Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials and Reagents
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
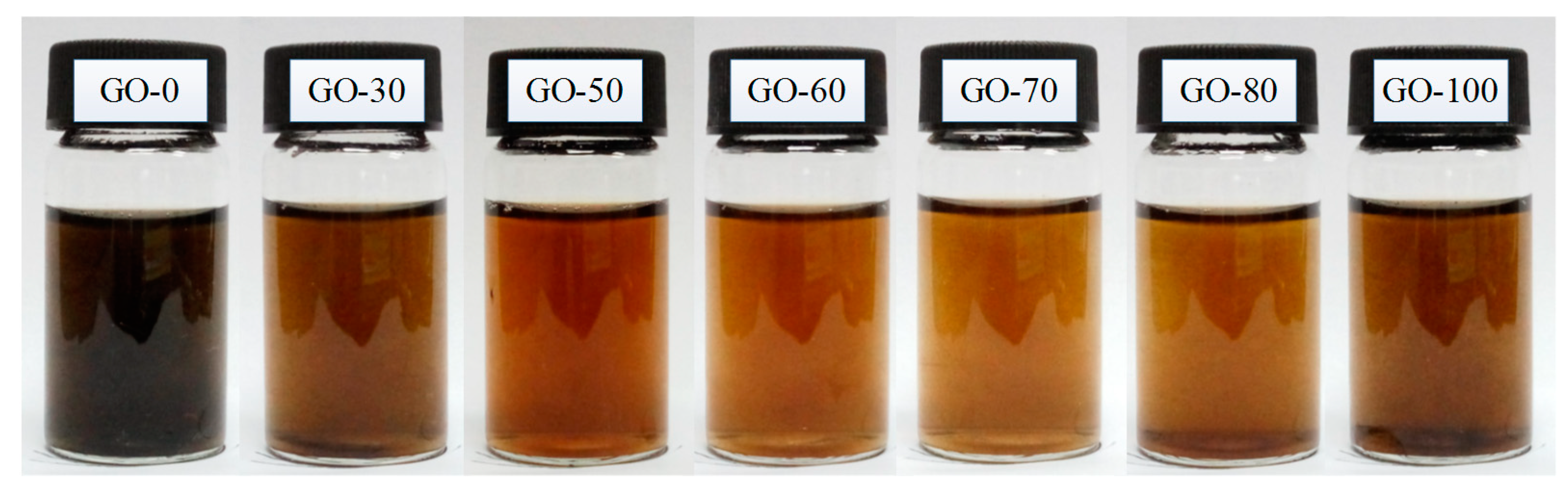
3.1. Color and Dispersion
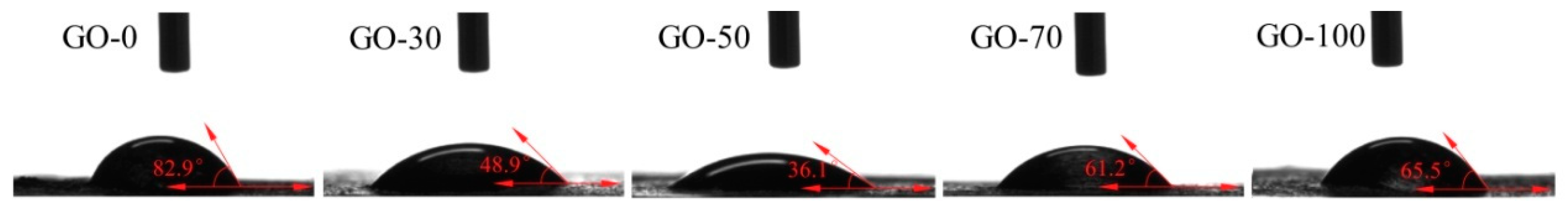
3.2. Contact Angle
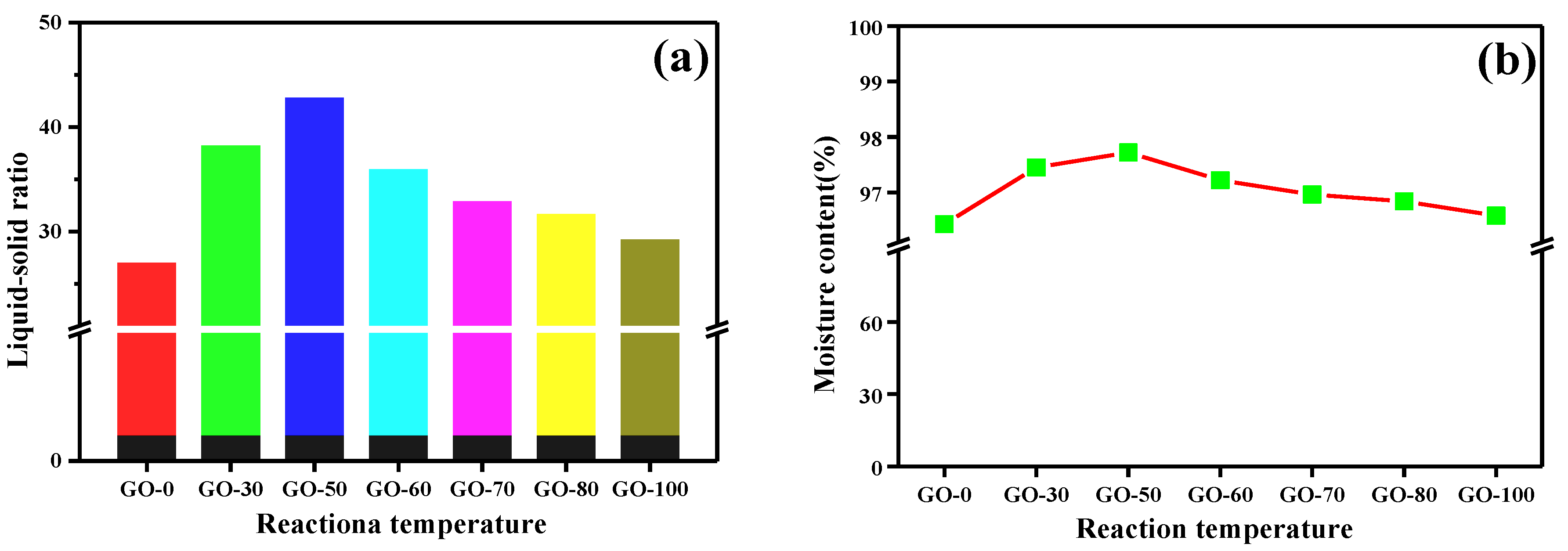
3.3. Moisture Content and Liquid-Solid Ratio
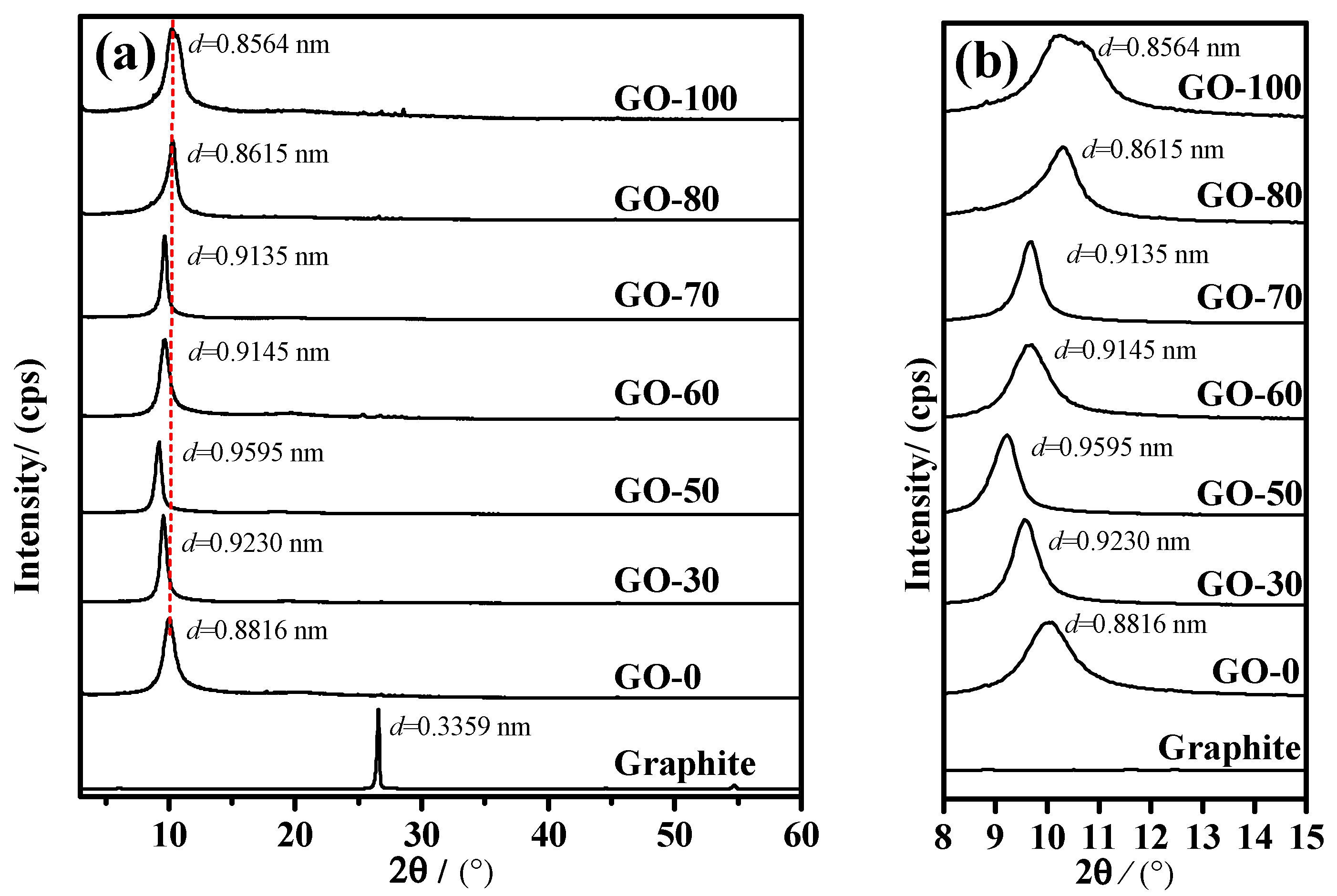
3.4. Structural Characterization
3.5. Gas Sensitivity Test
3.5.1. Static Resistance Analysis
3.5.2. Dynamic Resistance and Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohta, T.; Bostwick, A.; Mcchesney, J.; Seyller, T.; Horn, K.; Rotenberg, E. Controlling the Electronic Structure of Bilayer Graphene. Science 2006, 313, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Electronic structure of chiral graphene tubules. Appl. Phys. Lett. 1992, 60, 2204–2206. [Google Scholar] [CrossRef]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and Electronic Structure of Graphene-Oxide. Microsc. Microanal. 2009, 16, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.B.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 666. [Google Scholar] [CrossRef]
- Falkovsky, L.A. Optical properties of graphene. J. Exp. Theor. Phys. 2008, 115, 12004. [Google Scholar] [CrossRef]
