Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification
Abstract
1. Background
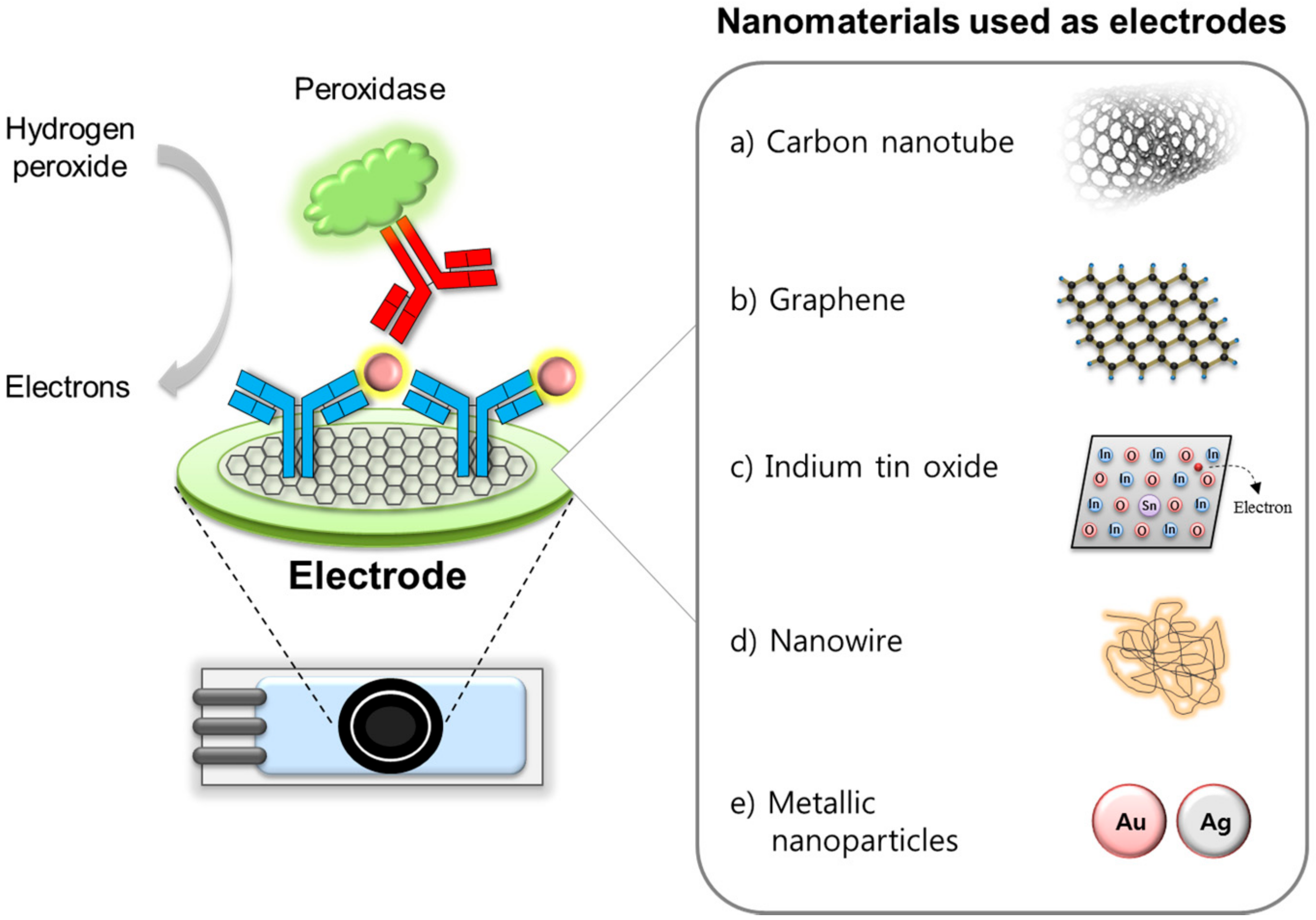
2. Functional Nanomaterials Used as Electrodes and Supporting Matrices
2.1. Carbon Nanotube and Graphene
2.2. Indium Tin Oxide
2.3. Nanowires
2.4. Metallic Nanoparticles
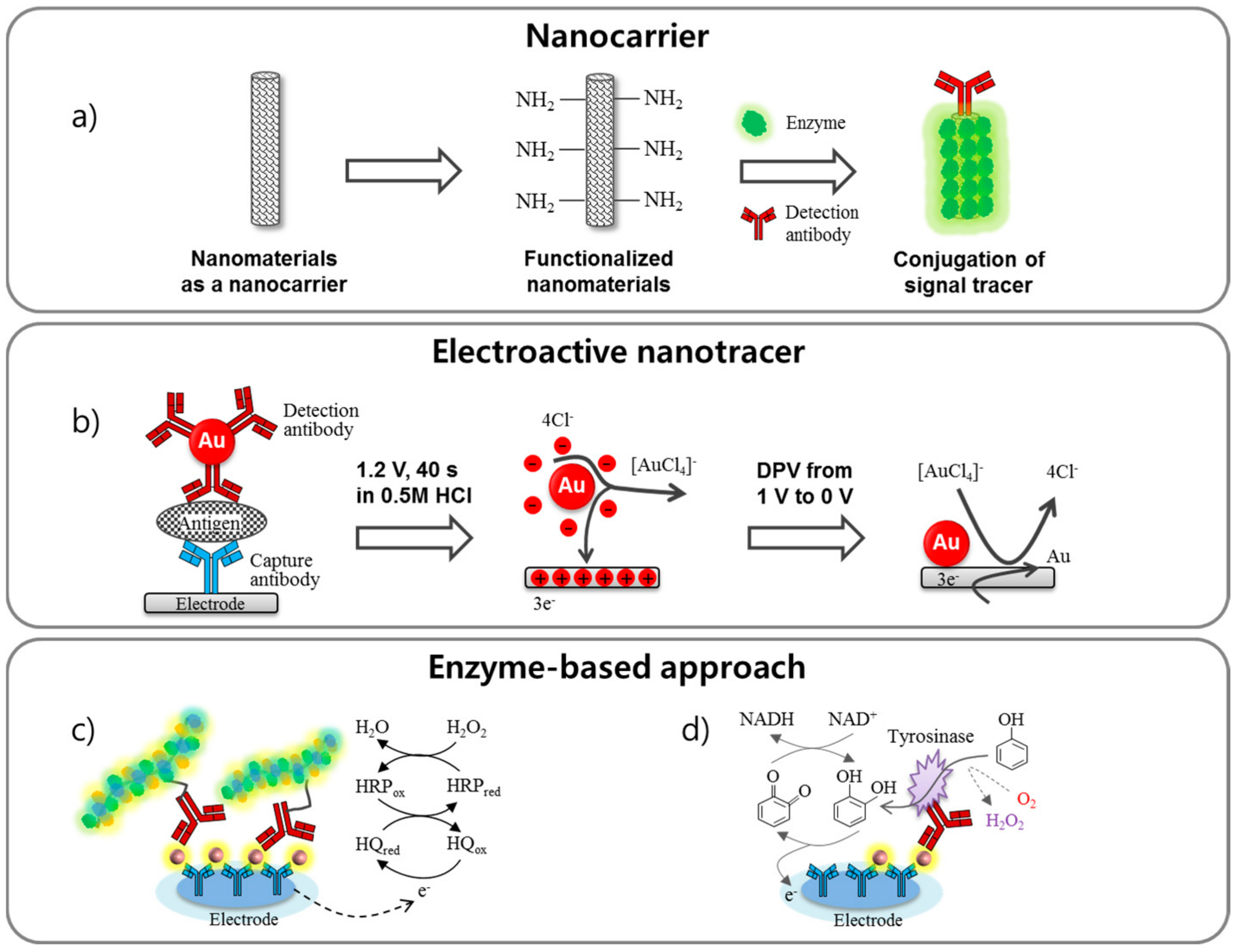
3. Signal Enhancement via Labeling Techniques
3.1. Nanocarriers
3.2. Electroactive Nanotracer
3.3. Enzyme-Based Approach
4. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ni, Y.; Kokot, S. Electrochemical cholesterol sensor based on cholesterol oxidase and MoS2-AuNPs modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 233, 100–106. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, S.; Feng, Q.; Wang, N. Silver nanowire-based electrochemical immunoassay for sensing immunoglobulin G with signal amplification using strawberry-like ZnO nanostructures as labels. Biosens. Bioelectron. 2013, 49, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Fähnrich, K.A.; Pravda, M.; Guilbault, G.G. Disposable amperometric immunosensor for the detection of polycyclic aromatic hydrocarbons (PAHs) using screen-printed electrodes. Biosens. Bioelectron. 2003, 18, 73–82. [Google Scholar] [CrossRef]
- Bosker, W.M.; Huestis, M.A. Oral Fluid Testing for Drugs of Abuse. Clin. Chem. 2009, 55, 1910–1931. [Google Scholar] [CrossRef] [PubMed]
- Schwilke, E.W.; Karschner, E.L.; Lowe, R.H.; Gordon, A.M.; Cadet, J.L.; Herning, R.I.; Huestis, M.A. Intra- and Intersubject Whole Blood/Plasma Cannabinoid Ratios Determined by 2-Dimensional, Electron Impact GC-MS with Cryofocusing. Clin. Chem. 2009, 55, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Wang, Y.; Jin, J.; Yang, R.; Wang, K.; Tan, W. Combination of DNA Ligase Reaction and Gold Nanoparticle-Quenched Fluorescent Oligonucleotides: A Simple and Efficient Approach for Fluorescent Assaying of Single-Nucleotide Polymorphisms. Anal. Chem. 2010, 82, 7684–7690. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.H.; Bonanni, A.; Ambrosi, A.; Poh, H.L.; Pumera, M. Impedimetric immunoglobulin G immunosensor based on chemically modified graphenes. Nanoscale 2012, 4, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Adornetto, G.; Palleschi, G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta 2012, 84, 74–83. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Feng, J.; Dong, Y.; Wang, P.; Chen, L.; Chen, Z.; Liu, H.; Wei, Q. Ultrasensitive amperometric immunosensor for PSA detection based on Cu2O@CeO2-Au nanocomposites as integrated triple signal amplification strategy. Biosens. Bioelectron. 2017, 87, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Wang, Y.; Ma, H.; Du, B.; Wei, Q. Facile synthesis of cuprous oxide nanowires decorated graphene oxide nanosheets nanocomposites and its application in label-free electrochemical immunosensor. Biosens. Bioelectron. 2017, 87, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tirado, E.; Gonzalez-Cortes, A.; Yanez-Sedeno, P.; Pingarron, J.M. Carbon nanotubes functionalized by click chemistry as scaffolds for the preparation of electrochemical immunosensors. Application to the determination of TGF-beta 1 cytokine. Analyst 2016, 141, 5730–5737. [Google Scholar] [CrossRef] [PubMed]
