Indicator Layers Based on Ethylene-Vinyl Acetate Copolymer (EVA) and Dicyanovinyl Azobenzene Dyes for Fast and Selective Evaluation of Vaporous Biogenic Amines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Starting Materials
2.2. Materials Characterization
2.3. Fabrication of Indicator Layers
2.4. Experimental Procedure
3. Results and Discussion
3.1. Preparation of Indicator Layers
3.2. Spectroscopic Measurements
3.3. Analytical Method Validation
3.4. Characterisation of Indicator Layers
3.4.1. FTIR Analysis
3.4.2. Morphology of Indicator Layers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El Halabieh, R.H.; Mermut, O.; Barrett, C.J. Using light to control physical properties of polymers and surfaces with azobenzene chromophores. Pure Appl. Chem. 2004, 76, 1445–1465. [Google Scholar] [CrossRef]
- Guezguez, I.; Ayadi, A.; Ordon, K.; Iliopoulos, K.; Branzea, D.G.; Migalska-Zalas, A.; Makowska-Janusik, M.; El-Ghayoury, A.; Sahraoui, B. Zinc induced a dramatic enhancement of the nonlinear optical properties of an Azo-based iminopyridine ligand. J. Phys. Chem. C 2014, 118, 7545–7553. [Google Scholar] [CrossRef]
- Essaidi, Z.; Niziol, J.; Sahraoui, B. Azo-azulene based compounds-nonlinear optical and photorefractive properties. Opt. Mater. 2011, 33, 1387–1390. [Google Scholar] [CrossRef] [Green Version]
- Yager, K.G.; Barrett, C.J. Novel photo-switching using azobenzene functional materials. J. Photochem. Photobiol. A Chem. 2006, 182, 250–261. [Google Scholar] [CrossRef]
- Spiridon, M.C.; Iliopoulos, K.; Jerca, F.A.; Jerca, V.V.; Vuluga, D.M.; Vasilescu, D.S.; Gindre, D.; Sahraoui, B. Novel pendant azobenzene/polymer systems for second harmonic generation and optical data storage. Dyes Pigment. 2015, 114, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Jerca, V.V.; Nicolescu, F.A.; Trusca, R.; Vasile, E.; Baran, A.; Anghel, D.F.; Vasilescu, D.S.; Vuluga, D.M. Oxazoline-functional polymer particles graft with azo-dye. React. Funct. Polym. 2011, 71, 373–379. [Google Scholar] [CrossRef]
- Jerca, V.V.; Jerca, F.A.; Rau, I.; Manea, A.M.; Vuluga, D.M.; Kajzar, F. Advances in understanding the photoresponsive behavior of azobenzenes substituted with strong electron withdrawing groups. Opt. Mater. 2015, 48, 160–164. [Google Scholar] [CrossRef]
- Yager, K.; Barrett, C. Light-Induced Nanostructure Formation using Azobenzene Polymers. In Polymeric Nanostructures and Their Applications; American Scientific Publishers: Valencia, CA, USA, 2006. [Google Scholar]
- Weber, R.W.; Hoffman, M.; Raine, D.A.; Nelson, H.S. Incidence of bronchoconstriction due to aspirin, azo dyes, non-azo dyes, and preservatives in a population of perennial asthmatics. J. Allergy Clin. Immunol. 1979, 64, 32–37. [Google Scholar] [CrossRef]
- Stevenson, J. Recent research on food additives: Implications for CAMH. Child Adolesc. Ment. Health 2010, 15, 130–133. [Google Scholar] [CrossRef]
- Číková, E.; Kuliček, J.; Janigová, I.; Omastová, M. Electrospinning of ethylene vinyl acetate/Poly(Lactic Acid) blends on a water surface. Materials 2018, 11, 1737. [Google Scholar] [CrossRef]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Zhang, J. UV aging behaviour of ethylene-vinyl acetate copolymers (EVA) with different vinyl acetate contents. Polym. Degrad. Stab. 2010, 95, 725–732. [Google Scholar] [CrossRef]
- Surmodics Bravo™ Durable Drug Delivery Polymer Coating—Technology. SurModics: Medical Device Coatings & In Vitro Diagnostics (IVD). Available online: http://www.surmodics.com/medical-device/coating-technologies/drug-delivery-coatings-overview/durable-materials/drug-delivery-coatings-technology/ (accessed on 15 November 2018).
