Selective Functionalization of High-Resolution Cu2O Nanopatterns via Galvanic Replacement for Highly Enhanced Gas Sensing Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Cu2O Nanopattern Fabrication
2.2. Galvanic Reaction
2.3. Characterization
2.4. Sensor Fabrication and Measurement
3. Results and Discussion
3.1. Fabrication of Pt Decorated High-Resolution Cu2O Nanochannel
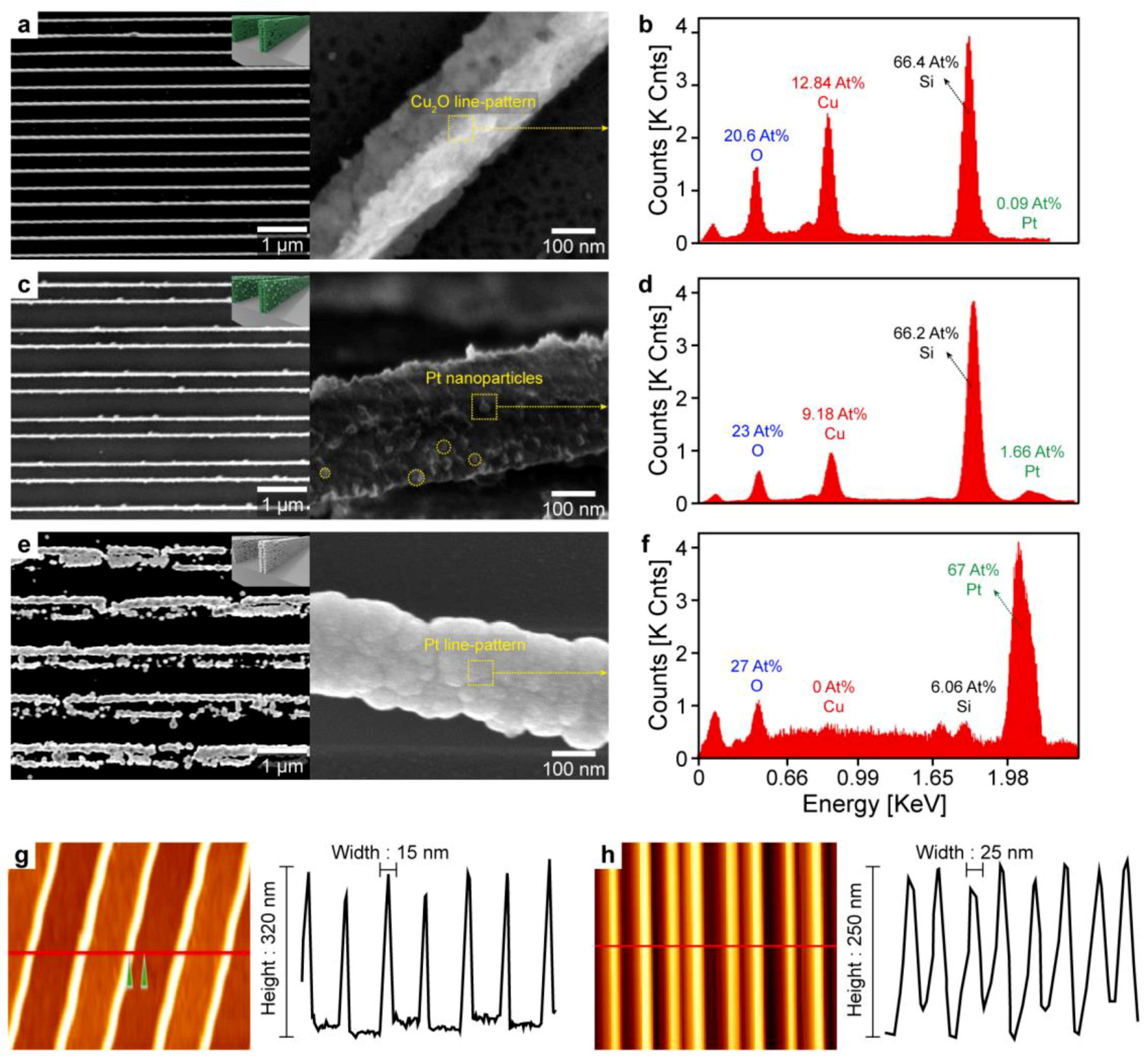
3.2. Morphology, Elements, and Dimension Characterizations
3.3. Chemical Binding States of Pt/Cu2O Nanochannel
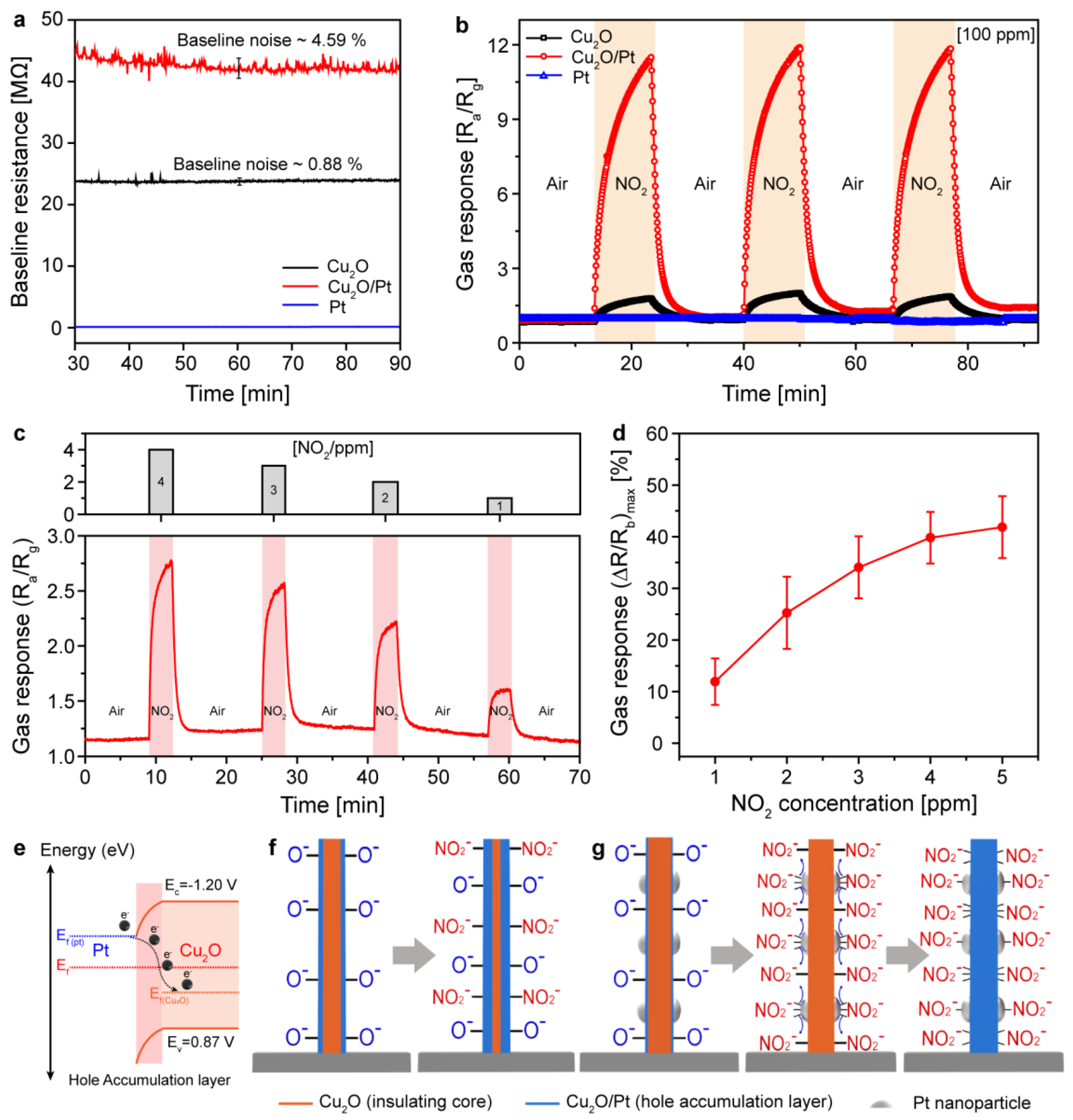
3.4. NO2 Sensing Performances and Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Velmathi, G.; Mohan, S.; Henry, R. Analysis of factors for improving functionality of tin oxide gas sensor. IETE Tech. Rev. 2016, 33, 122–129. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Choi, J.-K.; Woo, H.-S.; Kim, S.-J.; Jung, S.-Y.; Seong, T.-Y.; Kim, I.-D.; Lee, J.-H. Facile control of C2H5OH sensing characteristics by decorating discrete Ag nanoclusters on SnO2 nanowire networks. ACS Appl. Mater. Interfaces 2011, 3, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Matsunaga, N.; Hyodo, T.; Egashira, M. Improvement of SO2 sensing properties of WO3 by noble metal loading. Sens. Actuators B Chem. 2001, 77, 35–40. [Google Scholar] [CrossRef]
- Chang, Y.-E.; Youn, D.-Y.; Ankonina, G.; Yang, D.-J.; Kim, H.-G.; Rothschild, A.; Kim, I.-D. Fabrication and gas sensing properties of hollow SnO2 hemispheres. Chem. Commun. 2009, 27, 4019–4021. [Google Scholar] [CrossRef]
- Ahn, M.-W.; Park, K.-S.; Heo, J.-H.; Park, J.-G.; Kim, D.-W.; Choi, K.J.; Lee, J.-H.; Hong, S.-H. Gas sensing properties of defect-controlled ZnO-nanowire gas sensor. Appl. Phys. Lett. 2008, 93, 263103. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Yoo, H.-W.; Kim, J.Y.; Jung, W.-B.; Jin, M.L.; Kim, J.-S.; Jeon, H.-J.; Jung, H.-T. High-resolution p-type metal oxide semiconductor nanowire array as an ultrasensitive sensor for volatile organic compounds. Nano Lett. 2016, 16, 4508–4515. