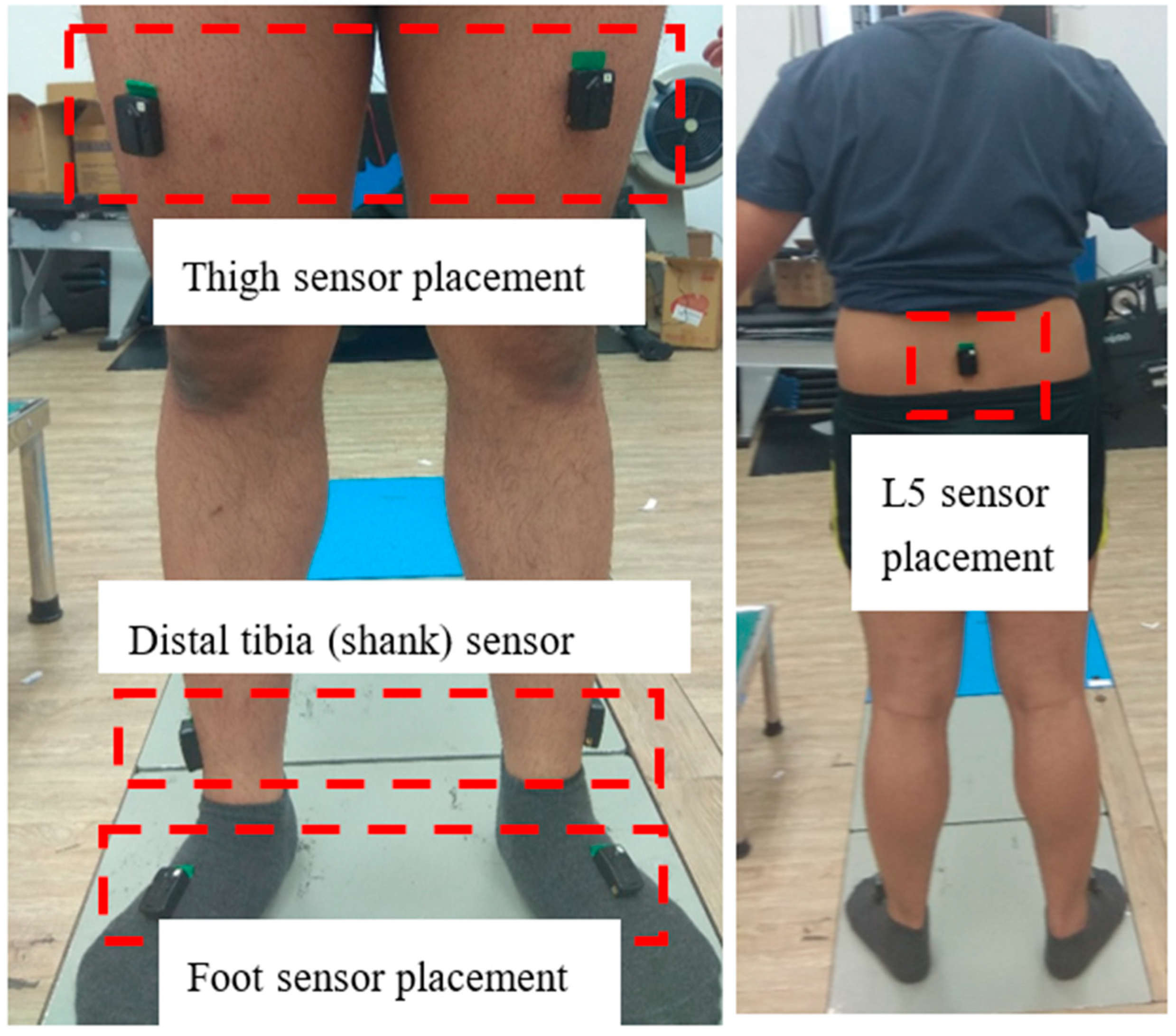
4.1. Discussion
This study aimed to perform a comprehensive analysis of multiple placements of wearable sensors for gait analysis and classification in patients with neurological disorders. Seven IMU sensors were placed at different locations, and gait-cycle-segmented data were used to create time domain features for classification purposes. The IC and FC gait event times were determined using filtered mediolateral acceleration data from the shank sensor. This algorithm has been commonly applied in studies using IMU sensors to define IC and FC gait event times, both in healthy individuals and in those with pathological disorders [
5], including application for patients with knee arthroplasty [
36], spinal cord injuries [
37], and Parkinson’s disease [
29]. All of these studies have been in agreement and confirmed the validity of using the shank angular velocity to define gait events with the golden standard, such as motion capture systems or foot switches. De Vroey et al. (2018) [
36] reported adequate to excellent intra-class correlation values overall for temporal gait parameters calculated using shank angular velocity and a camera system, also suggesting that IMU sensors can be used outside of laboratory assessments to examine the temporal gait parameters in the knee arthroplasty population.