- Belin, P.; Khalili, A.; Ginsburg, R.; Lieberman, R.M. Recent Innovations in Drug Delivery for Retinal Diseases. Adv. Ophthalmol. Optom. 2018, 3, 155–183. [Google Scholar] [CrossRef]
- Chevreau, J.; Krief, D.; Arab, O.A.; Zitoun, M.; Foulon, A.; Sergent, F.; Gondry, J. Factors associated with removal difficulties of etonogestrel-containing contraceptive implants (Nexplanon®). Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 224, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Selvan, P.; Piran, P.; Balucani, C.; Tark, B.; Adler, Z.; Levine, S.R. Stroke and Etonogestrel/Ethinyl Estradiol Ring (NuvaRing): Clinical, Radiological, and Prognostic Features. J. Stroke Cerebrovasc. Dis. 2017, 26, 608–617. [Google Scholar] [CrossRef]
- Bourges, J.L.; Bloquel, C.; Thomas, A.; Froussart, F.; Bochot, A.; Azan, F.; Gurny, R.; BenEzra, D.; Behar-Cohen, F. Intraocular implants for extended drug delivery: Therapeutic applications. Adv. Drug Deliv. Rev. 2006, 58, 1182–1202. [Google Scholar] [CrossRef] [PubMed]
- Yeǧin, S.; Üren, A. Biogenic amine content of boza: A traditional cereal-based, fermented Turkish beverage. Food Chem. 2008, 111, 983–987. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Namieśnik, J.; Płotka-Wasylka, J. Direct solid phase microextraction combined with gas chromatography—Mass spectrometry for the determination of biogenic amines in wine. Talanta 2018, 183, 276–282. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Troncoso, A.M.; García-Parrilla, M.D.C.; Callejón, R.M. Recent trends in the determination of biogenic amines in fermented beverages—A review. Anal. Chim. Acta 2016, 939, 10–25. [Google Scholar] [CrossRef]
- Castillero, P.; Roales, J.; Lopes-Costa, T.; Sánchez-Valencia, J.R.; Barranco, A.; González-Elipe, A.R.; Pedrosa, J.M. Optical gas sensing of ammonia and amines based on protonated porphyrin/TiO2 composite thin films. Sensors 2017, 17, 24. [Google Scholar] [CrossRef]
- Sutarlie, L.; Yang, K.L. Colorimetric responses of transparent polymers doped with metal phthalocyanine for detecting vaporous amines. Sens. Actuators B Chem. 2008, 134, 1000–1004. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Wang, H.; Xiao, S.; Wang, J.; Feng, L. Sensitivity enhancement of pH indicator and its application in the evaluation of fish freshness. Talanta 2015, 143, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Bueno, L.; Meloni, G.N.; Reddy, S.M.; Paixão, T.R. Use of plastic-based analytical device, smartphone and chemometric tools to discriminate amines. RSC Adv. 2015, 5, 20148–20154. [Google Scholar] [CrossRef] [Green Version]