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Mastelaro, V.R.; Ponpandian, N. Surface morphology-dependent room-temperature LaFeO3 nanostructure thin films as selective NO2 gas sensor prepared by radio frequency magnetron sputtering. ACS Appl. Mater. Interfaces 2014, 6, 13917–13927. [Google Scholar] [CrossRef]
- Joshi, N.; Silva, L.F.D.; Jadhav, H.; M’Peko, J.-C.; Torres, B.B.M.; Aguir, K.; Mastelaro, V.R., Jr.; Oliveira, N.O. One-step approach for preparing ozone gas sensors based on hierarchical NiCo2O4 structures. RSC Adv. 2016, 6, 92655–92662. [Google Scholar] [CrossRef]
- Joshi, N.; Silva, L.F.D.; Jadhav, H.S.; Shimizu, F.M.; Suman, P.H.; M’Peko, J.-C.; Orlandi, M.O.; Seo, J.G.; Mastelaro, V.R., Jr.; Oliveira, N.O. Yolk-shelled ZnCo2O4 microspheres: Surface properties and gas sensing application. Sens. Actuators B 2018, 257, 906–915. [Google Scholar] [CrossRef]
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Han, Y.; Kong, E.S.-W.; Yang, Z.; Zhang, Y. An ultrasensitive NO2 gas sensor based on a hierarchical Cu2O/CuO mesocrystal nanoflower. J. Mater. Chem. A 2018, 6, 17120–17131. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Yoo, H.-W.; Cho, S.-Y.; Jeon, H.-J.; Jung, H.-T. Well-defined and high resolution Pt nanowire arrays for a high performance hydrogen sensor by a surface scattering phenomenon. Anal. Chem. 2015, 87, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-dimensional nanostructured materials for gas sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H., Jr.; Oliveira, N.O.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, C.; Zhang, J.; Peng, Y.; Xie, E. Enhanced gas sensing performance of electrospun Pt-functionalized NiO nanotubes with chemical and electronic sensitization. ACS Appl. Mater. Interfaces 2013, 5, 7410–7416. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Wang, G.; Yang, J.; Park, J.; Wexler, D. Chemical synthesis, characterization and gas sensing performance of copper oxide nanoribbons. J. Mater. Chem. 2008, 18, 965–969. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Han, J.; Ng, H.-T.; Binder, C.; Partridge, C.; Meyyappan, M. Room temperature methane detection using palladium loaded single-walled carbon nanotube sensors. Chem. Phys. Lett. 2004, 391, 344–348. [Google Scholar] [CrossRef]
- Yamazoe, N. New Approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, K.H.; Baek, Y.-K.; Kim, D.W.; Jung, H.-T. New top-down approach for fabricating high-aspect-ratio complex nanostructures with 10 nm scale features. Nano Lett. 2010, 10, 3604–3610. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, P.-C.; Ryu, K.; Zhou, C. Devices and chemical sensing applications of metal oxide nanowires. J. Mater. Chem. 2009, 19, 828–839. [Google Scholar] [CrossRef]
- Jang, H.-J.; Hong, S.; Ham, S.; Shuford, K.L.; Park, S. Site-specific growth of a Pt shell on Au nanoplates: Tailoring their surface plasmonic behavior. Nanoscale 2014, 6, 7339–7345. [Google Scholar] [CrossRef]
- Seo, D.; Song, H. Asymmetric hollow nanorod formation through a partial galvanic replacement reaction. J. Am. Chem. Soc. 2009, 131, 18210–18211. [Google Scholar] [CrossRef]
- Pradhan, M.; Chowdhury, J.; Sarkar, S.; Sinha, A.K.; Pal, T. Hierarchical gold flower with sharp tips from controlled galvanic replacement reaction for high surface enhanced Raman scattering Activity. J. Phys. Chem. C 2012, 116, 24301–24313. [Google Scholar] [CrossRef]
- Zhang, W.; Rahmani, M.; Niu, W.; Ravaine, S.; Hong, M.; Lu, X. Tuning interior nanogaps of double-shelled Au/Ag nanoboxes for surface-enhanced Raman scattering. Sci. Rep. 2015, 5, 8382. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, S.J.; Lee, Y.; Kim, J.-S.; Jung, W.-B.; Yoo, H.-W.; Kim, J.; Jung, H.-T. highly enhanced gas adsorption properties in vertically aligned MoS2 layers. ACS Nano 2015, 9, 9314–9321. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Jeon, H.-J.; Yoo, H.-W.; Cho, K.M.; Jung, W.-B.; Kim, J.-S.; Jung, H.