Because this event detection method was crucial for separating the accelerometer and gyroscope data for classification purposes in the present study, the use of IMU sensors to define the IC and FC events in the study that validated the method was therefore imperative. Thus, the study conducted by K. Aminian et al. [
5] was significant for the conduction of the present study because it validated the algorithm for defining IC and FC events using shank angular velocity in healthy young and elderly subjects. The results also revealed high acceptability in elderly subjects and the method was recommended for use in clinical applications, such as the monitoring of rehabilitation progress, gait analysis in patients with neurological disorders, and fall risk assessment in elderly patients.
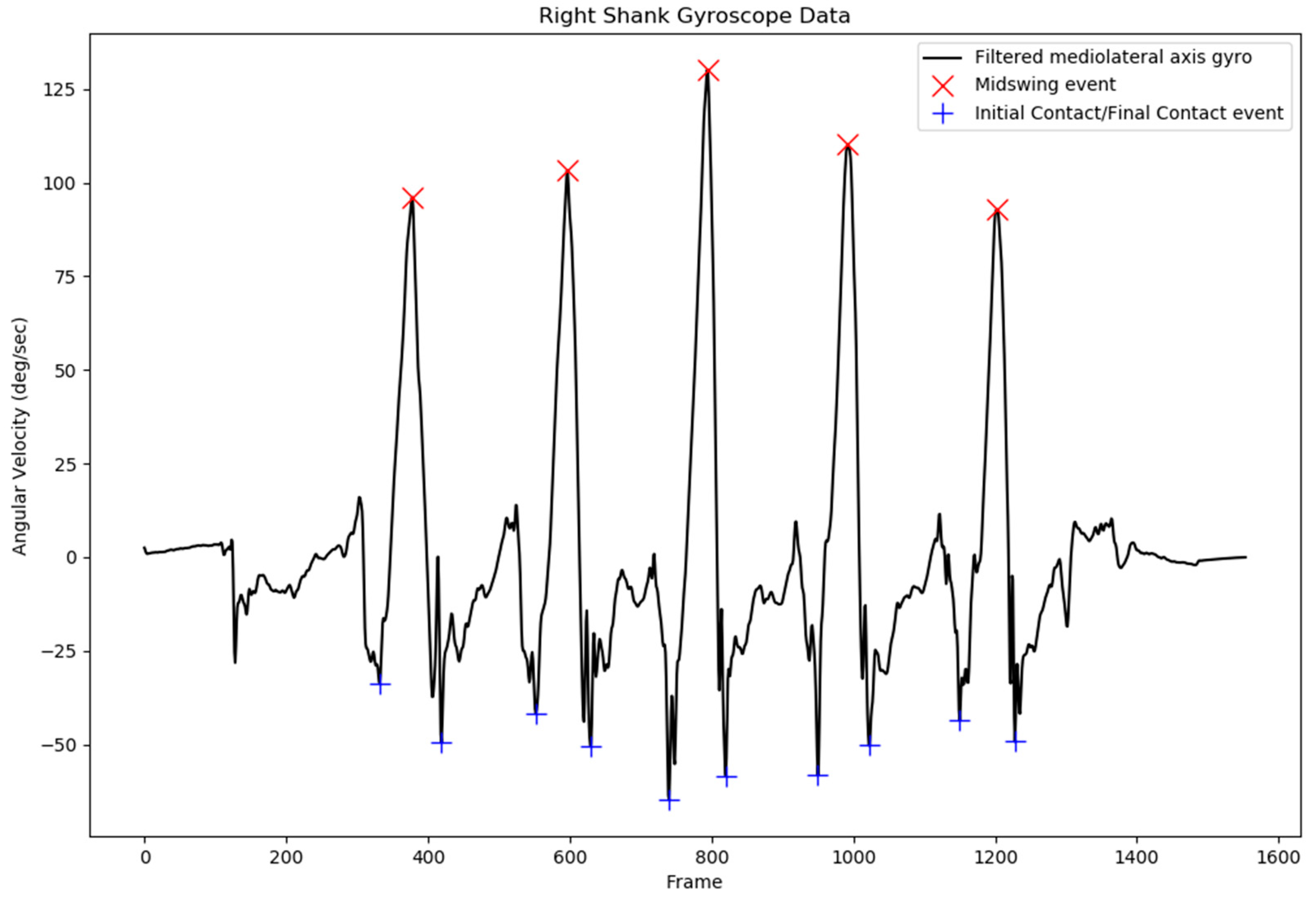
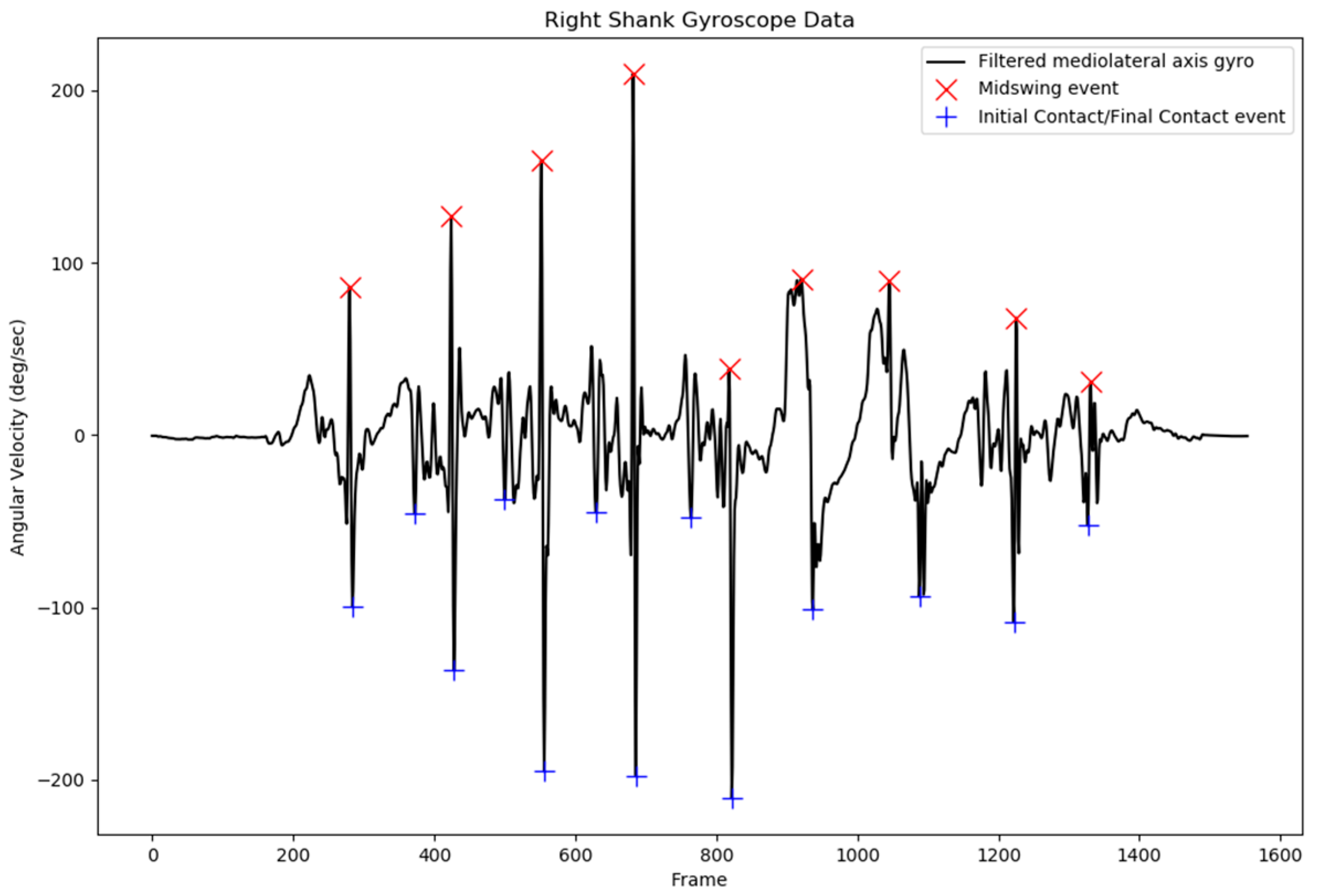
The results from the IC and FC event detection revealed that false peak detection, which can lead to false IC and FC event detection, can be prevented with the current additional parameters applied for peak detection. The pattern in the shank angular velocity of the nonaffected side of stroke subjects was similar to that of the shank angular velocity of healthy subjects, with a less abrupt signal, as indicated in
Figure 5. Although the shank angular velocity pattern of the affected side of stroke patients exhibited a more abrupt signal, the peak was still detected without any false peak detection. This phenomenon was also observed in another study involving a patient with a spinal cord injury; however, the study concluded that the gait event from the shank angular velocity could be used to detect the IC and FC events as accurately as a foot switch for both healthy subjects and those with pathological disorders [
37]. An example of the shank angular velocity of a stroke subject’s affected side is presented in
Figure 6.