- Schaude, C.; Meindl, C.; Fröhlich, E.; Attard, J.; Mohr, G.J. Developing a sensor layer for the optical detection of amines during food spoilage. Talanta 2017, 170, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, J.; Yu, J.; Cui, B. A colorimetric sensor for qualitative discrimination and quantitative detection of volatile amines. Sensors 2010, 10, 6463–6476. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W.; Zou, X.B.; Shi, J.Y.; Guo, Y.; Zhao, J.W.; Zhang, J.; Hao, L. Determination of pork spoilage by colorimetric gas sensor array based on natural pigments. Food Chem. 2014, 145, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Zór, K.; Kostesha, N.; Alstrøm, T.S.; Heiskanen, A.; El-Tanahi, H.; Sharoba, A.; Papkovsky, D.; Larsen, J.; Khalaf, H.; et al. Development and validation of a colorimetric sensor array for fish spoilage monitoring. Food Control 2016, 60, 346–352. [Google Scholar] [CrossRef]
- Lin, J.F.; Kukkola, J.; Sipola, T.; Raut, D.; Samikannu, A.; Mikkola, J.P.; Mohl, M.; Toth, G.; Su, W.F.; Laurila, T.; et al. Trifluoroacetylazobenzene for optical and electrochemical detection of amines. J. Mater. Chem. A 2015, 3, 4687–4694. [Google Scholar] [CrossRef]
- Dudnyk, I.; Janeček, E.R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Lin, T.; Wu, Y.; Li, Z.; Song, Z.; Guo, L.; Fu, F. Visual monitoring of food spoilage based on hydrolysis-induced silver metallization of au nanorods. Anal. Chem. 2016, 88, 11022–11027. [Google Scholar] [CrossRef]