- Tlili, C.; Cella, L.N.; Myung, N.V.; Shetty, V.; Mulchandani, A. Single-walled carbon nanotube chemoresistive label-free immunosensor for salivary stress biomarkers. Analyst 2010, 135, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.L.; Major, S.; Pandya, D.K. Transparent conductors—A status review. Thin Solid Films 1983, 102, 1–46. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezginturk, M.K. Label-free, ITO-based immunosensor for the detection of a cancer biomarker: Receptor for Activated C Kinase 1. Analyst 2016, 141, 5618–5626. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Morrow, T.J.; Keating, C.D. Nanowire sensors for multiplexed detection of biomolecules. Curr. Opin. Chem. Biol. 2008, 12, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Ahmed, M.U. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: A review. RSC Adv. 2016, 6, 24995–25014. [Google Scholar] [CrossRef]
- Li, M.; Wang, P.; Li, F.; Chu, Q.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of mesoporous core-shell Pd@Pt nanoparticles/amino group functionalized graphene nanocomposite. Biosens. Bioelectron. 2017, 87, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, W.-J.; Li, L.; Yang, Y.; Mao, L.-G.; Peng, Z. A label-free electrochemical immunosensor based on gold nanoparticles for direct detection of atrazine. Sens. Actuators B Chem. 2014, 191, 408–414. [Google Scholar] [CrossRef]
- Ronkainen, N.; Okon, S. Nanomaterial-Based Electrochemical Immunosensors for Clinically Significant Biomarkers. Materials 2014, 7, 4669–4709. [Google Scholar] [CrossRef] [PubMed]
- Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Role of carbon nanotubes in electroanalytical chemistry. Anal. Chim. Acta 2008, 622, 11–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, X.; Han, J.; Ma, J.; Ma, Z. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens. Bioelectron. 2013, 50, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, L.; Li, J. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, Y.; Feng, T.; Shi, W.; Li, X.; Ma, H. A graphene oxide-peptide fluorescence sensor tailor-made for simple and sensitive detection of matrix metalloproteinase 2. Chem. Commun. 2011, 47, 10680–10682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bai, L.; Shang, W.; Xie, W.; Ma, H.; Fu, Y.; Fang, D.; Sun, H.; Fan, L.; Han, M.; et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 2012, 22, 7461–7467. [Google Scholar] [CrossRef]