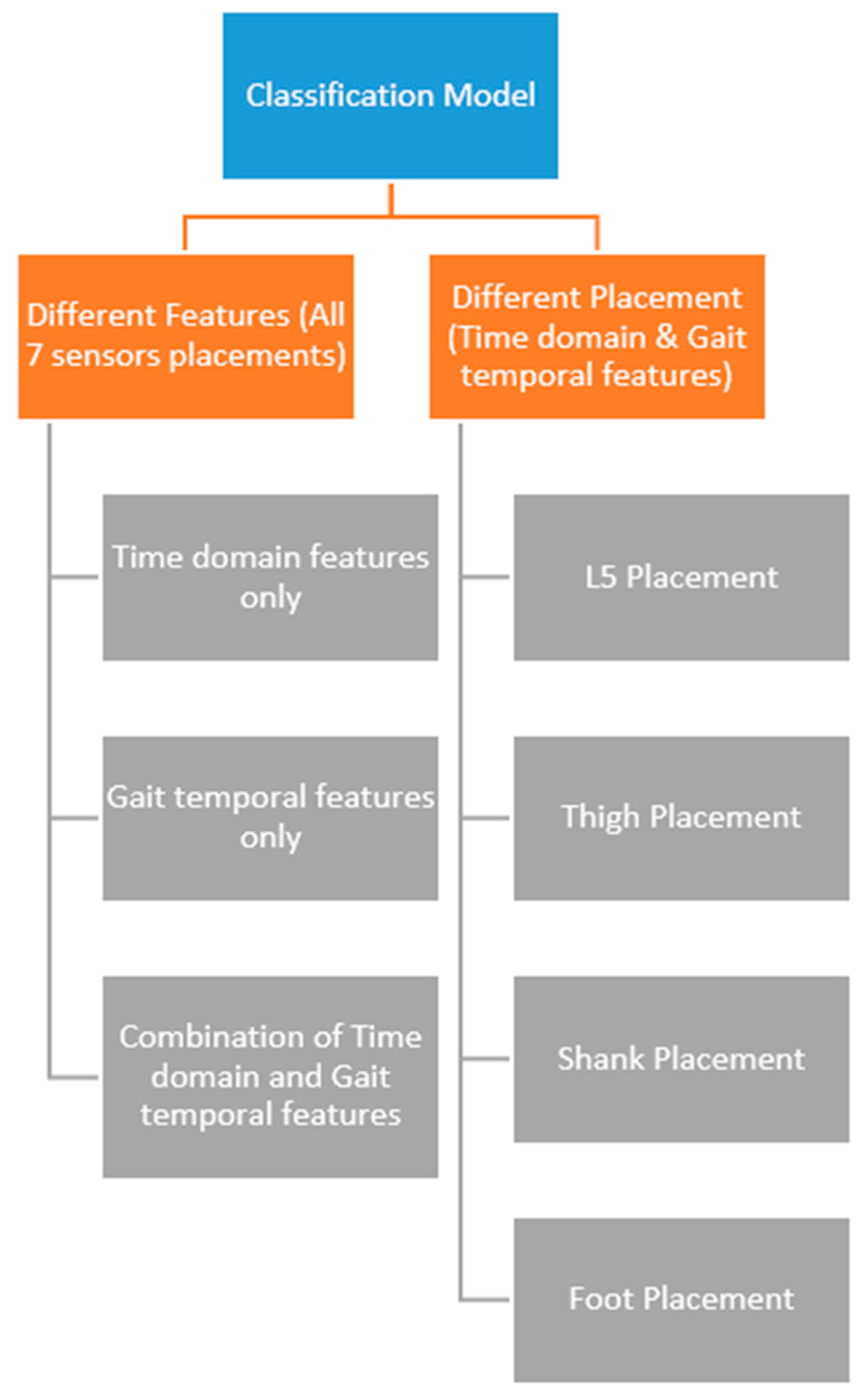
The IC and FC event detections were used to separate the acceleration and angular velocity data from the seven sensor positions for gait classification. This study performed several classification experiments using features from the time domain, gait temporal parameters, and a combination of time domain and gait temporal parameters. Classifications using only independent sensor locations with time domain and gait temporal features were also performed to examine the most accurate sensor placements for gait classification.