- Pedersen, T.G.; Flindt, C.; Pedersen, J.; Jauho, A.P.; Mortensen, N.A.; Pedersen, K. Optical properties of graphene antidot lattices. Phys. Rev. B Condens. Matter 2009, 77, 245431. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Zu, Y.; Tang, J.; Zhu, W.; Zhang, M.; Liu, G.; Liu, Y.; Zhang, W.; Jia, M. Graphite oxide-supported CaO catalysts for transesterification of soybean oil with methanol. Bioresour. Technol. 2011, 102, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Gao, W. The Chemistry of Graphene Oxide; Springer International Publishing: Berlin, Germany, 2015; pp. 61–95. [Google Scholar]
- Lobato, B.; Wendelbo, R.; Barranco, V.; Centeno, T.A. Graphite Oxide: An Interesting Candidate for Aqueous Supercapacitors. Electrochim. Acta 2014, 149, 245–251. [Google Scholar] [CrossRef]
- Chen, G.; Zhai, S.; Zhai, Y.; Zhang, K.; Yue, Q.; Wang, L.; Zhao, J.; Wang, H.; Liu, J.; Jia, J. Preparation of sulfonic-functionalized graphene oxide as ion-exchange material and its application into electrochemiluminescence analysis. Biosens. Bioelectron. 2011, 26, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Hontoria-Lucas, C.; López-Peinado, A.J.; López-González, J.D.D.; Rojas-Cervantes, M.L.; Martín-Aranda, R.M. Study of oxygen-containing groups in a series of graphite oxides: Physical and chemical characterization. Carbon 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Huang, Q.; Sun, H.J.; Yang, Y.H. Spectroscopy Characterization and Analysis of Graphite Oxide. Chin. J. Inorg. Chem. 2011, 27, 1721–1726. [Google Scholar]
