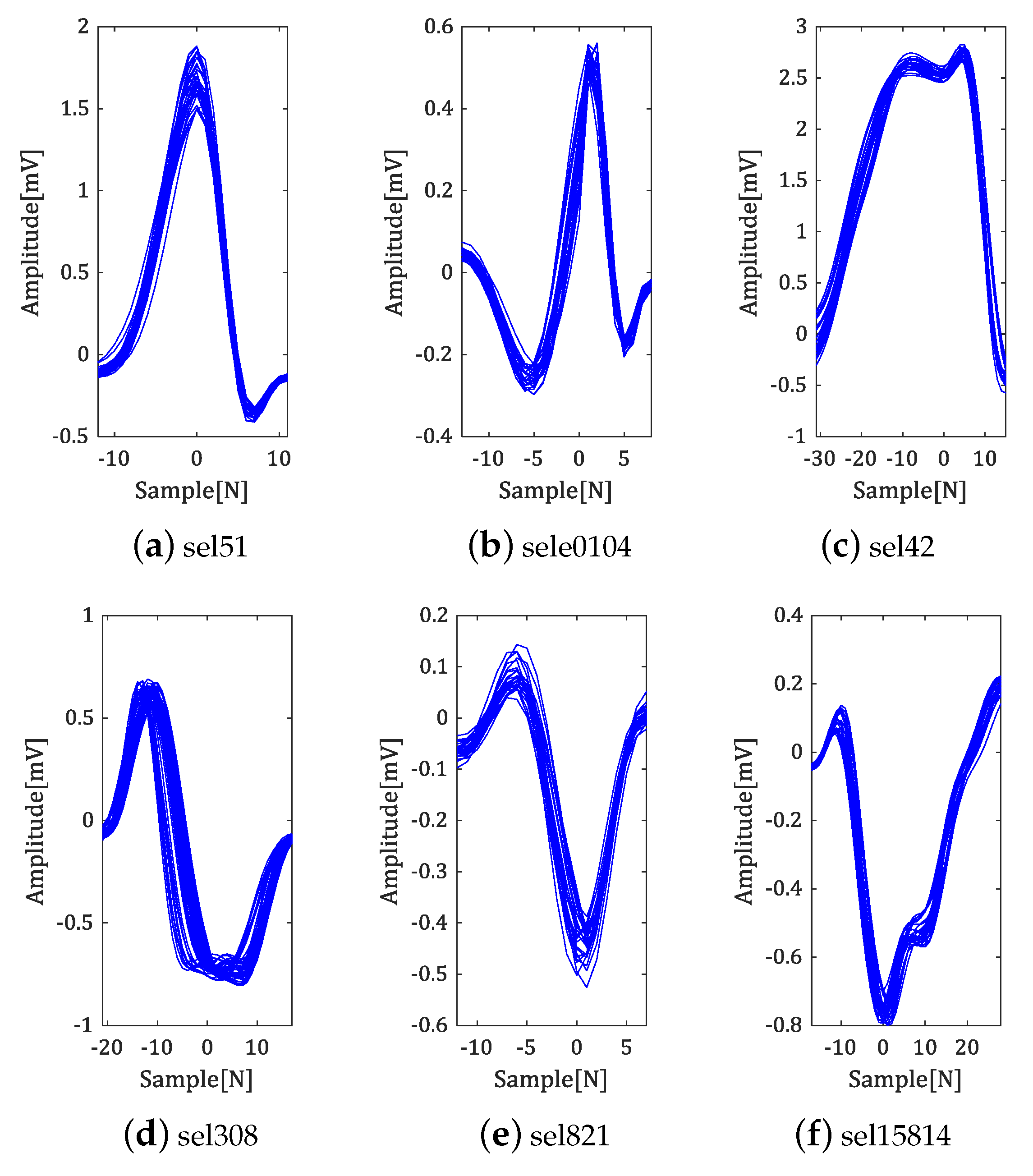
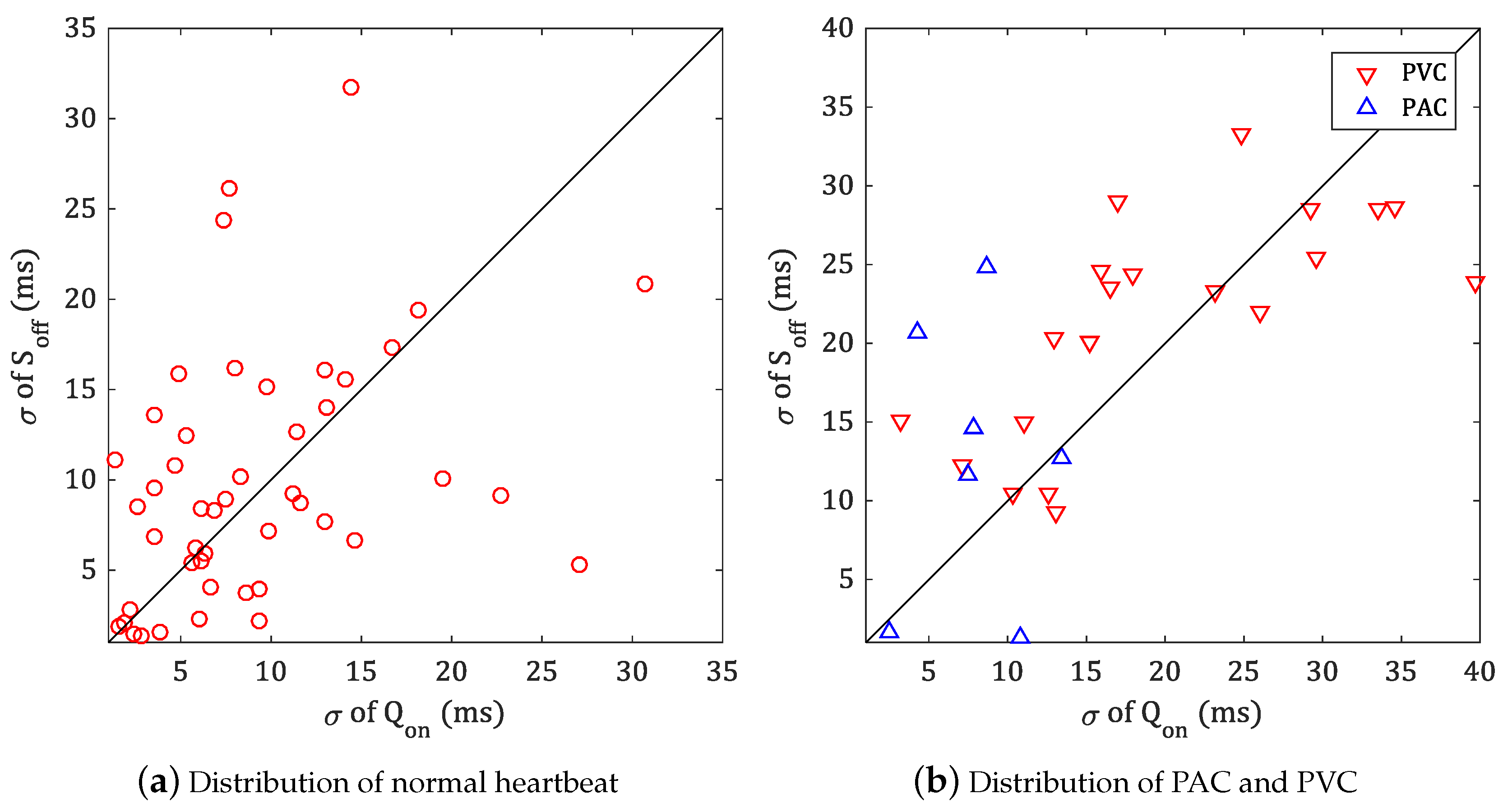
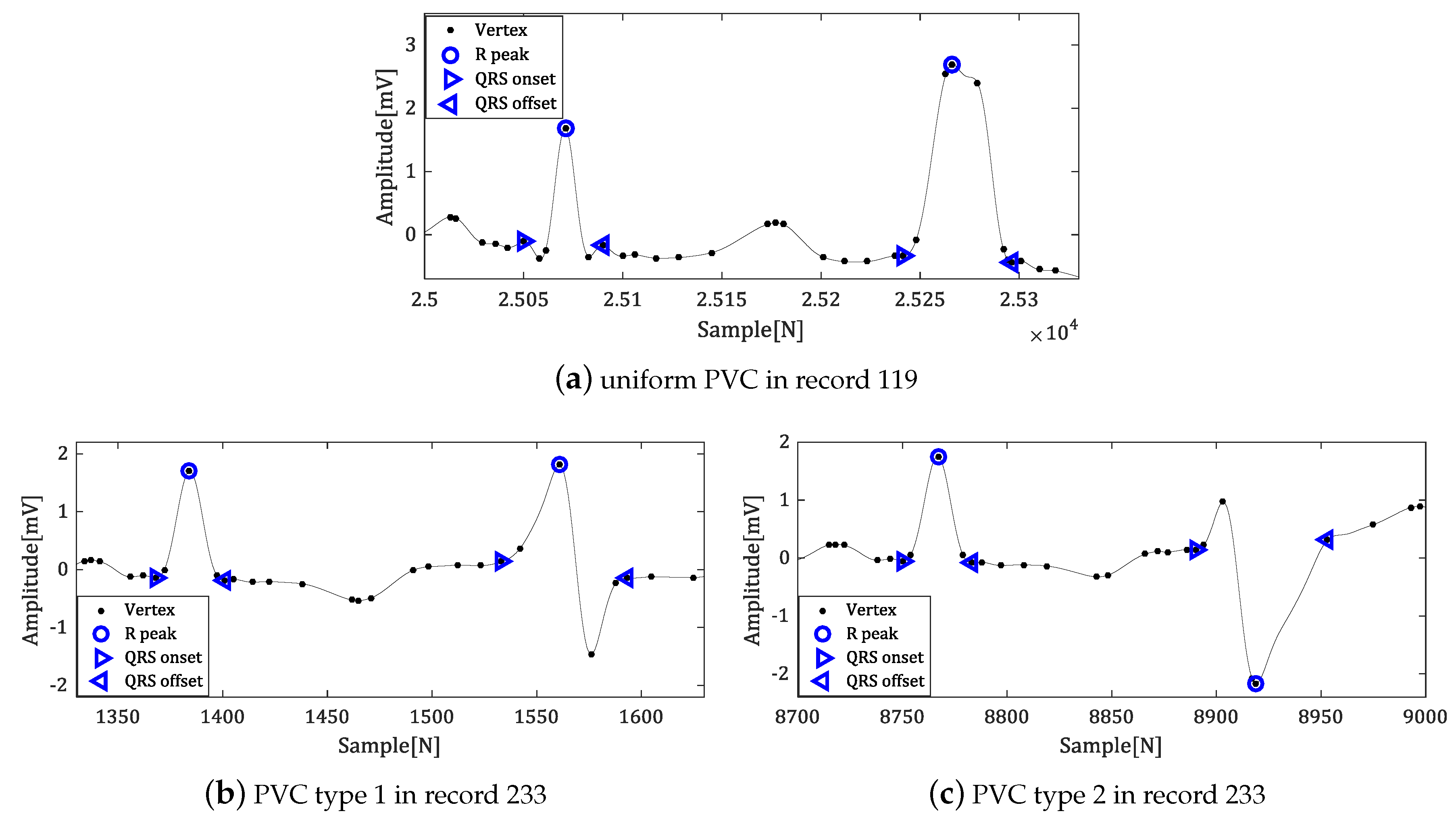
5. Experiment and Analysis of Results
5.1. Experiment in QT-DB
5.2. Experiment in MIT-BIH ADB
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| PVC | Premature Ventricular Contraction |
| PAC | Premature Atrial Contraction |
| SVP | Supraventricular premature |
| LBBB | Left Bundle Branch Block |
| RBBB | Right Bundle Branch Block |
| PM | PaceMaker |
| MLII | Modified Lead II |
References
- Huszar, R.J. Basic Dysrhythmias: Interpretation & Management; Mosby Jems/Elsevier: Maryland Heights, MO, USA, 2007. [Google Scholar]
- Chan, H.; Chou, W.; Chen, S.; Fang, S.; Liou, C.; Hwang, Y. Continuous and online analysis of heart rate variability. J. Med. Eng. Technol. 2005, 29, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Azuaje, F.; McSharry, P. Advanced Methods and Tools for ECG Data Analysis; Artech House, Inc.: Norwood, MA, USA, 2006. [Google Scholar]
- Oussama, B.M.; Saadi, B.M.; Zine-Eddine, H.S. Extracting Features from ECG and Respiratory Signals for Automatic Supervised Classification of Heartbeat Using Neural Networks. Asian J. Inf. Technol. 2015, 14, 53–59. [Google Scholar]
- Salai, M.; Vassányi, I.; Kósa, I. Stress Detection Using Low Cost Heart Rate Sensors. J. Healthc. Eng. 2016, 2016, 5136705. [Google Scholar] [CrossRef] [PubMed]