- Sun, X.; Ma, Z. Highly stable electrochemical immunosensor for carcinoembryonic antigen. Biosens. Bioelectron. 2012, 35, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, Z. Fabrication of an ultrasensitive electrochemical immunosensor for CEA based on conducting long-chain polythiols. Biosens. Bioelectron. 2013, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Zhang, H.; Tamanna, T.; Yu, A. Ultrasensitive Immunoassay Based on Electrochemical Measurement of Enzymatically Produced Polyaniline. Anal. Chem. 2014, 86, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, A.J.S.; Lee, J.-J.; Rahman, M.A. Electrochemical Sensors Based on Carbon Nanotubes. Sensors 2009, 9, 2289–2319. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Dai, Z.; Lin, Y.; Du, D. Electrochemical Immunoassays Based on Graphene: A Review. Electroanalysis 2016, 28, 4–12. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhu, J.; Xu, J.; Chen, H.; Xu, D. An electrochemical impedimetric arrayed immunosensor based on indium tin oxide electrodes and silver-enhanced gold nanoparticles. Microchim. Acta 2008, 163, 63–70. [Google Scholar] [CrossRef]
- Choi, Y.-B.; Jeon, W.-Y.; Kim, H.-H. A Simple Interfacial Platform for Homogeneous Electrochemical Immunoassays Using a Poly(Vinylimidazole)-Modified Electrode. Sensors 2017, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Park, S.; Jon, S.; Yang, H. Amperometric immunosensing using an indium tin oxide electrode modified with multi-walled carbon nanotube and poly(ethylene glycol)-silane copolymer. Chem. Commun. 2007, 25, 2610–2612. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.H.; Bae, S.C.; Lee, C.-W.; Jeong, S.; Kim, K.S. Ultrathin Single-Crystalline Silver Nanowire Arrays Formed in an Ambient Solution Phase. Science 2001, 294, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, J.; Zhang, L.; Hu, X.; Cao, X. Ultrasensitive Detection Using Surface Enhanced Raman Scattering from Silver Nanowire Arrays in Anodic Alumina Membranes. J. Nanosci. Nanotechnol. 2009, 9, 4812–4816. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-Y.; Hu, B.; Yu, X.-C.; Zhao, R.-L.; Ren, X.-F.; Liu, S.-L.; Liu, J.-W.; Feng, M.; Xu, A.-W.; Yu, S.-H. Ordering of Disordered Nanowires: Spontaneous Formation of Highly Aligned, Ultralong Ag Nanowire Films at Oil–Water–Air Interface. Adv. Funct. Mater. 2010, 20, 958–964. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Wang, R.; Ruan, C.; Kanayeva, D.; Lassiter, K.; Li, Y. TiO2 Nanowire Bundle Microelectrode Based Impedance Immunosensor for Rapid and Sensitive Detection of Listeria monocytogenes. Nano Lett. 2008, 8, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, C.; Kwon, D.; Kim, D.; Meyyappan, M.; Jeon, S.; Lee, J.-S. Silicon nanowire biosensors for detection of cardiac troponin I (cTnI) with high sensitivity. Biosens. Bioelectron. 2016, 77, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Lu, N.; Dai, P.; Li, T.; Pei, H.; Gao, X.; Gong, Y.; Wang, Y.; Fan, C. Silicon-Nanowire-Based CMOS-Compatible Field-Effect Transistor Nanosensors for Ultrasensitive Electrical Detection of Nucleic Acids. Nano Lett. 2011, 11, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Bangar, M.A.; Shirale, D.J.; Chen, W.; Myung, N.V.; Mulchandani, A. Single Conducting Polymer Nanowire Chemiresistive Label-Free Immunosensor for Cancer Biomarker. Anal. Chem. 2009, 81, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Hui, N.; Sun, X.; Song, Z.; Niu, S.; Luo, X. Gold nanoparticles and polyethylene glycols functionalized conducting polyaniline nanowires for ultrasensitive and low fouling immunosensing of alpha-fetoprotein. Biosens. Bioelectron. 2016, 86, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Granot, E.; Katz, E.; Basnar, B.; Willner, I. Enhanced Bioelectrocatalysis Using Au-Nanoparticle/Polyaniline Hybrid Systems in Thin Films and Microstructured Rods Assembled on Electrodes. Chemi. Mater. 2005, 17, 4600–4609. [Google Scholar] [CrossRef]