- Mastnak, T.; Lobnik, A.; Mohr, G.J.; Turel, M. Design and characterization of dicyanovinyl reactive dyes for the colorimetric detection of thiols and biogenic amines. Sensors 2018, 18, 814. [Google Scholar] [CrossRef] [PubMed]
- Mayr, T.; Abel, T.; Kraker, E.; Köstler, S.; Haase, A.; Konrad, C.; Tscherner, M.; Lamprecht, B. An optical sensor array on a flexible substrate with integrated organic opto-electric devices. Procedia Eng. 2010, 5, 1005–1008. [Google Scholar] [CrossRef]
- Coatings, S.; Silsesquioxane, O. Preparation and Properties of High Hardness Ultraviolet Curable Polyethylene Terephthalates. Coatings 2018, 8, 411. [Google Scholar]
- Alam, A.U.; Qin, Y.; Nambiar, S.; Yeow, J.T.; Howlader, M.M.; Hu, N.X.; Deen, M.J. Polymers and organic materials-based pH sensors for healthcare applications. Prog. Mater. Sci. 2018, 96, 174–216. [Google Scholar] [CrossRef]
- Wengeler, L.; Schmidt-Hansberg, B.; Peters, K.; Scharfer, P.; Schabel, W. Investigations on knife and slot die coating and processing of polymer nanoparticle films for hybrid polymer solar cells. Chem. Eng. Process. Process Intensif. 2011, 50, 478–482. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Lavín, Á.; Vicente, J.; Holgado, M.; Laguna, M.; Casquel, R.; Santamaría, B.; Maigler, M.; Hernández, A.; Ramírez, Y. On the determination of uncertainty and limit of detection in label-free