- Covello, R.; Fortino, G.; Gravina, R.; Aguilar, A.; Breslin, J.G. Novel method and real-time system for detecting the Cardiac Defense Response based on the ECG. In Proceedings of the 2013 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Gatineau, QC, Canada, 4–5 May 2013; pp. 53–57. [Google Scholar]
- Poungponsri, S.; Yu, X.H. An adaptive filtering approach for electrocardiogram (ECG) signal noise reduction using neural networks. Neurocomputing 2013, 117, 206–213. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef]
- Nygårds, M.E.; Sörnmo, L. Delineation of the QRS complex using the envelope of the e.c.g. Med. Biol. Eng. Comput. 1983, 21, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.P.; Almeida, R.; Olmos, S.; Rocha, A.P.; Laguna, P. A wavelet-based ECG delineator: Evaluation on standard databases. IEEE Trans. Biomed. Eng. 2004, 51, 570–581. [Google Scholar] [CrossRef]
- Dokur, Z.; Ölmez, T. ECG beat classification by a novel hybrid neural network. Comput. Methods Programs Biomed. 2001, 66, 167–181. [Google Scholar] [CrossRef]
- Hu, Y.H.; Palreddy, S.; Tompkins, W.J. A patient-adaptable ECG beat classifier using a mixture of experts approach. IEEE Trans. Biomed. Eng. 1997, 44, 891–900. [Google Scholar]
- Tsipouras, M.G.; Fotiadis, D.I.; Sideris, D. Arrhythmia classification using the RR-interval duration signal. In Proceedings of the Computers in Cardiology, Memphis, TN, USA, 22–25 September 2002; pp. 485–488. [Google Scholar]