- Arya, S.K.; Dey, A.; Bhansali, S. Polyaniline protected gold nanoparticles based mediator and label free electrochemical cortisol biosensor. Biosens. Bioelectron. 2011, 28, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Kaushik, A.; Arya, S.K.; Bhansali, S. Mediator free highly sensitive polyaniline-gold hybrid nanocomposite based immunosensor for prostate-specific antigen (PSA) detection. J. Mater. Chem. 2012, 22, 14763–14772. [Google Scholar] [CrossRef]
- Ozdemir, C.; Yeni, F.; Odaci, D.; Timur, S. Electrochemical glucose biosensing by pyranose oxidase immobilized in gold nanoparticle-polyaniline/AgCl/gelatin nanocomposite matrix. Food Chem. 2010, 119, 380–385. [Google Scholar] [CrossRef]
- Elnathan, R.; Kwiat, M.; Pevzner, A.; Engel, Y.; Burstein, L.; Khatchtourints, A.; Lichtenstein, A.; Kantaev, R.; Patolsky, F. Biorecognition layer engineering: Overcoming screening limitations of nanowire-based FET devices. Nano Lett. 2012, 12, 5245–5254. [Google Scholar] [CrossRef] [PubMed]
- Xuan Viet, N.; Chikae, M.; Ukita, Y.; Maehashi, K.; Matsumoto, K.; Tamiya, E.; Hung Viet, P.; Takamura, Y. Gold-linked electrochemical immunoassay on single-walled carbon nanotube for highly sensitive detection of human chorionic gonadotropin hormone. Biosens. Bioelectron. 2013, 42, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, M.; Zhou, H.; Zhang, S. Efficient double-quenching of electrochemiluminescence from CdS:Eu QDs by hemin-graphene-Au nanorods ternary composite for ultrasensitive immunoassay. Sci. Rep. 2016, 6, 30577. [Google Scholar] [CrossRef] [PubMed]
- Elshafey, R.; Siaj, M.; Tavares, A.C. Au nanoparticle decorated graphene nanosheets for electrochemical immunosensing of p53 antibodies for cancer prognosis. Analyst 2016, 141, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, C.M.; Dong, S.L.; Du, C.X.; Zhang, X.Y.; Li, L.H.; Wei, Y. Enhanced conductivity of rGO/Ag NPs composites for electrochemical immunoassay of prostate-specific antigen. Biosens. Bioelectron. 2017, 87, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Takahara, Y.K.; Ikeda, S.; Ishino, S.; Tachi, K.; Ikeue, K.; Sakata, T.; Hasegawa, T.; Mori, H.; Matsumura, M.; Ohtani, B. Asymmetrically Modified Silica Particles: A Simple Particulate Surfactant for Stabilization of Oil Droplets in Water. J. Am. Chem. Soc. 2005, 127, 6271–6275. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Liu, C.; Shen, J.; Gao, D.; Zhu, J.-J.; Chen, H.-Y. Gold Nanoparticle–Colloidal Carbon Nanosphere Hybrid Material: Preparation, Characterization and Application for an Amplified Electrochemical Immunoassay. Adv. Funct. Mater. 2008, 18, 2197–2204. [Google Scholar] [CrossRef]
