Response of a Zn2TiO4 Gas Sensor to Propanol at Room Temperature
Abstract
:1. Introduction
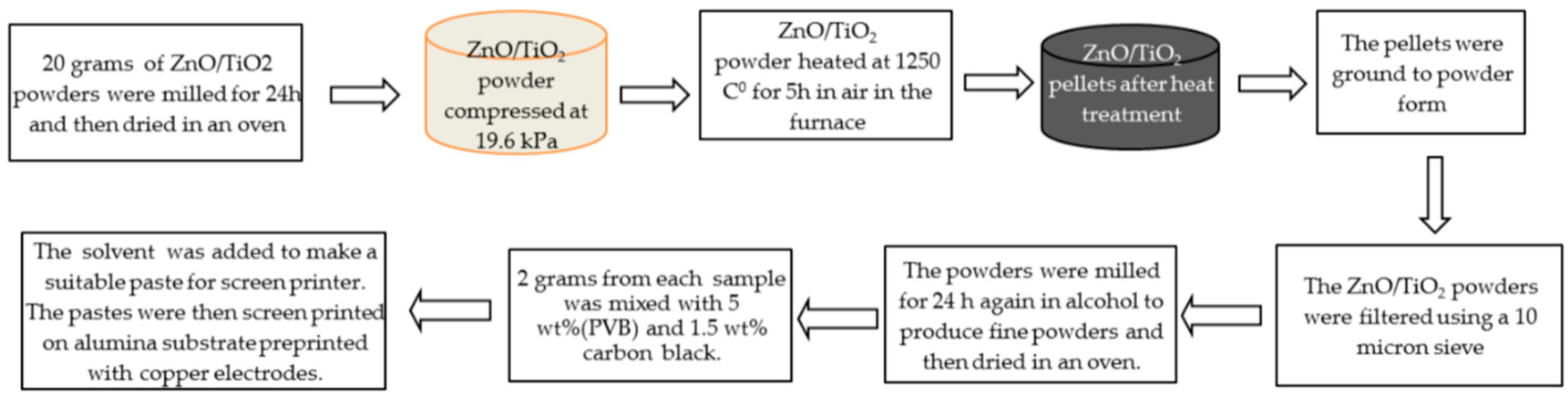