- Kumar, A.; Komaragiri, R.; Kumar, M. Design of wavelet transform-based electrocardiogram monitoring system. ISA Trans. 2018, 80, 381–398. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.Y.; Kim, J.H.; Yun, B.J.; Park, K.H. Curvature-based ECG signal compression for effective communication on WPAN. J. Commun. Netw. 2012, 14, 21–26. [Google Scholar] [CrossRef]
- Mamaghanian, H.; Khaled, N.; Atienza, D.; Vandergheynst, P. Compressed Sensing for Real-Time Energy-Efficient ECG Compression on Wireless Body Sensor Nodes. IEEE Trans. Biomed. Eng. 2011, 58, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Israel, S.A.; Irvine, J.M.; Cheng, A.; Wiederhold, M.D.; Wiederhold, B.K. ECG to identify individuals. Pattern Recognit. 2005, 38, 133–142. [Google Scholar] [CrossRef]
- Arteaga-Falconi, J.S.; Osman, H.A.; Saddik, A.E. ECG Authentication for Mobile Devices. IEEE Trans. Instrum. Meas. 2016, 65, 591–600. [Google Scholar] [CrossRef]
- Lin, H.Y.; Liang, S.Y.; Ho, Y.L.; Lin, Y.H.; Ma, H.P. Discrete-wavelet-transform-based noise removal and feature extraction for ECG signals. IRBM 2014, 35, 351–361. [Google Scholar] [CrossRef]
- Martinez, A.; Alcaraz, R.; Rieta, J.J. Application of the phasor transform for automatic delineation of single-lead ECG fiducial points. Physiol. Meas. 2010, 31, 1467. [Google Scholar] [CrossRef]
- Manriquez, A.I.; Zhang, Q. An algorithm for robust detection of QRS onset and offset in ECG signals. In Proceedings of the Computers in Cardiology, Bologna, Italy, 14–17 September 2008; pp. 857–860. [Google Scholar]
