Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Synthesis of Cu2O NPs
2.3. Synthesis of Cu2O-GO Composite Nanomaterials
2.4. Fabrication of Cu2O-RGO-Modified GCE
2.5. Characterization
2.6. Electrochemical Experiments
2.7. Analysis of Real Samples
3. Result and Discussion
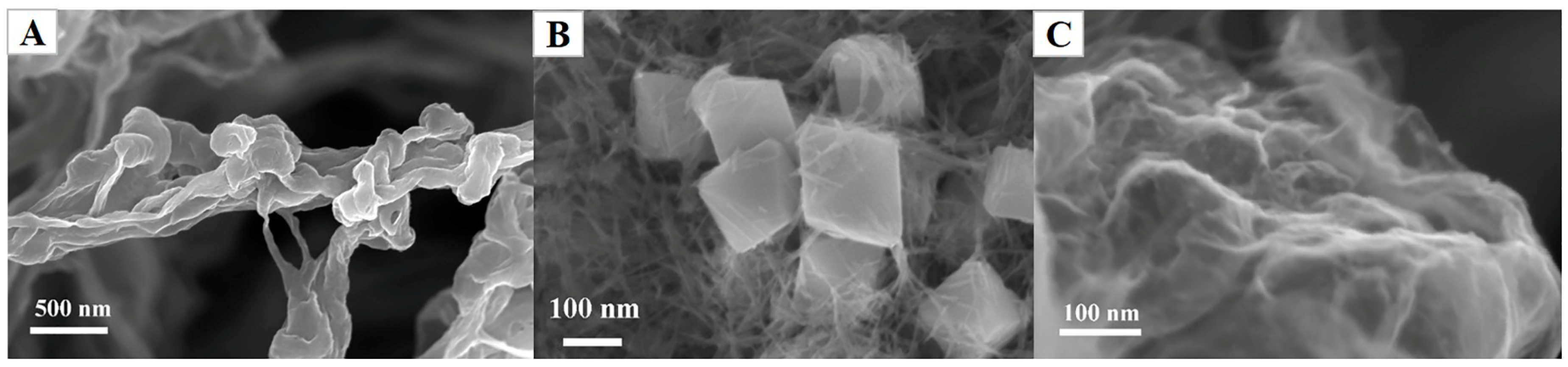
3.1. Morphologic Characterization of Cu2O-GO Nanocomposites
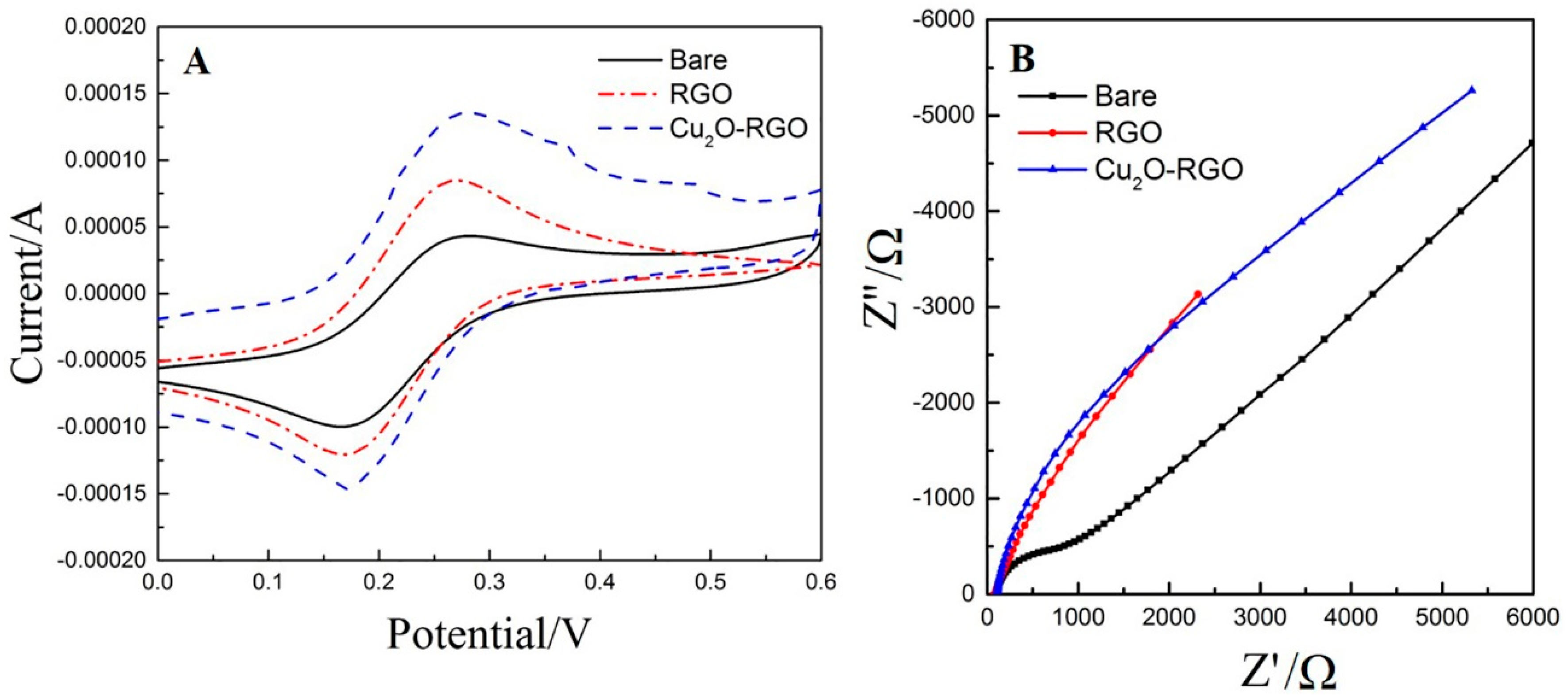
3.2. Electrochemical Characterization
3.3. Optimization of Electrochemical Reduction Condition
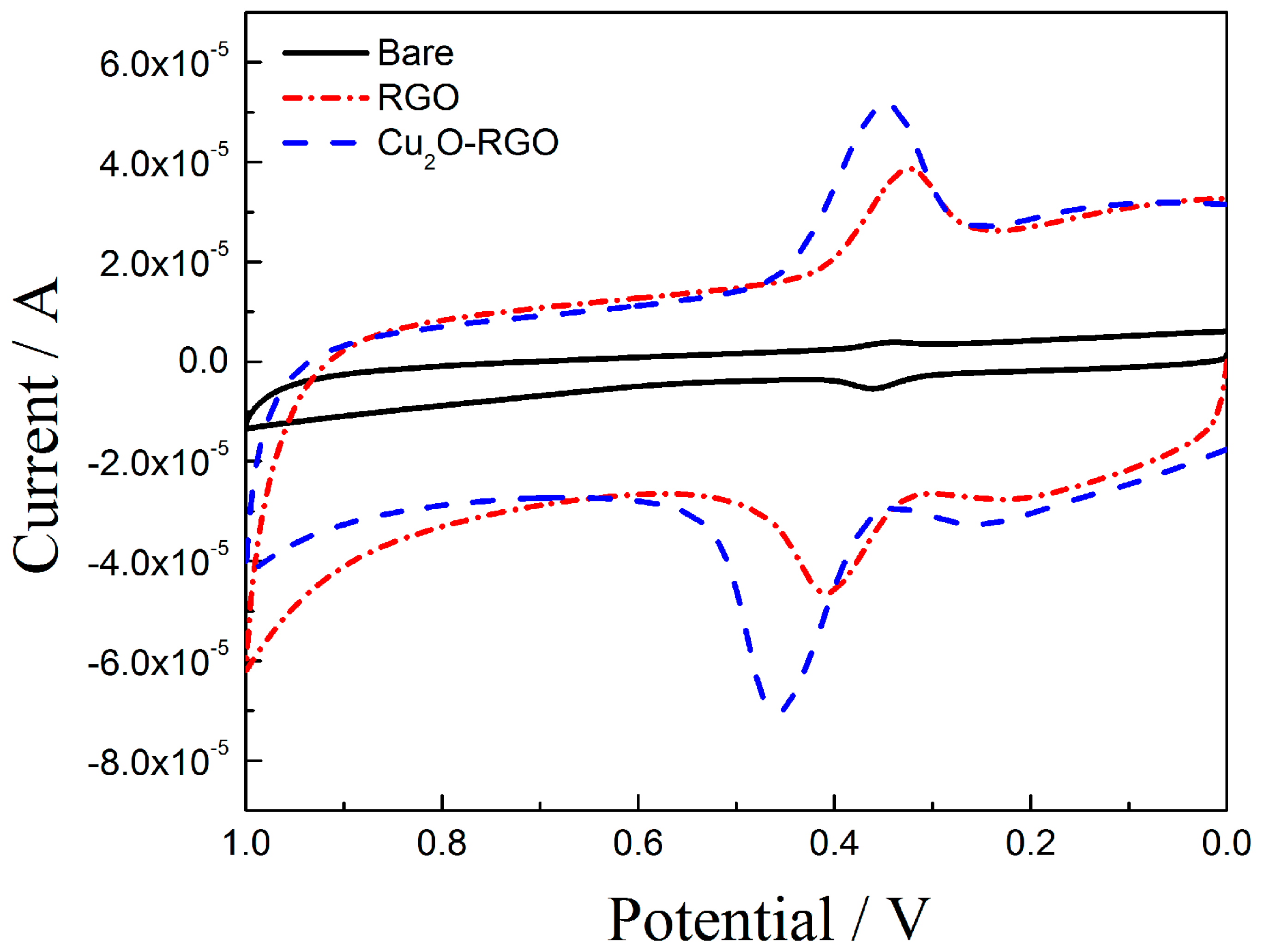
3.4. The DA sensing of Modified Electrodes
3.5. Optimization of the Detection Condition of DA
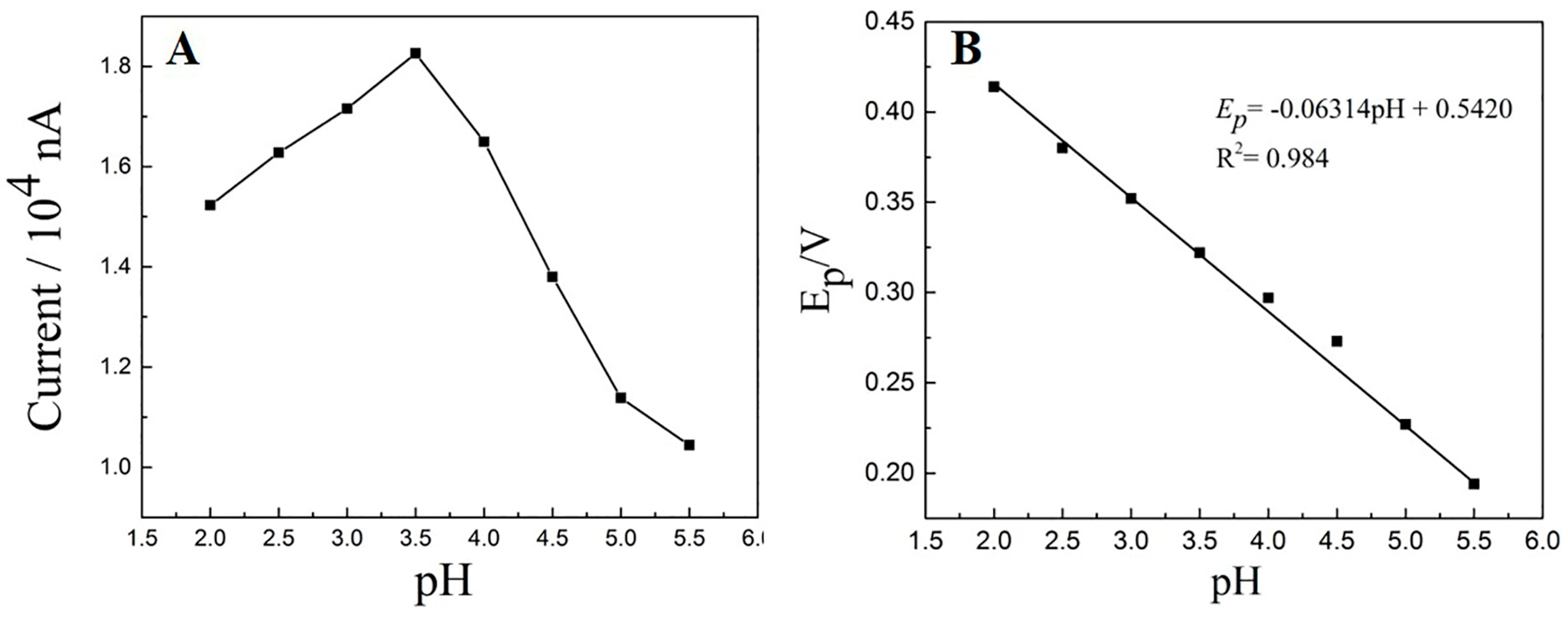
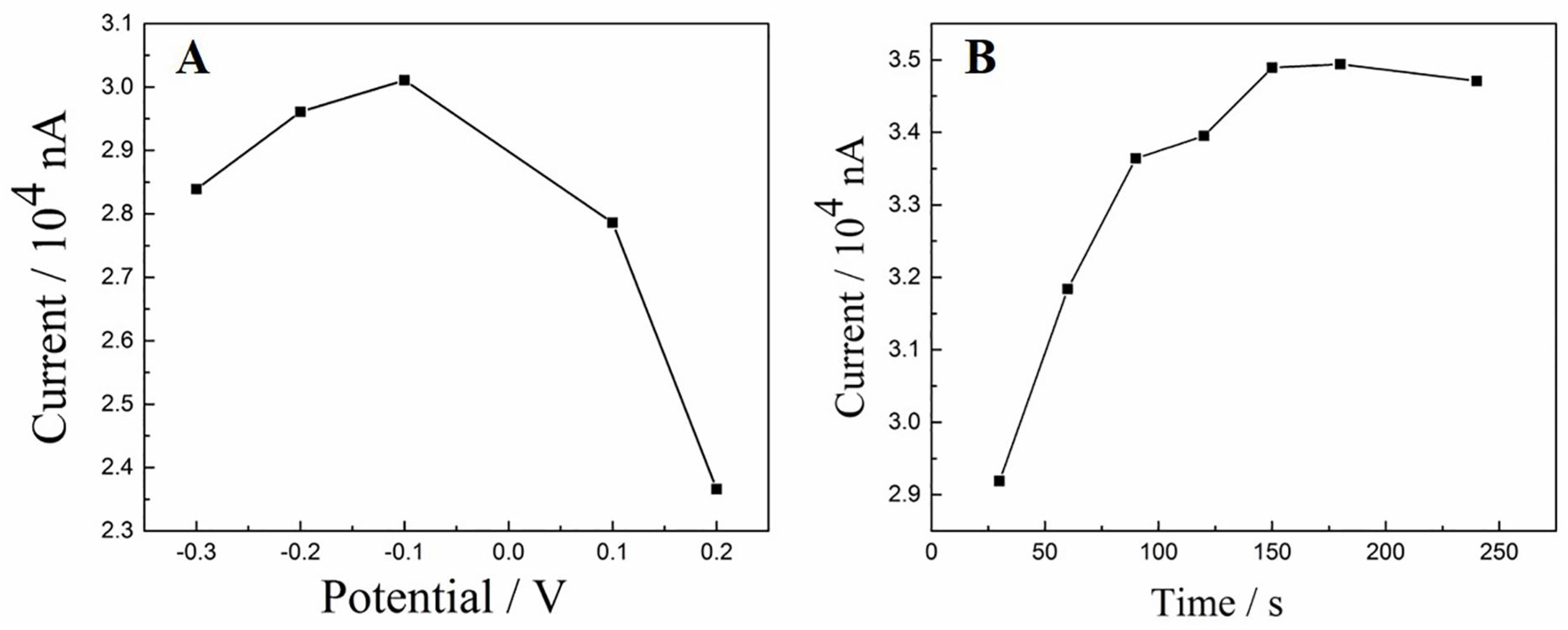
3.5.1. The Influence of pH
3.5.2. Effect of Accumulation Conditions
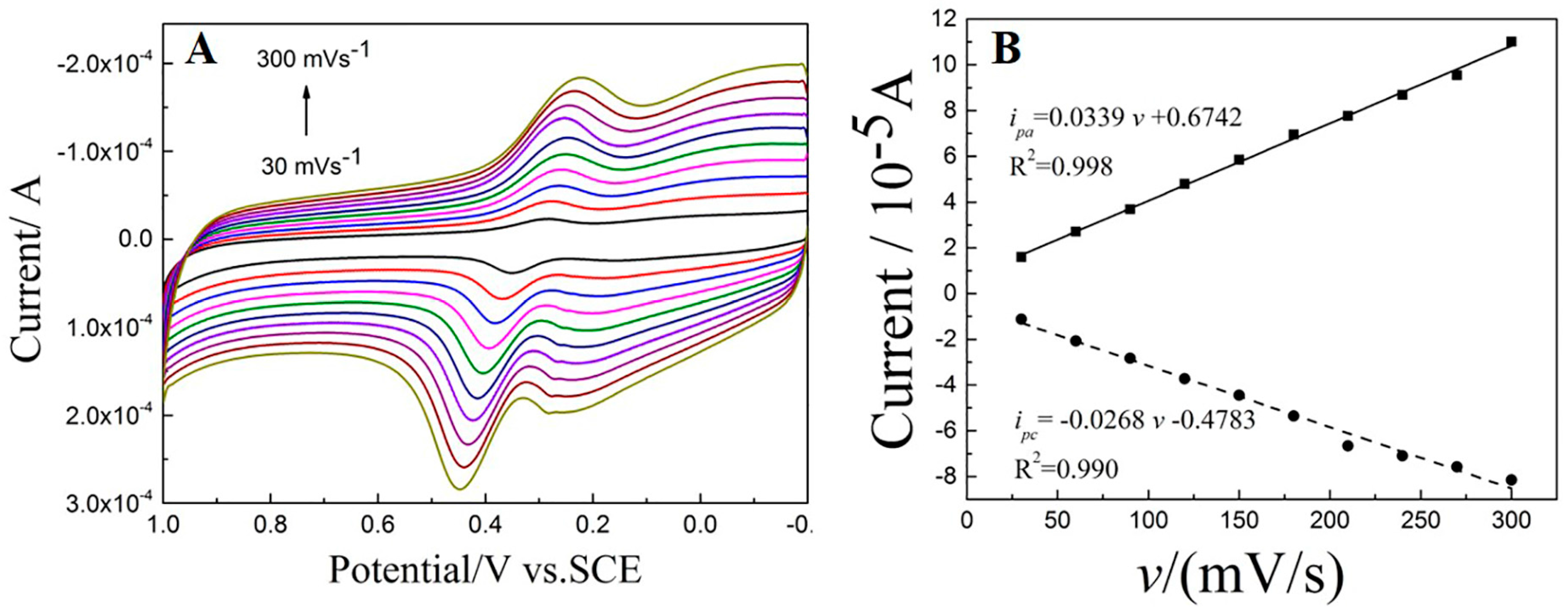
3.5.3. The Influence of Scan Rate
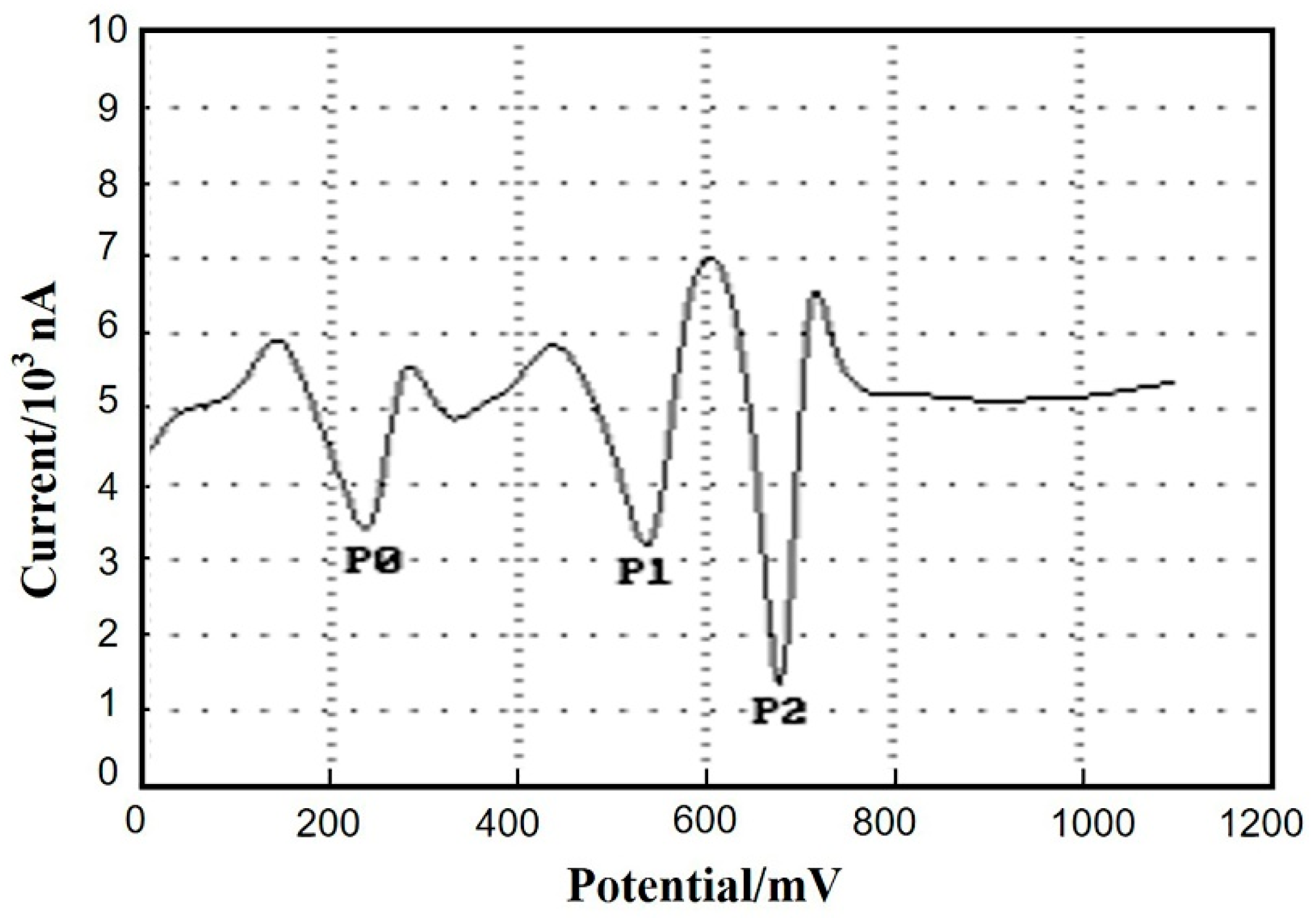
3.6. Interference Studies
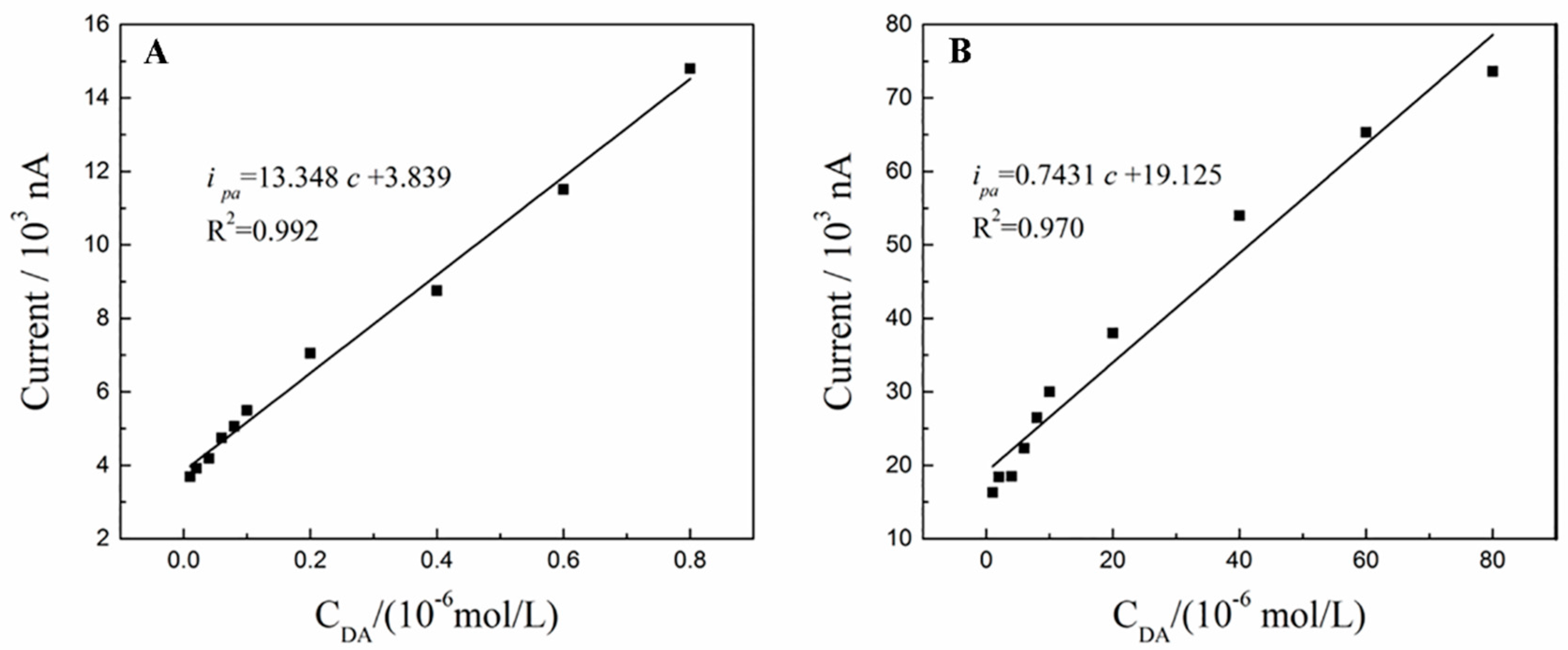
3.7. Calibration Curve and Detection Limit
3.8. Practical Applications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, A.; Honma, I.; Zhou, H. Amperometric biosensor based on tyrosinase-conjugated polysacchride hybrid film: Selective determination of nanomolar neurotransmitters metabolite of 3,4-dihydroxyphenylacetic acid (DOPAC) in biological fluid. Biosens. Bioelectron. 