- Zhao, X.; Johnson, J.K. An Effective Potential for Adsorption of Polar Molecules on Graphite. Mol. Simul. 2005, 31, 1–10. [Google Scholar] [CrossRef]
- Zhang, B.; Li, F.; Wu, T.; Sun, D.; Li, Y. Adsorption of p-nitrophenol from aqueous solutions using nanographite oxide. Colloids Surf. A Physicochem. Eng. Asp. 2015, 464, 78–88. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liao, S.H.; Yen, M.Y.; Liu, P.I.; Pu, N.W.; Wang, C.A.; Ma, C.C. Preparation of covalently functionalized graphene using residual oxygen-containing functional groups. ACS Appl. Mater. Interfaces 2010, 2, 3092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Huang, Z.; Zhang, H. Effects of oxygen-containing functional groups on the supercapacitor performance of incompletely reduced graphene oxides. Int. J. Hydrogen Energy 2016, 42, 7186–7194. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Zhao, D.; Li, Q. The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale 2016, 8, 7449–7458. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L.; Dtsch, B. The structure of graphite oxide: Investigation of its surface chemical groups. Chem. Ges 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Nakajima, T.; Matsuo, Y. Formation process and structure of graphite oxide. Carbon 1994, 32, 469–475. [Google Scholar] [CrossRef]