- Madeiro, J.P.; Cortez, P.C.; Marques, J.A.; Seisdedos, C.R.; Sobrinho, C.R. An innovative approach of QRS segmentation based on first-derivative, Hilbert and Wavelet Transforms. Med. Eng. Phys. 2012, 34, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Laguna, P.; Jané, R.; Caminal, P. Automatic Detection of Wave Boundaries in Multilead ECG Signals: Validation with the CSE Database. Comput. Biomed. Res. 1994, 27, 45–60. [Google Scholar] [CrossRef]
- Laguna, P.; Mark, R.G.; Goldberg, A.; Moody, G.B. A database for evaluation of algorithms for measurement of QT and other waveform intervals in the ECG. In Proceedings of the Computers in Cardiology, Lund, Sweden, 7–10 September 1997; pp. 673–676. [Google Scholar]
- Moody, G.B.; Mark, R.G. The MIT-BIH Arrhythmia Database on CD-ROM and software for use with it. In Proceedings of the Computers in Cardiology, Chicago, IL, USA, 23–26 September 1990; pp. 185–188. [Google Scholar]
- Merone, M.; Soda, P.; Sansone, M.; Sansone, C. ECG databases for biometric systems: A systematic review. Expert Syst. Appl. 2017, 67, 189–202. [Google Scholar] [CrossRef]
- Elhaj, F.A.; Salim, N.; Harris, A.R.; Swee, T.T.; Ahmed, T. Arrhythmia recognition and classification using combined linear and nonlinear features of ECG signals. Comput. Methods Programs Biomed. 2016, 127, 52–63. [Google Scholar] [CrossRef]
- Friesen, G.M.; Jannett, T.C.; Jadallah, M.A.; Yates, S.L.; Quint, S.R.; Nagle, H.T. A comparison of the noise sensitivity of nine QRS detection algorithms. IEEE Trans. Biomed. Eng. 1990, 37, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Berkaya, S.K.; Uysal, A.K.; Gunal, E.S.; Ergin, S.; Gunal, S.; Gulmezoglu, M.B. A survey on ECG analysis. Biomed. Signal Process. Control 2018, 43, 216–235. [Google Scholar] [CrossRef]