2005, 21, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Taei, M.; Khayamian, T.; Arabzadeh, A. Highly selective determination of ascorbic acid, dopamine, and uric acid by differential pulse voltammetry using poly (sulfonazo III) modified glassy carbon electrode. Sens. Actuators B: Chem. 2010, 147, 213–221. [Google Scholar] [CrossRef]
- Revin, S.B.; John, S.A. Highly sensitive determination of uric acid in the presence of major interferents using a conducting polymer film modified electrode. Bioelectrochemistry 2012, 88, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.R.; Dadfarnia, S.; Shabani, A.M.H.; Shahbazikhah, P. Chemometric-assisted kinetic–spectrophotometric method for simultaneous determination of ascorbic acid, uric acid, and dopamine. Anal. Biochem. 2011, 410, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, X. Simultaneous electroanalysis of dopamine, ascorbic acid and uric acid by poly (vinyl alcohol) covalently modified glassy carbon electrode. Sens. Actuators B: Chem. 2006, 115, 134–139. [Google Scholar] [CrossRef]
- Ulubay, Ş.; Dursun, Z. Cu nanoparticles incorporated polypyrrole modified GCE for sensitive simultaneous determination of dopamine and uric acid. Talanta 2010, 80, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Cheng, C.; Yuan, H.; Du, J.; Xiao, D.; Xie, S.; Choi, M.M.F. Simultaneous determination of l-ascorbic acid, dopamine and uric acid with gold nanoparticles–β-cyclodextrin–graphene-modified