biosensors. Sensors 2018, 18, 2038. [Google Scholar] [CrossRef] [PubMed]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. (Eds.) Handbook of Chemometrics and Qualimetrics: Part A, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Koren, K.; Hutter, L.; Enko, B.; Pein, A.; Borisov, S.M.; Klimant, I. Tuning the dynamic range and sensitivity of optical oxygen-sensors by employing differently substituted polystyrene-derivatives. Sens. Actuators B Chem. 2013, 176, 344–350. [Google Scholar] [CrossRef] [Green Version]
- European Parliament and Council of the European Union. 2002/61/EC: Directive of 19 July 2002 amending for the nineteenth time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (azocolourants). Off. J. Eur. Communities 2002, L243, 15–18. [Google Scholar]
- O’Riordan, T.C.; Voraberger, H.; Kerry, J.P.; Papkovsky, D.B. Study of migration of active components of phosphorescent oxygen sensors for food packaging applications. Anal. Chim. Acta 2005, 530, 135–141. [Google Scholar] [CrossRef]

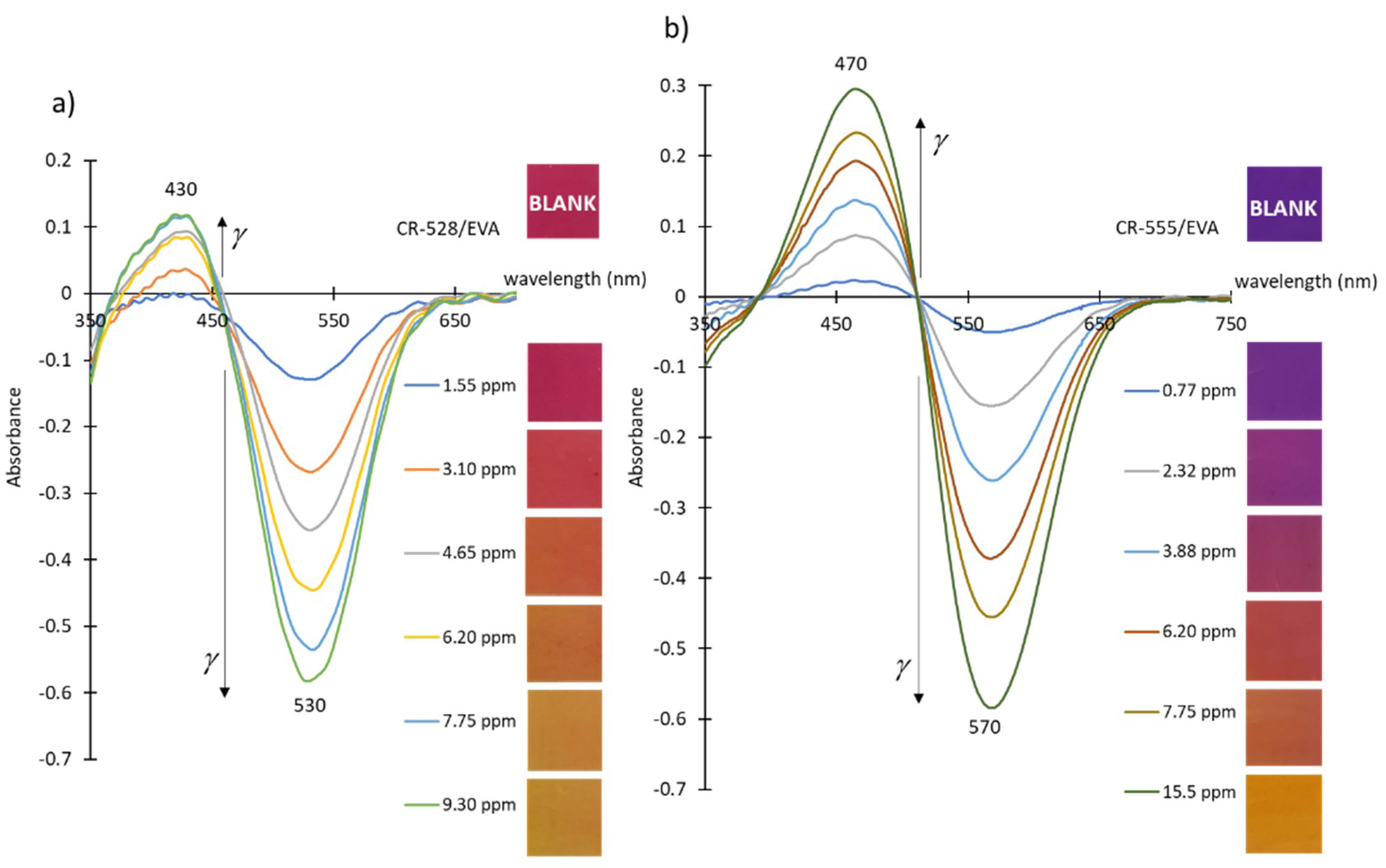
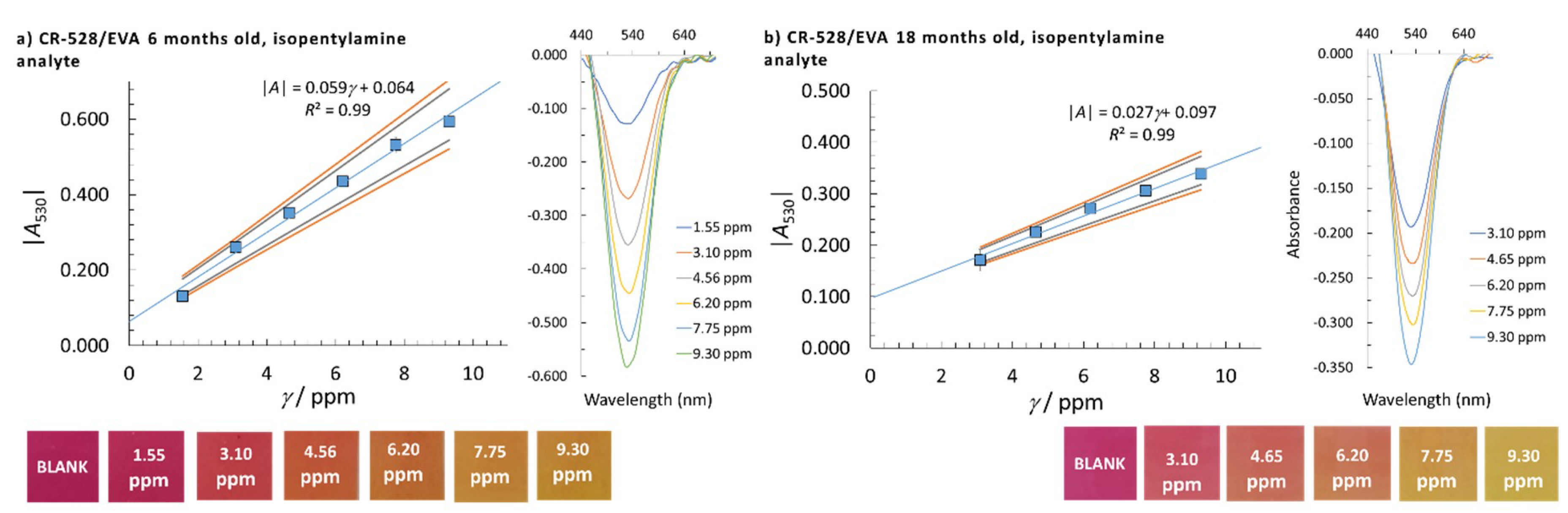

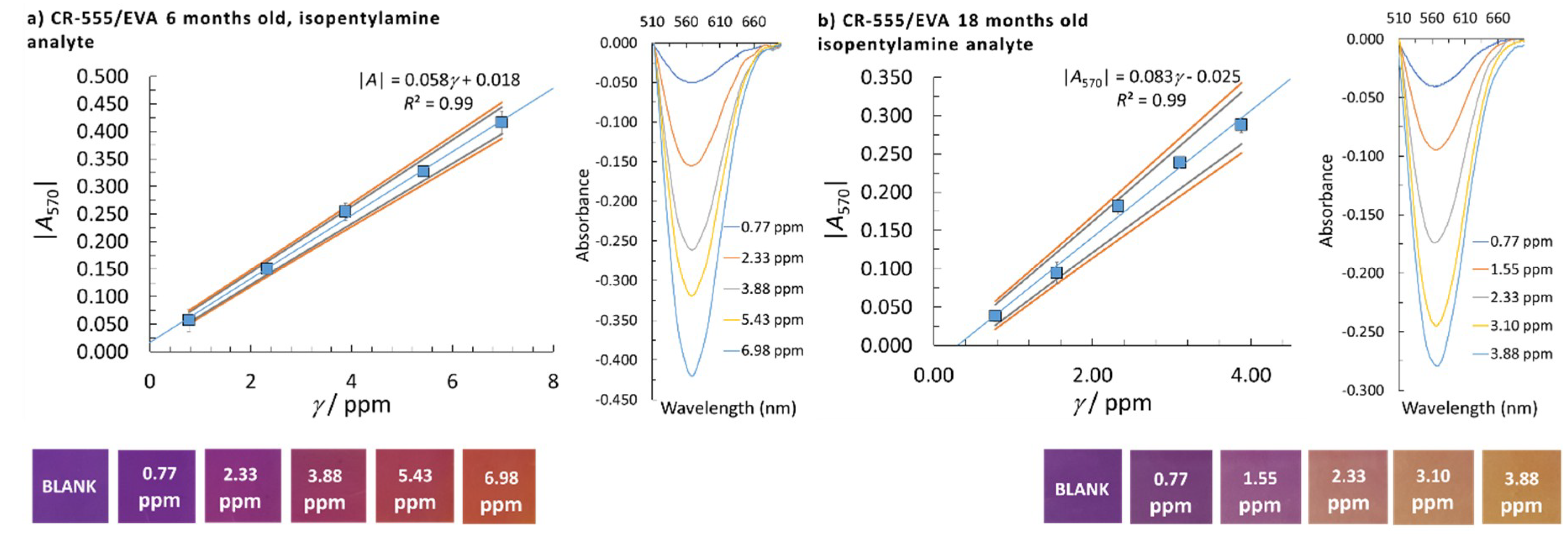
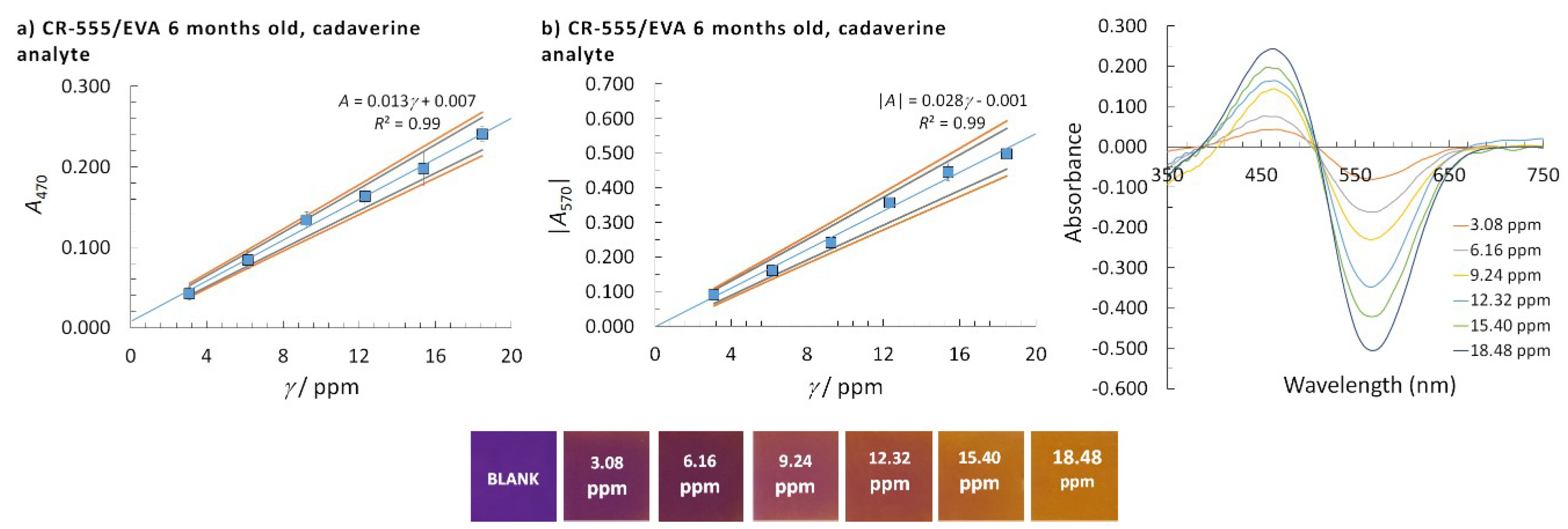
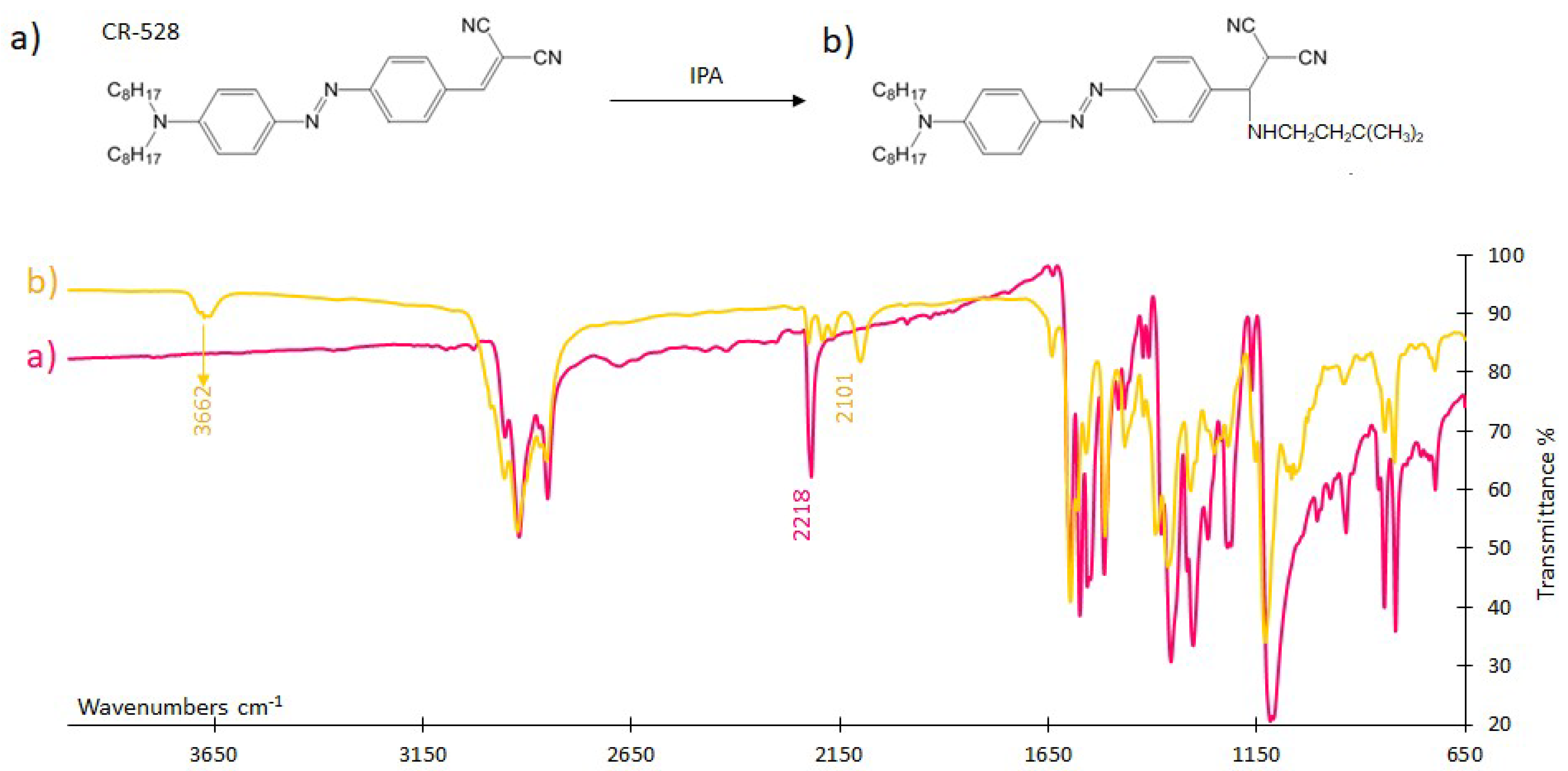
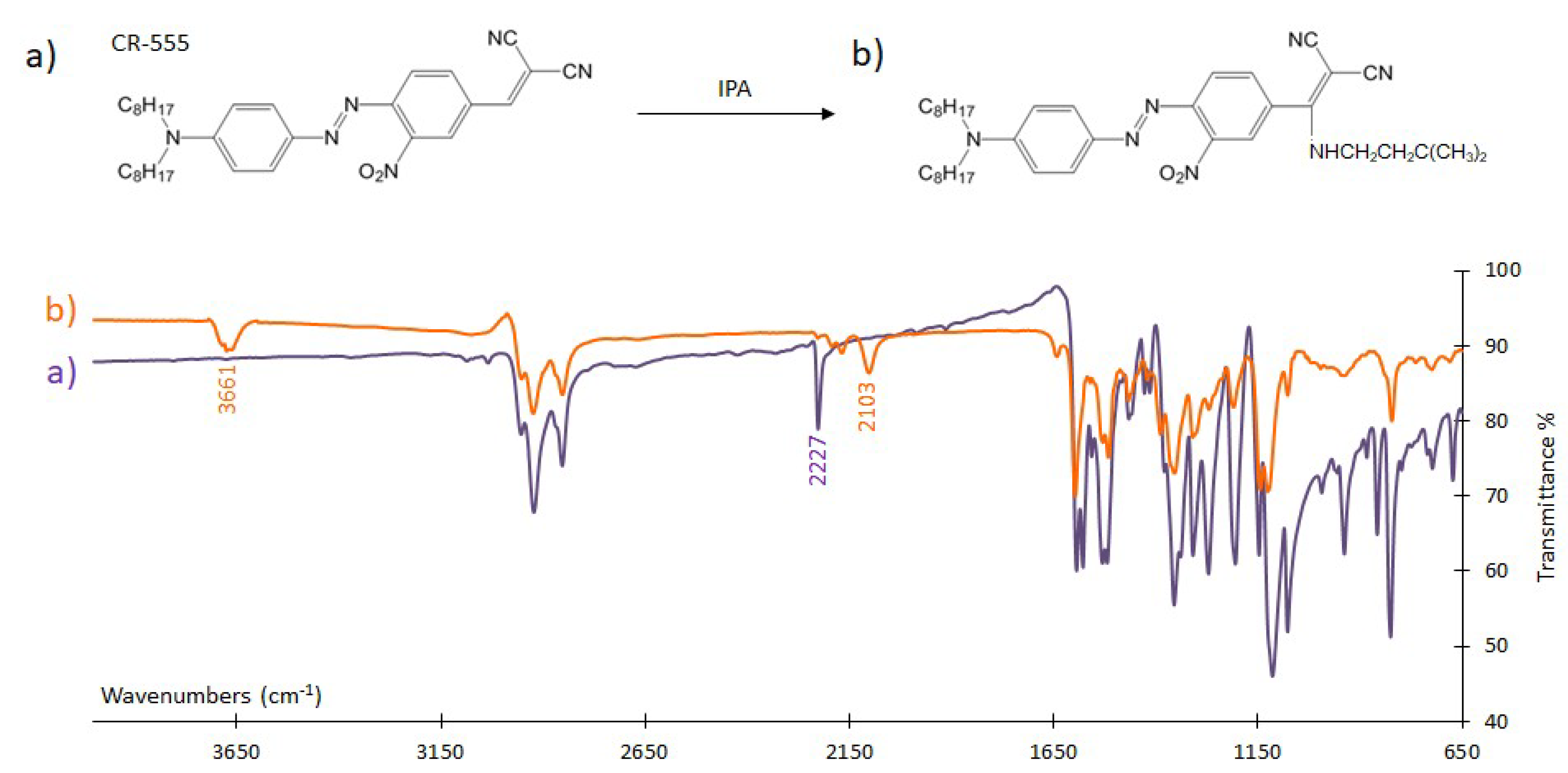
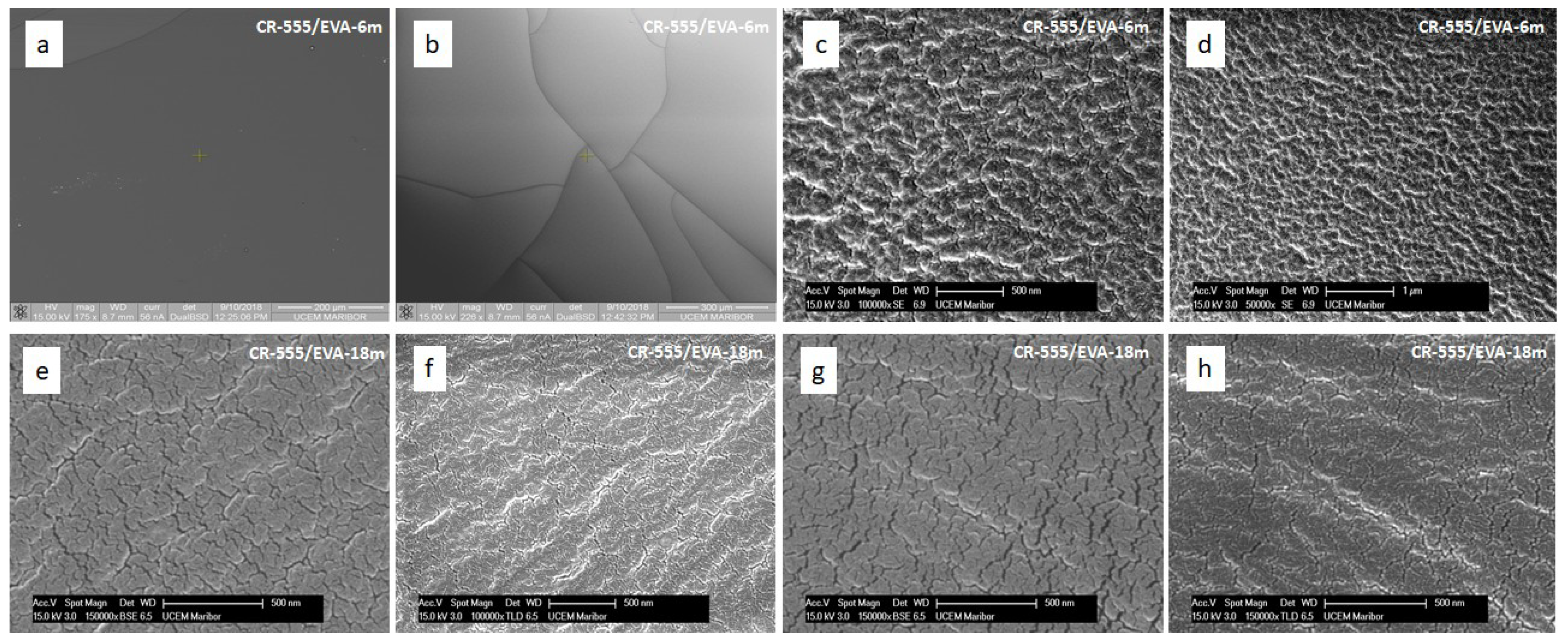
| Sensor/Probe—Matrix/Support | Analyte | Response Time | Application | LOD | Ref. |