- Nakajima, T.; Mabuchi, A.; Hagiwara, R. A new structure model of graphite oxide. Carbon 1988, 26, 357–361. [Google Scholar] [CrossRef]
- Beckett, R.J.; Croft, R.C. The Structure of Graphite Oxide. J. Phys. Chem. 1952, 56, 929–941. [Google Scholar] [CrossRef]
- Mermoux, M.; Chabre, Y.; Rousseau, A. FTIR and 13 C NMR study of graphite oxide. Carbon 1991, 29, 469–474. [Google Scholar] [CrossRef]
- Avdeev, V.V.; Sorokina, N.E.; Nikol’ Skaya, I.V.; Monyakina, L.A.; Voronkina, A.V. Synthesis of Intercalation Compounds in the System Graphite-HNO3-H2SO4. Inorg. Mater. 1997, 33, 584–587. [Google Scholar]
- Vieira, M.A.; Gonçalves, G.R.; Cipriano, D.F.; Schettino, M.A., Jr.; Filho, E.A.S.; Cunha, A.G.; Emmerich, F.G.; Freitas, J.C.C. Synthesis of graphite oxide from milled graphite studied by solid-state 13 C nuclear magnetic resonance. Carbon 2016, 98, 496–503. [Google Scholar] [CrossRef]
- Hamwi, A.; Marchand, V. Some chemical and electrochemical properties of graphite oxide. J. Phys. Chem. Solids 1996, 57, 867–872. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lee, Y.P.; Lahaye, R.J.; Park, M.H.; An, K.H.; Kim, I.J.; Yang, C.W.; Park, C.Y.; Ruoff, R.S.; Lee, Y.H. Evidence of Graphitic AB Stacking Order of Graphite Oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G.; Zhang, F. Deoxygenation of Exfoliated Graphite Oxide under Alkaline Conditions: A Green Route to Graphene Preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Boehm, H.P.; Clauss, A.; Hofmann, U. Graphite oxide and its membrane properties. J. Chim. Phys. 1961, 58, 141–147. [Google Scholar] [CrossRef]
- He, H.; Riedl, T.; Lerf, A.; Klinowski, J. Solid-State NMR Studies of the Structure of Graphite Oxide. J. Phys. Chem. 1996, 100, 19954–19958. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Lerf, A.; Buchsteiner, A.; Pieper, J.; Schöttl, S.; Dekany, I.; Szabo, T.; Boehm, H.P. Hydration behavior and dynamics of water molecules in graphite oxide. J. Phys. Chem. Solids 2006, 67, 1106–1110. [Google Scholar] [CrossRef]
- Dékány, I.; Krüger-Grasser, R.; Weiss, A. Selective liquid sorption properties of hydrophobized graphite oxide nanostructures. Colloid Polym. Sci. 1998, 276, 570–576. [Google Scholar]
- Ramesh, P.; Bhagyalakshmi, S.; Sampath, S. Preparation and physicochemical and electrochemical characterization of exfoliated graphite oxide. J. Colloid Interface Sci. 2004, 274, 95. [Google Scholar] [CrossRef] [PubMed]
- Peckett, J.W.; Trens, P.; Gougeon, R.D.; Pöppl, A.; Harris, R.K.; Hudson, M.J. Electrochemically oxidised graphite: Characterisation and some ion exchange properties. Carbon 2000, 38, 345–353. [Google Scholar] [CrossRef]