-T. Highly enhanced fluorescence signals of quantum dot–polymer composite arrays formed by hybridization of ultrathin plasmonic Au nanowalls. Nano Lett. 2015, 15, 7273–7280. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Jeon, H.-J.; Kim, J.-S.; Ok, J.M.; Jung, H.-T. Hierarchical ordering of quantum dots and liquid with tunable super-periodicity into high aspect ratio moiré superlattice structure. Adv. Funct. Mater. 2014, 24, 6939–6947. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Lu, X. Tailoring galvanic replacement reaction for the preparation of Pt/Ag bimetallic hollow nanostructures with controlled number of voids. ACS Nano 2012, 6, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Fu, Z.-W.; Qin, D. Galvanic replacement-free deposition of Au on Ag for core-shell nanocubes with enhanced chemical stability and SERS activity. J. Am. Chem. Soc. 2014, 136, 8153–8156. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, P.; Zhang, B.; Wu, G.; Zhao, H.; Fu, E.; Wang, H.-L. Self-supported Pt nanoclusters via galvanic replacement from Cu2O nanocubes as efficient electrocatalysts. Nanoscale 2013, 5, 7397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Du, M.; Yu, D.L.; Wang, Y.; Wang, L.; Zou, M.; Zhang, M.; Fu, Y.Q. A new strategy for the surface-free-energy-distribution induced selective growth and controlled formation of Cu2O-Au hierarchical heterostructures with a series of morphological evolutions. J. Mater. Chem. A 2013, 1, 919–929. [Google Scholar] [CrossRef]
- Ji, R.; Sun, W.; Chu, Y. One-step hydrothermal synthesis of Ag/Cu2O heterogeneous nanostructure over Cu foil and their SERS applications. RSC Adv. 2014, 4, 6055–6059. [Google Scholar] [CrossRef]
- Murata, N.; Suzuki, T.; Kobayashi, M.; Togoh, F.; Asakura, K. Characterization of Pt-doped SnO2 catalyst for a high-performace micro gas sensor. Phys. Chem. Chem. Phys. 2013, 15, 17938–17946. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Lee, Y.; Koh, H.-J.; Jung, H.; Kim, J.-S.; Yoo, H.-W.; Kim, J.; Jung, H.