electrode by square wave voltammetry. Talanta 2012, 93, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Habibi, B.; Pournaghi-Azar, M.H. Simultaneous determination of ascorbic acid, dopamine and uric acid by use of a MWCNT modified carbon-ceramic electrode and differential pulse voltammetry. Electrochim. Acta 2010, 55, 5492–5498. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Yin, Y.; Yang, R.; Li, J.; Qu, L. Nano-sized copper oxide/multi-wall carbon nanotube/Nafion modified electrode for sensitive detection of dopamine. J. Electroanal. Chem. 2013, 703, 45–51. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Wang, A.; Cai, W.; Deng, B.; Zhang, Z. An Electrochemical Sensor Based on Reduced Graphene Oxide and ZnO Nanorods-Modified Glassy Carbon Electrode for Uric Acid Detection. Arabian J. Sci. Eng. 2016, 41, 135–141. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Zhang, Y.; Wang, P.; Wei, Q.; Du, B. Sensitive electrochemical sensor for simultaneous determination of dopamine, ascorbic acid, and uric acid enhanced by amino-group functionalized mesoporous Fe3O4@graphene sheets. Electrochim. Acta 2014, 116, 244–249. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal M, K.; Mansha, M.; Mansha, M.; Alsharaa, A.; Jillani S M, S.; Basheeret, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. TrAC Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Krystyna, J.; Pawel, K. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar]