- Alcaraz, R.; Hornero, F.; Rieta, J.J. Dynamic time warping applied to estimate atrial fibrillation temporal organization from the surface electrocardiogram. Med. Eng. Phys. 2013, 35, 1341–1348. [Google Scholar] [CrossRef]
- Yun, B.J. Curvature-Based Vertex Selection for Reducing Contour Information. Ph.D. Thesis, Korea Advanced Institute of Science and Technology, Daejeon, Korea, 2002. [Google Scholar]
- Mokhtarian, F.; Suomela, R. Robust image corner detection through curvature scale space. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 1376–1381. [Google Scholar] [CrossRef]
- O’Connell, K.J. Object-adaptive vertex-based shape coding method. IEEE Trans. Circuits Syst. Video Technol. 1997, 7, 251–255. [Google Scholar] [CrossRef]
- Bellman, R.; Dreyfus, S. Applied Dynamic Programming; Princeton Legacy Library, Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- The CSE Working Party. Recommendations for measurement standards in quantitative electrocardiography. Eur. Heart J. 1985, 6, 815–825. [Google Scholar]
- Yazdani, S.; Vesin, J.M. Extraction of QRS fiducial points from the ECG using adaptive mathematical morphology. Digit. Signal Process. 2016, 56, 100–109. [Google Scholar] [CrossRef]