- Li, F.; Feng, Y.; Wang, Z.; Yang, L.; Zhuo, L.; Tang, B. Direct electrochemistry of horseradish peroxidase immobilized on the layered calcium carbonate–gold nanoparticles inorganic hybrid composite. Biosens. Bioelectron. 2010, 25, 2244–2248. [Google Scholar] [CrossRef] [PubMed]
- Paun, C.; Safonova, O.V.; Szlachetko, J.; Abdala, P.M.; Nachtegaal, M.; Sa, J.; Kleymenov, E.; Cervellino, A.; Krumeich, F.; van Bokhoven, J.A. Polyhedral CeO2 Nanoparticles: Size-Dependent Geometrical and Electronic Structure. J. Phys. Chem. C 2012, 116, 7312–7317. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Song, S.; Zhang, H. Pt@CeO2 Multicore@Shell Self-Assembled Nanospheres: Clean Synthesis, Structure Optimization and Catalytic Applications. J. Am. Chem. Soc. 2013, 135, 15864–15872. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H.; Zhuo, Y.; Yuan, R.; Chai, Y.-Q. An amplified electrochemical immunosensor based on in situ-produced 1-naphthol as electroactive substance and graphene oxide and Pt nanoparticles functionalized CeO2 nanocomposites as signal enhancer. Biosens. Bioelectron. 2015, 69, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peng, B.; Hernandez-Viezcas, J.A.; Rico, C.; Sun, Y.; Peralta-Videa, J.R.; Tang, X.; Niu, G.; Jin, L.; Varela-Ramirez, A.; et al. Stress Response and Tolerance of Zea mays to CeO2 Nanoparticles: Cross Talk among H2O2, Heat Shock Protein and Lipid Peroxidation. ACS Nano 2012, 6, 9615–9622. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, J.; Song, G.; Zhu, J.J. Fabrication of silica/PDMS hybrid nanoparticles by a novel solvent adjustment route. J. Nanosci. Nanotechnol. 2012, 12, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yuan, R.; Chai, Y. Magnetic Core−Shell Fe3O4@Ag Nanoparticles Coated Carbon Paste Interface for Studies of Carcinoembryonic Antigen in Clinical Immunoassay. J. Phys. Chem. B 2006, 110, 11640–11646. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Hirsch, T.; Baeumner, A.J. Nanomaterials as versatile tools for signal amplification in (bio)analytical applications. TrAC Trends Anal. Chem. 2016, 79 (Suppl. C), 306–316. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chiu, N.-F.; Lin, D.S.; Chu-Su, Y.; Liang, Y.-H.; Lin, C.-W. High-Sensitivity Detection of Carbohydrate Antigen 15-3 Using a Gold/Zinc Oxide Thin Film Surface Plasmon Resonance-Based Biosensor. Anal. Chem. 2010, 82, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, S.; Jiang, L.-P.; Hou, W.; Shen, Q.; Zhu, J.-J. Graphene–CdS Nanocomposites: Facile One-Step Synthesis and Enhanced Photoelectrochemical Cytosensing. Chem. A Eur. J. 2012, 18, 4974–4981. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.; Hernández-Albors, A.; Marco, M.P. Electrochemical coding strategies using metallic nanoprobes for biosensing applications. TrAC Trends Anal. Chem. 2016, 79 (Suppl. C), 9–22. [Google Scholar] [CrossRef]