- Hasanzadeh, M.; Shadjou, N.; Guardia, M.D.L. Current advancement in electrochemical analysis of neurotransmitters in biological fluids. TrAC Trends Anal. Chem. 2016, 86, 107–121. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, A.J. Introducing Cu2O thin films as a hole-transport layer in efficient planar perovskite solar cell structures. J. Phys. Chem. C. 2016, 120, 1428–1437. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Luo, L.; Chen, X.; Wang, Z.; Wu, E.; Su, D.; Huang, W. TiO2/Cu2O core/ultrathin shell nanorods as efficient and stable photocatalysts for water reduction. Angew. Chem. Int. Ed. 2015, 54, 15260–15265. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, J.; Chang, J.; Wu, D.; He, J.; Wang, K.; Xu, F.; Jiang, K. Mesocrystalline Cu2O hollow nanocubes: synthesis and application in non-enzymatic amperometric detection of hydrogen peroxide and glucose. CrystEngComm 2012, 14, 6639–6646. [Google Scholar] [CrossRef]
- Albo, J.; Irabien, A. Cu2O-loaded gas diffusion electrodes for the continuous electrochemical reduction of CO2 to methanol. J. Catal. 2015, 343, 232–239. [Google Scholar] [CrossRef]
- Albo, J.; Sáez, A.; Garikoitz, B.; Castañoa, P.; Irabienb, A. Methanol electrosynthesis from CO2 at Cu2O/ZnO prompted by pyridine-based aqueous solutions pyridine-based aqueous solutions. J. CO2 Util. 2017, 18, 164–172. [Google Scholar] [CrossRef]