- Ghaffari, A.; Homaeinezhad, M.; Akraminia, M.; Atarod, M.; Daevaeiha, M. A robust wavelet-based multi-lead electrocardiogram delineation algorithm. Med. Eng. Phys. 2009, 31, 1219–1227. [Google Scholar] [CrossRef]
- Manriquez, A.I.; Zhang, Q. An algorithm for QRS onset and offset detection in single lead electrocardiogram records. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; pp. 541–544. [Google Scholar]
- Dumont, J.; Hernandez, A.I.; Carrault, G. Parameter optimization of awavelet-based electrocardiogram delineator with an evolutionary algorithm. In Proceedings of the Computers in Cardiology, Lyon, France, 25–28 September 2005; pp. 707–710. [Google Scholar]
- Jane, R.; Blasi, A.; Garcia, J.; Laguna, P. Evaluation of an automatic threshold-based detector of waveform limits in Holter ECG with the QT database. In Proceedings of the Computers in Cardiology, Lund, Sweden, 7–10 September 1997; pp. 295–298. [Google Scholar]


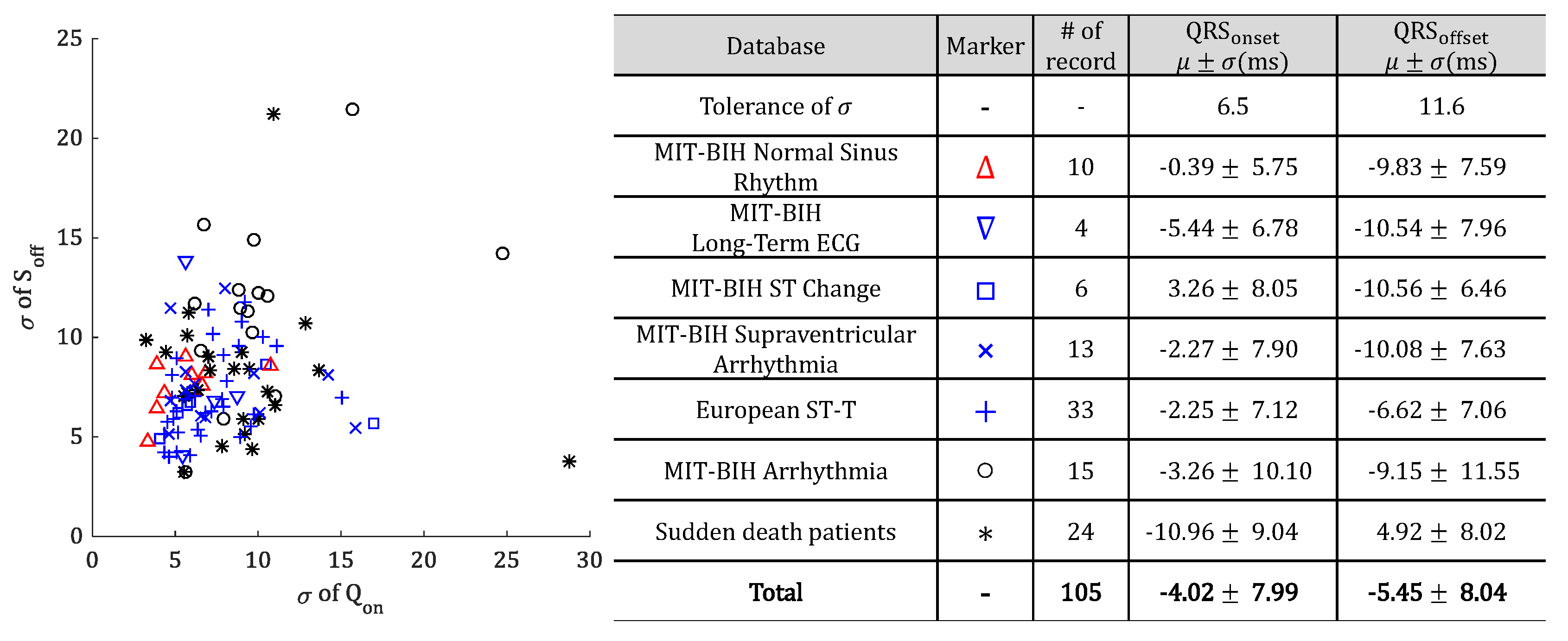
| Method | Ref | QRS Onset (ms) | QRS Offset (ms) |
|---|---|---|---|
| This work | - | −4.02 ± 7.99 | −5.45 ± 8.04 |
| Yazdani and Vesin | [36] | 6.16 ± 8.3 | 1.5 ± 4.2 |
| Martinez et al. | [20] | −0.2 ± 7.2 | 2.5 ± 8.9 |
| Ghaffari et al. | [37] | −0.6 ± 8.0 | 0.3 ± 8.8 |
| Manriquez and Zhang | [21] | −2.6 ± 7.1 | 0.7 ± 8.0 |
| Manriquez and Zhang | [38] | 0.58 ± 7.18 | −0.95 ± 8.25 |
| Dumont et al. | [39] | 0.3 ± 6.6 | −1.9 ± 8.3 |
| Martinez et al. | [10] | 4.6 ± 7.7 | 0.8 ± 8.7 |
| Jane et al. | [40] | −7.82 ± 10.86 | −3.64 ± 10.74 |
| Laguna et al. | [23] | −3.6 ± 8.6 | −1.1 ± 8.3 |