- De Wit, M.; Vanneste, E.; Blockhuys, F.; Verreyt, G.; Tachelet, W.; Nagels, L.J.; Geise, H.J. Chemically Sensitive Sensor Comprising Arylene Alkenylene Oligomers. U.S. Patent 6,042,788, 28 March 2000. [Google Scholar]
- Drewniak, S.; Muzyka, R.; Stolarczyk, A.; Pustelny, T.; Kotyczka-Moraå Ska, M.; Setkiewicz, M. Studies of Reduced Graphene Oxide and Graphite Oxide in the Aspect of Their Possible Application in Gas Sensors. Sensors 2016, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kwak, Y.; Lee, I.Y.; Maeng, S.; Kim, G.H. Highly responsive hydrogen gas sensing by partially reduced graphite oxide thin films at room temperature. Carbon 2012, 50, 4061–4067. [Google Scholar] [CrossRef]
- Yeh, T.F.; Syu, J.M.; Cheng, C.; Chang, T.H.; Teng, H. Graphite Oxide as a Photocatalyst for Hydrogen Production from Water. Adv. Funct. Mater. 2010, 20, 2255–2262. [Google Scholar] [CrossRef]
- Xu, C.; Cao, Y.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, G.S.; Yu, Z.Z.; Qi, J.S. The effect of graphite oxide on the thermoelectric properties of polyaniline. Carbon 2012, 50, 3064–3073. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Peng, T. Structure Development during the Cation Exchange Processes of Graphite Oxide. Acta Phys. -Chim. 2016, 33, 413–418. [Google Scholar]