- Schizodimou, A.; Kyriacou, G. Acceleration of the reduction of carbon dioxide in the presence of multivalent cations. Electrochim. Acta 2012, 78, 171–176. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors. Biosens. Bioelectron. 2017, 89, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Feng J Cai, L.; Fang, Q.; Chen, J.; Wang, A. Facile synthesis of monodisperse porous Cu2O nanospheres on reduced graphene oxide for non-enzymatic amperometric glucose sensing. Electrochim. Acta 2014, 115, 103–108. [Google Scholar] [CrossRef]
- Mei, L.; Song, P.; Feng, J.; Shen, J.; Wang, W.; Wang, A. Nonenzymatic amperometric sensing of glucose using a glassy carbon electrode modified with a nanocomposite consisting of reduced graphene oxide decorated with Cu2O. Microchim. Acta 2015, 182, 1701–1708. [Google Scholar] [CrossRef]
- Jiang, B.; Wei, X.; Wu, F.; Wu, K.; Chen, L.; Yuan, G.; Dong, C.; Ye, Y. A non-enzymatic hydrogen peroxide sensor based on a glassy carbon electrode modified with cuprous oxide and nitrogen-doped graphene in a nafion matrix. Microchim. Acta 2014, 181, 1463–1470. [Google Scholar] [CrossRef]
- Bahr, J.L.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: a bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Zeng, R.; Ding, C. Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem. 2015, 180, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Feng, Y. Acetylene black paste electrode modified with graphene as the voltammetric sensor for selective determination of tryptophan in the presence of high concentrations of tyrosine. Mater. Sci. Eng. C 2014, 35, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Li, J. Simultaneous voltammetric determination of 2-nitrophenol and 4-nitrophenol based on an acetylene black paste electrode modified with a graphene-chitosan composite. Microchim. Acta 2014, 181, 1077–1084. [Google Scholar] [CrossRef]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemically reduced graphene oxide modified acetylene black paste electrode for the sensitive determination of bisphenol A. J. Electroanal. Chem. 2013, 707, 7–14. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Xu, F.; Deng, M.; Li, G.; Chen, S.; Wang, L. Electrochemical behavior of cuprous oxide–reduced graphene oxide nanocomposites and their application in nonenzymatic hydrogen peroxide sensing. Electrochim. Acta 2013, 88, 59–65. [Google Scholar] [CrossRef]
- Yogeswaran, U.; Chen, S.-M. Multi-walled carbon nanotubes with poly (methylene blue) composite film for the enhancement and separation of electroanalytical responses of catecholamine and ascorbic acid. Sens. Actuators B Chem. 2008, 130, 739–749. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Lai, C.; Zhang, Y.; Deng, J.; Li, H.; Yao, S. A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a nanocomposite of ferrocene thiolate stabilized Fe3O4@Au nanoparticles with graphene sheet. Biosens. Bioelectron. 