| Tolerance | [35] | 6.5 | 11.6 |
| Record of PAC | ♯ of Beat | of Onset (ms) | of Offset (ms) | Record of PVC | ♯ of Beat | of Onset (ms) | of Offset (ms) |
|---|---|---|---|---|---|---|---|
| 100 | 33 | 2.45 | 1.59 | 114 | 43 | 12.64 | 10.48 |
| 207 | 106 | 7.89 | 14.60 | 116 | 109 | 13.04 | 9.29 |
| 209 | 383 | 7.50 | 11.61 | 119 | 444 | 3.21 | 15.14 |
| 220 | 94 | 10.85 | 1.28 | 201 | 198 | 7.09 | 12.23 |
| 222 | 212 | 15.28 | 16.78 | 208 | 992 | 11.02 | 14.96 |
| 232 | 1381 | 8.66 | 24.80 | 221 | 396 | 10.31 | 10.45 |
| Average | 8.77 | 11.78 | Average | 9.55 | 12.09 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Jeong, Y.; Park, D.; Yun, B.-J.; Park, K.H. Efficient Fiducial Point Detection of ECG QRS Complex Based on Polygonal Approximation. Sensors 2018, 18, 4502. https://doi.org/10.3390/s18124502
Lee S, Jeong Y, Park D, Yun B-J, Park KH. Efficient Fiducial Point Detection of ECG QRS Complex Based on Polygonal Approximation. Sensors. 2018; 18(12):4502. https://doi.org/10.3390/s18124502
Chicago/Turabian StyleLee, Seungmin, Yoosoo Jeong, Daejin Park, Byoung-Ju Yun, and Kil Houm Park. 2018. "Efficient Fiducial Point Detection of ECG QRS Complex Based on Polygonal Approximation" Sensors 18, no. 12: 4502. https://doi.org/10.3390/s18124502
APA StyleLee, S., Jeong, Y., Park, D., Yun, B.-J., & Park, K. H. (2018). Efficient Fiducial Point Detection of ECG QRS Complex Based on Polygonal Approximation. Sensors, 18(12), 4502. https://doi.org/10.3390/s18124502