- Akanda, M.R.; Ju, H. A Tyrosinase-Responsive Nonenzymatic Redox Cycling for Amplified Electrochemical Immunosensing of Protein. Anal. Chem. 2016, 88, 9856–9861. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xiong, E.; Zhang, X.; Zhang, X.; Chen, J. Nanomaterials as signal amplification elements in DNA-based electrochemical sensing. Nano Today 2014, 9, 197–211. [Google Scholar] [CrossRef]
- Akter, R.; Kyun Rhee, C.; Aminur Rahman, M. Sensitivity enhancement of an electrochemical immunosensor through the electrocatalysis of magnetic bead-supported non-enzymatic labels. Biosens. Bioelectron. 2014, 54, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Pang, J.; Yin, H.; Ai, S. G-quadruplex functionalized nano mesoporous silica for assay of the DNA methyltransferase activity. Anal. Chim. Acta 2015, 879, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Li, N.; Ma, H.; Li, Y.; Hu, L.; Du, B.; Wei, Q. Electrochemical immunosensor for detection of prostate specific antigen based on an acid cleavable linker into MSN-based controlled release system. Biosens. Bioelectron. 2016, 85, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang, L.; Shao, Y.; Wang, J.; Engelhard, M.H.; Lin, Y. Functionalized graphene oxide as a nanocarrier in a multienzyme labeling amplification strategy for ultrasensitive electrochemical immunoassay of phosphorylated p53 (S392). Anal. Chem. 2011, 83, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Li, M.; Qing, Y.; Dai, N.; Guan, W.; Liang, W.; Wang, D. Signal-on electrochemical immunoassay for APE1 using ionic liquid doped Au nanoparticle/graphene as a nanocarrier and alkaline phosphatase as enhancer. Analyst 2014, 139, 6563–6568. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Yoshikawa, H.; Tamiya, E.; Yasin, H.M.; Ahmed, M.U. A highly sensitive gold nanoparticle bioprobe based electrochemical immunosensor using screen printed graphene biochip. RSC Adv. 2014, 4, 58460–58466. [Google Scholar] [CrossRef]
- Kim, D.; Daniel, W.L.; Mirkin, C.A. Microarray-Based Multiplexed Scanometric Immunoassay for Protein Cancer Markers Using Gold Nanoparticle Probes. Anal. Chem. 2009, 81, 9183–9187. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, J.; Wang, M.; Yan, F.; Ju, H. Triple Signal Amplification of Graphene Film, Polybead Carried Gold Nanoparticles as Tracing Tag and Silver Deposition for Ultrasensitive Electrochemical Immunosensing. Anal. Chem. 2012, 84, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, J.; Ju, H.; Yan, F. Nanogold/mesoporous carbon foam-mediated silver enhancement for graphene-enhanced electrochemical immunosensing of carcinoembryonic antigen. Biosens. Bioelectron. 2014, 52, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Bolado, P.; Hernández-Santos, D.; González-García, M.B.; Costa-García, A. Alkaline Phosphatase-Catalyzed Silver Deposition for Electrochemical Detection. Anal. Chem. 2007, 79, 5272–5277. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, H.; Jiang, W.; Zhou, Y.; Ai, S. Electrochemical immunosensor for N6-methyladenosine detection in human cell lines based on biotin-streptavidin system and silver-SiO2 signal amplification. Biosens. Bioelectron. 