The cross-validation accuracy and testing accuracy after feature selection were compared between the classification experiment and within-classification experiment (for different classification algorithms). The results revealed that for classifications involving only time domain features, the Naïve Bayes and MLP classifier outperformed other algorithms with 84.78% testing accuracy; however, MLP had better average precision at 0.86. Moreover, when the features changed from time domain to gait temporal parameters, the RF classifier achieved the highest testing accuracy (76.08%).
The third classification experiment combined time domain features and gait temporal parameters, and the results revealed that the MLP classifier outperformed other algorithms with 84.78% testing accuracy; MLP also had better average precision at 0.88. The results from the first three classification experiments, which used different types of features on the same sensor placement configuration, revealed that the classification with a combination of time domain and gait temporal features showed the best result with 84.78% testing accuracy and average precision and recall of 0.88 and 0.85, respectively. This testing accuracy was achieved with the MLP algorithm.
Classification with only gait temporal parameters demonstrated the lowest testing accuracy (76.08%) (with the RF method), in comparison with a combination of time domain and gait temporal features (84.78% testing accuracy with the MLP algorithm) or even with only time domain feature classification. Other studies have also reported that time domain and frequency domain features achieved higher accuracy than other classification methods using group-specific features based on the Hidden Markov Model (HMM) [
12]. Although these studies used a classification algorithm (SVM) not used in the present study, the comparison of classification algorithms used in this study indicated that even using the same algorithm, the classification methods based on time domain features still outperformed those using gait temporal features and a combination of features (such as the RF method in this study). Moreover, an SVM classifier was already explored in a study classifying healthy young and older gaits [
34] and in another classifying gaits between normal, hemiplegia, and PD patients [
35] with both of them showing good results. Combined with HMM-based features, an SVM classifier was also explored in two previous studies by Mannini et al. which classified gaits between elderly, post-stroke, and Huntington’s disease subjects [
12] and classified human physical activity [
22]. However, these results are in agreement with those reported in the present study because the classification results from a combination of features were more favorable than the results from using only gait temporal parameters.
Another study also used time domain features and gait temporal parameters and discovered that their method was capable of classifying three types of neurodegenerative diseases (Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis) with up to 90.63% accuracy [
38]. In contrast with our results, this study reported relatively higher accuracy for the features extracted from gait temporal parameters. The fact that both the method utilized for defining the IC and FC events and the classification algorithm differed from those used in the present study may be a reason as to why their study achieved higher accuracy. Moreover, their study extracted the time domain features from gait temporal parameters, which also influenced their results. This study also found that among the set of all features, the gait temporal parameters feature was shown to have the worst classification performance with the lowest testing accuracy (60.8%). That means that the classification which only used gait temporal parameters was able to differentiate those groups but the performance was far worse than those of other classifications which utilized the other feature sets like time domain features from the acceleration and gyroscope.
The second part of the classification experiment was a comparison of classification using different sensor placements using the same type of features (combination of time domain and gait temporal features). In this part, four sensor placement groups (L5, foot, shank, and thigh) were used for different independent classification experiments. For the foot, shank, and thigh, the features used were derived from sensors on the left and right side. The results revealed that the shank-based placement achieved the highest result with 89.13% testing accuracy with the Decision Tree algorithm. This result was in agreement with the authors’ hypothesis which stated that shank-based placement might have better accuracy since the gait event definition was based on the shank sensor. Moreover, shank-based placement also outperformed all of the classification experiments which used all sensors.
These results could be attributable to the fact that all of the definitions of gait event were based on the angular velocity of the shank sensor, leading to the classification results based on the shank and thigh sensors demonstrating the highest accuracy. The results from the highest accuracy, precision, and recall from each classification method and sensor placement are shown in
Table 8.
Another study that also used the same algorithm to define the IC and FC gait events compared the configuration of various sensor placements for detecting IC and FC events and calculated the spatiotemporal parameters of gait in children with cerebral palsy (CP). Their results revealed that a shank-and-thigh sensor configuration yielded more robust results in children with CP and a higher level of disability [
26]. This result is in agreement with our results, which revealed that the shank and thigh sensor placement group achieved the highest accuracy among the sensor placements; moreover, other results proved that the algorithm using shank angular velocity to define IC and FC gait event times is more robust than other sensor placement configurations.