2013, 48, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Afkhami, A.; Hashemi, P.; Ghanei, M. Simultaneous and sensitive determination of melatonin and dopamine with Fe3O4 nanoparticle-decorated reduced graphene oxide modified electrode. RSC Adv. 2015, 5, 21659–21669. [Google Scholar] [CrossRef]
- Yao, Z.; Yang, X.; Niu, Y.; Wu, F.; Hu, Y.; Yang, Y. Voltammetric dopamine sensor based on a gold electrode modified with reduced graphene oxide and Mn3O4 on gold nanoparticles. Microchim. Acta 2017, 184, 2081–2088. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, J.; Zhang, F. Elimination of ascorbic acid and sensitive detection of uric acid at the MnO2 nanorods/graphene-based modified electrode. Int. J. Electrochem. Sci. 2013, 2031, 9967–9976. [Google Scholar]
- Liu, S.Q.; Ouyang, G.W.; Dai, G.P.; Luo, T.X.; Liang, Y. Preparation of Nickel Oxide-Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode and Its Application for the Determination of Dopamine. J. Anal. Sci. 2014, 30, 853–857. [Google Scholar]
- He, F.Y.; Liu, M.S.; Zhu, Z.F.; Yang, F.Z.; Sun, G.Y. Electrochemical Behavior and Determination of Dopamine at ZnO Nanorods Intercalated Graphite Modified Electrode. Chin. J. Appl. Chem. 2011, 28, 320–325. [Google Scholar]

| Modified Electrodes | Linear Range (M) | Detection Limit (M) |