|---|---|---|---|---|---|
| pH indicators—sol-gel | ethylenediamine gas | 30 min | Fish freshness | 10 ppm | [24] |
| pH indicators—cellulose acetate membranes | triethylamine, isobutylamine, isopentylamine vapours | 10 min | Meat spoilage | 1–5 ppm | [25] |
| iron(II) phthalocyanine—poly(dimethylsiloxane) | Saturated * hexylamine vapours | 15 min | Environmental monitoring | 11,600 ppm * | [23] |
| chemically sensitive dyes—inert substrate plate | Trimethylamine vapours | 15 min | Preliminary study | 50 ppb | [27] |
| pH indicator dye—cellulose microparticles | total volatile basic nitrogen (TVB-N) | 1.5 h | Meat spoilage | Not given | [26] |
| Natural pigments—silica gel plates | cadaverine, putrescine in pork | 3 days | Pork spoilage | 4 ppm | [28] |
| 16 chemo-sensitive compounds—silica gel plates | trimethylamine, dimethylamine, cadaverine, putrescine | 2–6 h | Fish spoilage | 20–200 ppm | [29] |
| 4-(dioctylamino)-4′-(trifluoroacetyl)azobenzene in tetrahydrofuran—PET foil | cadaverine | 2 h | Food spoilage | Not given | [30] |
| red-cabbage extract and pectin | cadaverine | 1 day | Meat and seafood freshness | 1 ppm | [31] |
| Au nanorods—agarose | trimethylamine | 3 h | Food spoilage | Not given | [32] |
| CR-528/EVA | CR-555/EVA | CR-555/EVA | ||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | Isopentylamine | Isopentylamine | Cadaverine | |||||