-T. Superior chemical sensing performance of black phosphorus: comparison with MoS2 and graphene. Adv. Mater. 2016, 28, 7020–7028. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Guo, Z.; Yan, W.; Xin, Y. Enhancing light harvesting and charge separation of Cu2O photocathodes with spatially separated noble-metal cocatalysts towards highly efficient water splitting. J. Mater. Chem. A 2018, 6, 20393–20401. [Google Scholar] [CrossRef]
- Cao, D.; Wang, C.; Zheng, F.; Dong, W.; Fang, L.; Shen, M. High-efficiency ferroelectric-film solar cells with an n-type Cu2O cathode buffer layer. Nano Lett. 2012, 12, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Liang, J.-R.; Li, C.-Q.; Yan, W.-J.; Hu, M. Room temperature NO2 gas sensing of Au-loaded tungsten oxide nanowires/porous silicon hybrid structure. Chin. Phys. B 2015, 25, 028102. [Google Scholar] [CrossRef]
- Mane, A.A.; Moholkar, A.V. Palladium (Pd) sensitized molybdenum trioxide (MoO3) nanobelts for nitrogen dioxide (NO2) gas detection. Solid-State Electron. 2018, 139, 21–30. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Cho, S.-Y.; Jung, H.-T. Selective Functionalization of High-Resolution Cu2O Nanopatterns via Galvanic Replacement for Highly Enhanced Gas Sensing Performance. Sensors 2018, 18, 4438. https://doi.org/10.3390/s18124438
Kim JY, Cho S-Y, Jung H-T. Selective Functionalization of High-Resolution Cu2O Nanopatterns via Galvanic Replacement for Highly Enhanced Gas Sensing Performance. Sensors. 2018; 18(12):4438. https://doi.org/10.3390/s18124438
Chicago/Turabian StyleKim, Ju Ye, Soo-Yeon Cho, and Hee-Tae Jung. 2018. "Selective Functionalization of High-Resolution Cu2O Nanopatterns via Galvanic Replacement for Highly Enhanced Gas Sensing Performance" Sensors 18, no. 12: 4438. https://doi.org/10.3390/s18124438
APA StyleKim, J. Y., Cho, S.-Y., & Jung, H.-T. (2018). Selective Functionalization of High-Resolution Cu2O Nanopatterns via Galvanic Replacement for Highly Enhanced Gas Sensing Performance. Sensors, 18(12), 4438. https://doi.org/10.3390/s18124438