2017, 90, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Narayanan, J.; Pardasani, D.; Srivastava, D.N.; Upadhyay, S.; Goel, A.K. Ultrasensitive electrochemical immunoassay for surface array protein, a Bacillus anthracis biomarker using Au-Pd nanocrystals loaded on boron-nitride nanosheets as catalytic labels. Biosens. Bioelectron. 2016, 80, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Y.; Liu, G.; Dong, B.; Zhou, J.; Wang, A.; Wang, J.; Jin, R.; Lv, H.; Dou, Z.; Huang, W. Microbial synthesis of Pd/Fe(3)O(4), Au/Fe(3)O(4) and PdAu/Fe(3)O(4) nanocomposites for catalytic reduction of nitroaromatic compounds. Sci. Rep. 2015, 5, 13515. [Google Scholar] [CrossRef] [PubMed]
- Pozun, Z.D.; Rodenbusch, S.E.; Keller, E.; Tran, K.; Tang, W.; Stevenson, K.J.; Henkelman, G. A Systematic Investigation of p-Nitrophenol Reduction by Bimetallic Dendrimer Encapsulated Nanoparticles. J. Phys. Chem. C 2013, 117, 7598–7604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, C.; Huang, H.; Zhang, L.; Jiang, Z.; Chen, G.; Jia, Y.; Kuang, Q.; Xie, Z.; Zheng, L. Surfactant-Concentration-Dependent Shape Evolution of Au–Pd Alloy Nanocrystals from Rhombic Dodecahedron to Trisoctahedron and Hexoctahedron. Small 2013, 9, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Gao, Z.; Xu, M.; Cao, X.; Wu, X.; Chen, G.; Tang, D. DNAzyme-functionalized gold–palladium hybrid nanostructures for triple signal amplification of impedimetric immunosensor. Biosens. Bioelectron. 2014, 54, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Gan, N.; Cao, Y.; Hu, F.; Li, T.; Zheng, L. An Ultrasensitive Electrochemical Immunosensor for Alpha-Fetoprotein Using an Envision Complex-Antibody Copolymer as a Sensitive Label. Materials 2012, 5, 2757–2772. [Google Scholar] [CrossRef]
- Lai, G.; Cheng, H.; Xin, D.; Zhang, H.; Yu, A. Amplified inhibition of the electrochemical signal of ferrocene by enzyme-functionalized graphene oxide nanoprobe for ultrasensitive immunoassay. Anal. Chim. Acta 2016, 902, 189–195. [Google Scholar] [CrossRef] [PubMed]

| Materials | Examples | Advantages | Limitations | Limit of Detection | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| (a) Carbon-based | SWCNT | Large surface area to volume ratio (S/V) Low charge-carried Density Delocalized π-orbitals | Difficult manipulation during sensor fabrication process Difficult chemical functionalization | hCG (2.4 pg/mL) | 10–2000 pg/mL | [54] |
| MWCNT | Excellent conducting and electro-catalytic properties | Need to functionalize surface for increasing biocompatibility | PSA (3.33 fg/mL) | 10 fg/mL–100 ng/mL | [22] | |
| Graphene | High S/V Large active sites Fast electron transfer High thermal Conductivity Better mechanical Flexibility Good biocompatibility | Hard to dissolve in water | CEA (0.10 pg/mL) | 0.01 pg/mL–1.0 ng/mL | [55] | |
| (b) ITO | Low cost/High Transmittance Good electrical Conductivity Ease of surface Modification | Slow kinetics of electron-transfer upon coating surface with antibodies | RACK1 (30 fg/mL) | 14.25–712.5 fg/mL | [19] | |