| Probe age/months | 6 | 18 | 6 | 18 | 6 | 18 | 6 | 6 |
| Detection at λ/nm | 530 | 530 | 470 | 470 | 570 | 570 | 470 | 570 |
| LOD/ppm | 0.98 | 1.23 | 0.61 | 0.54 | 0.44 | 0.41 | 1.86 | 1.80 |
| Linear concentration range/ppm | 1.55–9.30 | 3.10–9.30 | 0.77–7.75 | 0.77–3.88* | 0.77–6.98 | 0.77–3.88 | 3.08–18.48 | 3.08–18.48 |
| Calibration curve slope/ppm–1 | 0.059 | 0.027 | 0.028 | 0.038 | 0.058 | 0.083 | 0.013 | 0.028 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastnak, T.; Lobnik, A.; Mohr, G.J.; Finšgar, M. Indicator Layers Based on Ethylene-Vinyl Acetate Copolymer (EVA) and Dicyanovinyl Azobenzene Dyes for Fast and Selective Evaluation of Vaporous Biogenic Amines. Sensors 2018, 18, 4361. https://doi.org/10.3390/s18124361
Mastnak T, Lobnik A, Mohr GJ, Finšgar M. Indicator Layers Based on Ethylene-Vinyl Acetate Copolymer (EVA) and Dicyanovinyl Azobenzene Dyes for Fast and Selective Evaluation of Vaporous Biogenic Amines. Sensors. 2018; 18(12):4361. https://doi.org/10.3390/s18124361
Chicago/Turabian StyleMastnak, Tinkara, Aleksandra Lobnik, Gerhard J. Mohr, and Matjaž Finšgar. 2018. "Indicator Layers Based on Ethylene-Vinyl Acetate Copolymer (EVA) and Dicyanovinyl Azobenzene Dyes for Fast and Selective Evaluation of Vaporous Biogenic Amines" Sensors 18, no. 12: 4361. https://doi.org/10.3390/s18124361