- Wang, P.; Sun, H.; Peng, T. The Evolution Rule of Three-Dimensional Structures of Graphite During Oxidation. Nano Brief Rep. Rev. 2015, 10, 1550014. [Google Scholar] [CrossRef]
- Sun, H.; Peng, T. The Preparation of Graphene Materials via Oxidation-Reduction; Science Press: Henderson, NV, USA, 2015. [Google Scholar]
- Cotton, F.A.; Wilkinson, G. Advance Inorganic Chemistry. J. Chem. Educ. 1988, 9, 417–420. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ding, J.N.; Liu, Y.B.; Yuan, N.Y.; Ding, G.Q.; Fan, Y.; Yu, C.T. The influence of temperature, time and concentration on the dispersion of reduced graphene oxide prepared by hydrothermal reduction. Diam. Relat. Mater. 2012, 21, 11–15. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445–502. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Li, J. Ammonia adsorption on graphene and graphene oxide: A first-principles study. Front. Environ. Sci. Eng. 2013, 7, 403–411. [Google Scholar]
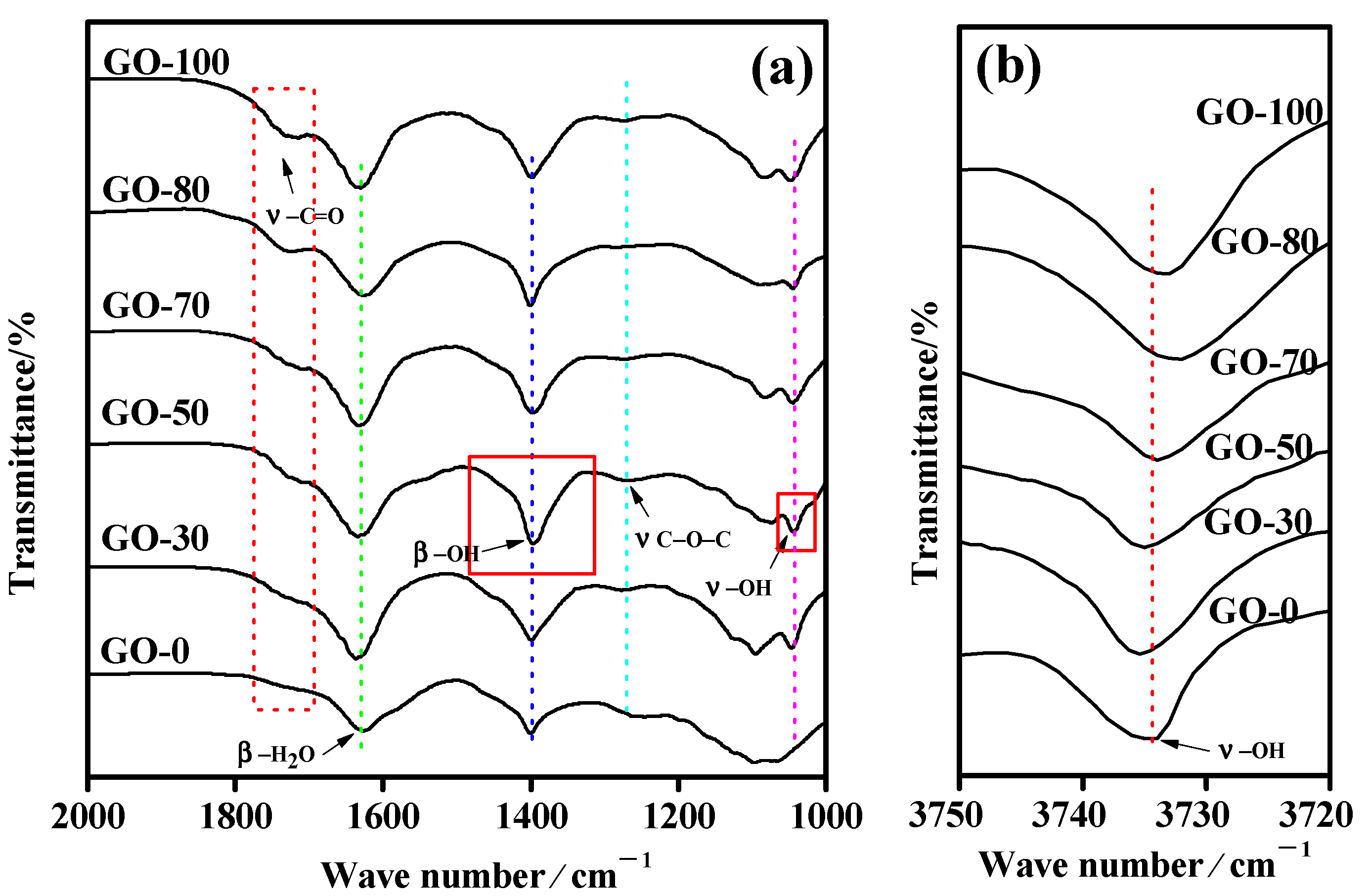


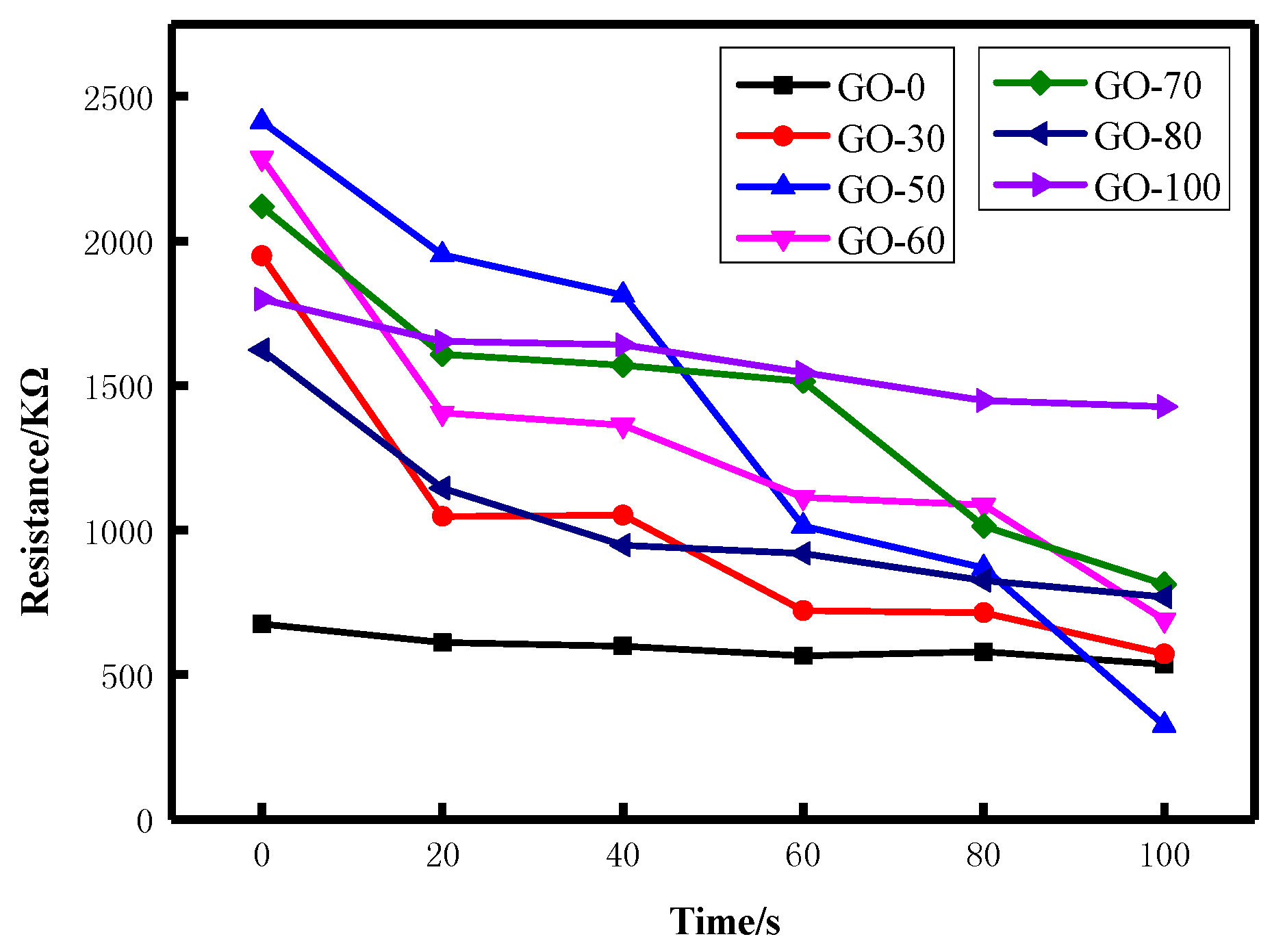

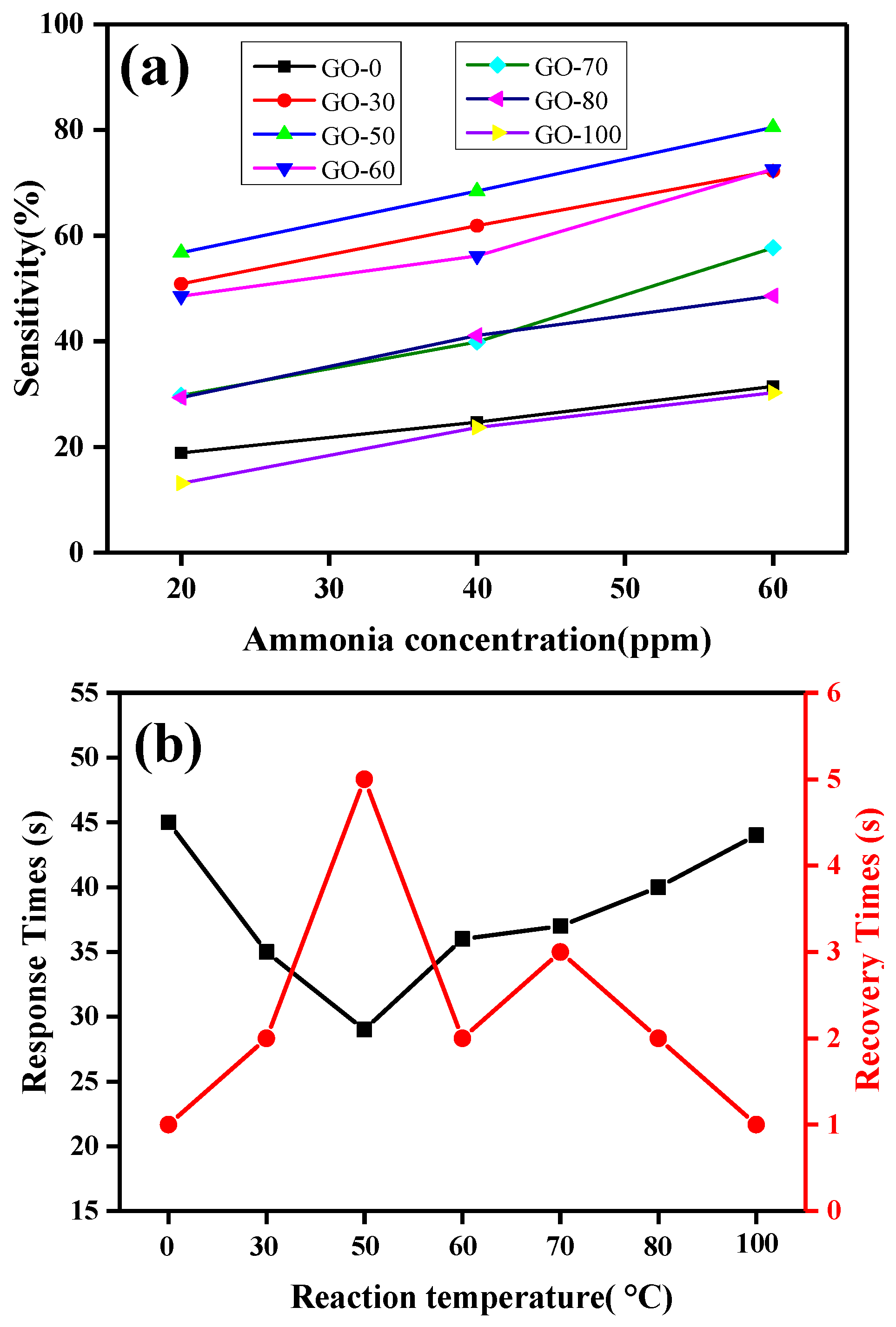
| Sample | Relative Percentage Content /% | C/O | |||
|---|---|---|---|---|---|
| C=C | C−OH | Epoxy Groups | O−C=O | ||
| GO-0 | 53.75 | 23.08 | 18.03 | 5.14 | 2.09 |
| GO-30 | 43.80 | 28.77 | 19.95 | 7.48 | 1.90 |