| (c) Nanowire | Metal | Rapid response, electro-catalytic capability and reproducibility | Decrease in electrostatic potential with distance | IgG (4 pg/mL) | 0.01–200 ng/mL | [6] |
| Metal oxides | Facilitation of electron-transfer kinetics | The same as above | Listeria Monocytogenes (102 cfu/mL) | No linear range can be found | [43] | |
| Semi-conductor | Ultrasensitive/Real-time Label-free in NWFETs | The same as above | cTnI (5 pg/mL) | 5–200 pg/mL | [44] | |
| Conducting polymers | Maintenance of conductance under neutral pH Improvement of the charge transfer and stability | The same as above | AFP (7 fg/mL) | 0.01 pg/mL–1.0 ng/mL | [48] | |
| (d) Metallic nanoparticle | Au, Ag, composites | Efficient electron Transfer Increase in S/V Supplying superior conductivity | Electrical instability in high salt concentration Inconsistent upon signal amplification | Atrazine (16 pg/mL) p53 (88 fg/mL) PSA (30 fg/mL) | 0.05–0.5 ng/mL 0.1 pg/mL–10 ng/mL 0.1 pg/mL–100 ng/mL | [23] [56] [13] |
| Strategies | Example | Effects | Limit of Detection | Linear Range | Ref. |
|---|---|---|---|---|---|
| (a) Nanocarrier | MSN | Encapsulation of electron mediator | PSA (0.31 pg/mL) | 0.001–5.0 ng/mL | [76] |
| GO | High loading capacity of ALP | Human apurinic/APE 1 (40 fg/mL) | 0.1–80 pg/mL | [78] | |
| (b) Electroactive nanotracer | Colloidal gold | Redox properties in acidic condition Facilitation of chemical reaction | hCG (5 pg/mL) | 0–500 pg/mL | [79] |
| Nanogold | Superior catalytic activity to colloidal gold | CEA (24 fg/mL) | 0.05 pg/mL–1.0 ng/mL | [82] | |
| Silver nanoparticle | Production of sharper peak compared to gold nanoparticle | N6-methyladenosine (78 pM) | 0.2–500 nM | [84] | |
| Bimetallic nanostructures | Enhanced catalytic capability Excellent adsorption and charge transfer trait | Bacillus anthracis (1 pg/mL) | 5 pg/mL–100 ng/mL | [85] | |
| (c) Enzyme-based approach | Antibody-enzyme network structure | Increasing the number of enzyme molecules | AFP (2 pg/mL) | 5–200 pg/mL | [90] |
| (d) Redox cycling | Facilitation by electron mediators | Converting the oxidized state of signal species with reducing agents | CEA (sub pg/mL) | 1.0 pg/mL–0.1 μg/mL | [71] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.-s.; Paik, J.K.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H. Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors 2018, 18, 207. https://doi.org/10.3390/s18010207
Cho I-H, Lee J, Kim J, Kang M-s, Paik JK, Ku S, Cho H-M, Irudayaraj J, Kim D-H. Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors. 2018; 18(1):207. https://doi.org/10.3390/s18010207
Chicago/Turabian StyleCho, Il-Hoon, Jongsung Lee, Jiyeon Kim, Min-soo Kang, Jean Kyung Paik, Seockmo Ku, Hyun-Mo Cho, Joseph Irudayaraj, and Dong-Hyung Kim. 2018. "Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification" Sensors 18, no. 1: 207. https://doi.org/10.3390/s18010207
APA StyleCho, I.-H., Lee, J., Kim, J., Kang, M.-s., Paik, J. K., Ku, S., Cho, H.-M., Irudayaraj, J., & Kim, D.-H. (2018). Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors, 18(1), 207. https://doi.org/10.3390/s18010207