|---|---|---|
| Cu2O-RGO/GCE | 1 × 10−8~1 × 10−6; 1 × 10−6~8 × 10−5 | 6.0 × 10−9 |
| Fe3O4@Au-Gr/GCE [35] | 5 × 10−7~5 × 10−5 | 6.5 × 10−7 |
| Fe3O4-RGO/CPE [36] | 2 × 10−8~5.8 × 10−6 | 6.5 × 10−9 |
| Mn3O4-RGO/GCE [37] | 1 × 10−6~1.45 × 10−3 | 2.5 × 10−7 |
| MnO2 NR-RGO/GCE [38] | 5 × 10−8~4 × 10−4 | 1.0 × 10−8 |
| NiO-RGO/GCE [39] | 5 × 10−7~3.2 × 10−5 | 3.8 × 10−8 |
| ZnO NR-RGO/Graphite [40] | 5 × 10−7~1 × 10−4 | 2.5 × 10−7 |
| No. | Standard Value (μM) | Determination Value (μM) | Added (μM) | Total Found | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 13.14 | 12.72 | 10.00 | 23.16 | 104.4 | −3.20 |
| 2 | 27.63 | 27.94 | 30.00 | 56.91 | 96.5 | 1.12 |
| 3 | 48.62 | 47.38 | 50.00 | 96.77 | 98.8 | −2.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection. Sensors 2018, 18, 199. https://doi.org/10.3390/s18010199
He Q, Liu J, Liu X, Li G, Deng P, Liang J. Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection. Sensors. 2018; 18(1):199. https://doi.org/10.3390/s18010199
Chicago/Turabian StyleHe, Quanguo, Jun Liu, Xiaopeng Liu, Guangli Li, Peihong Deng, and Jing Liang. 2018. "Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection" Sensors 18, no. 1: 199. https://doi.org/10.3390/s18010199
APA StyleHe, Q., Liu, J., Liu, X., Li, G., Deng, P., & Liang, J. (2018). Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection. Sensors, 18(1), 199. https://doi.org/10.3390/s18010199