| GO-50 | 42.58 | 30.01 | 19.72 | 7.69 | 1.85 |
| GO-60 | 42.23 | 27.92 | 21.33 | 8.52 | 1.86 |
| GO-70 | 44.78 | 25.27 | 21.40 | 8.54 | 1.89 |
| GO-80 | 45.42 | 21.33 | 22.93 | 10.31 | 2.06 |
| GO-100 | 45.83 | 20.18 | 22.96 | 11.03 | 2.23 |
| GO-0 | GO-30 | GO-50 | GO-60 | GO-70 | GO-80 | GO-100 | |
|---|---|---|---|---|---|---|---|
| Response time (s) | 45 | 35 | 29 | 36 | 37 | 40 | 44 |
| Recovery time (s) | 1 | 2 | 5 | 2 | 3 | 2 | 1 |
| Sensitivity value (%) | 18.895 | 50.867 | 56.795 | 48.539 | 29.742 | 29.346 | 13.123 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Peng, T.; Yuan, M.; Sun, H.; Dai, S.; Wang, L. Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity. Sensors 2018, 18, 3745. https://doi.org/10.3390/s18113745
Luo L, Peng T, Yuan M, Sun H, Dai S, Wang L. Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity. Sensors. 2018; 18(11):3745. https://doi.org/10.3390/s18113745
Chicago/Turabian StyleLuo, Liming, Tongjiang Peng, Mingliang Yuan, Hongjuan Sun, Shichan Dai, and Long Wang. 2018. "Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity" Sensors 18, no. 11: 3745. https://doi.org/10.3390/s18113745
APA StyleLuo, L., Peng, T., Yuan, M., Sun, H., Dai, S., & Wang, L. (2018). Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity. Sensors, 18(11), 3745. https://doi.org/10.3390/s18113745