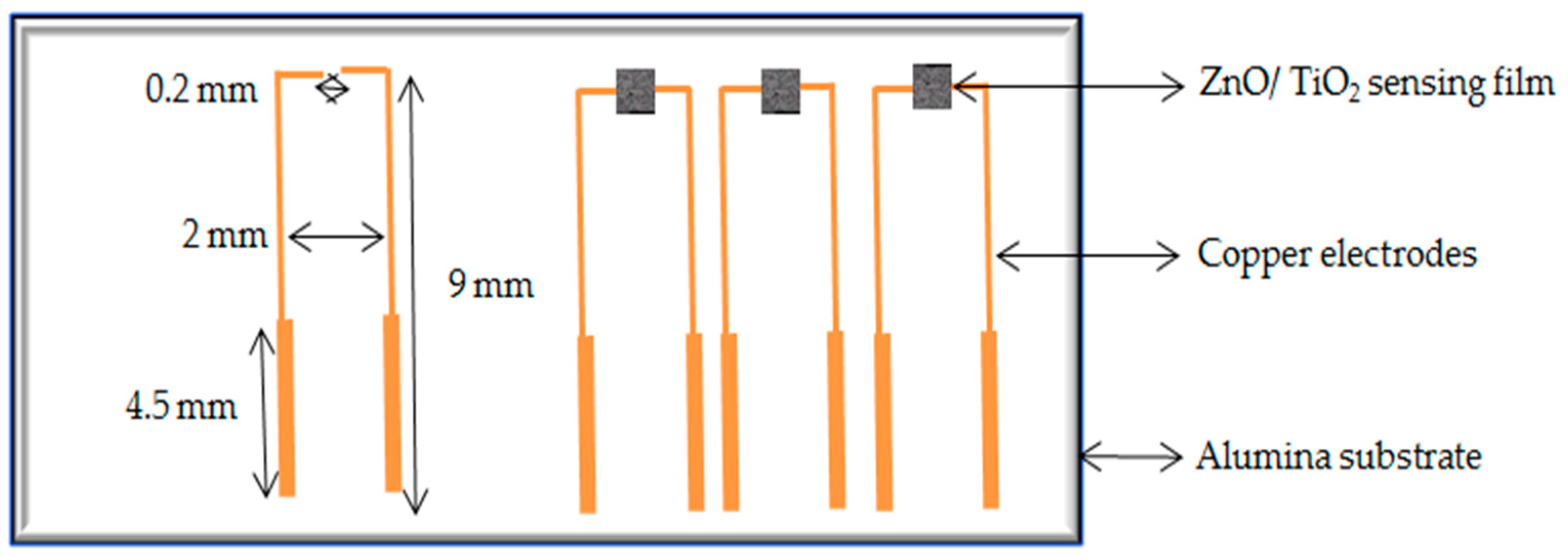
2. Experimental Work
3. Results and Discussion
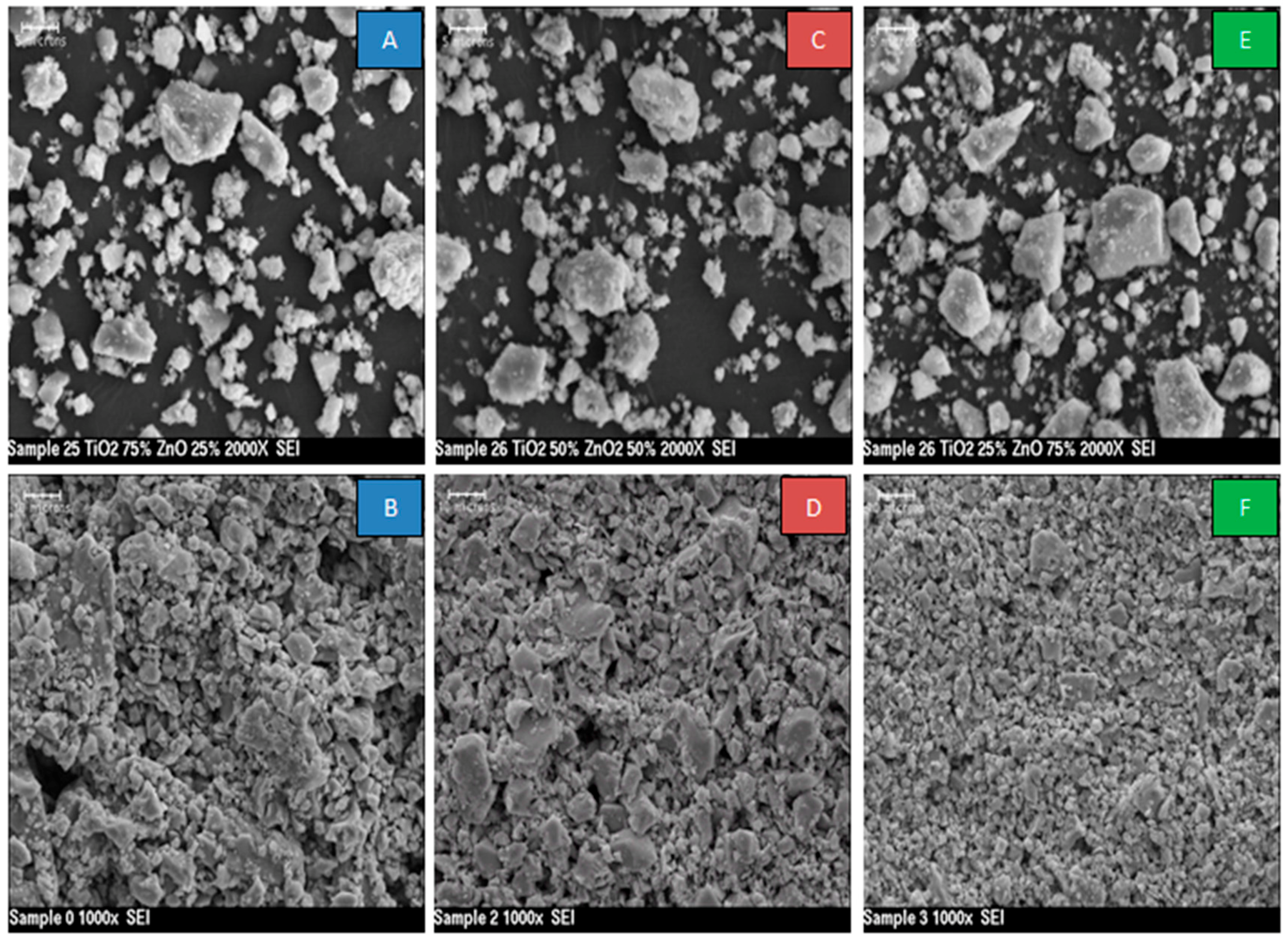
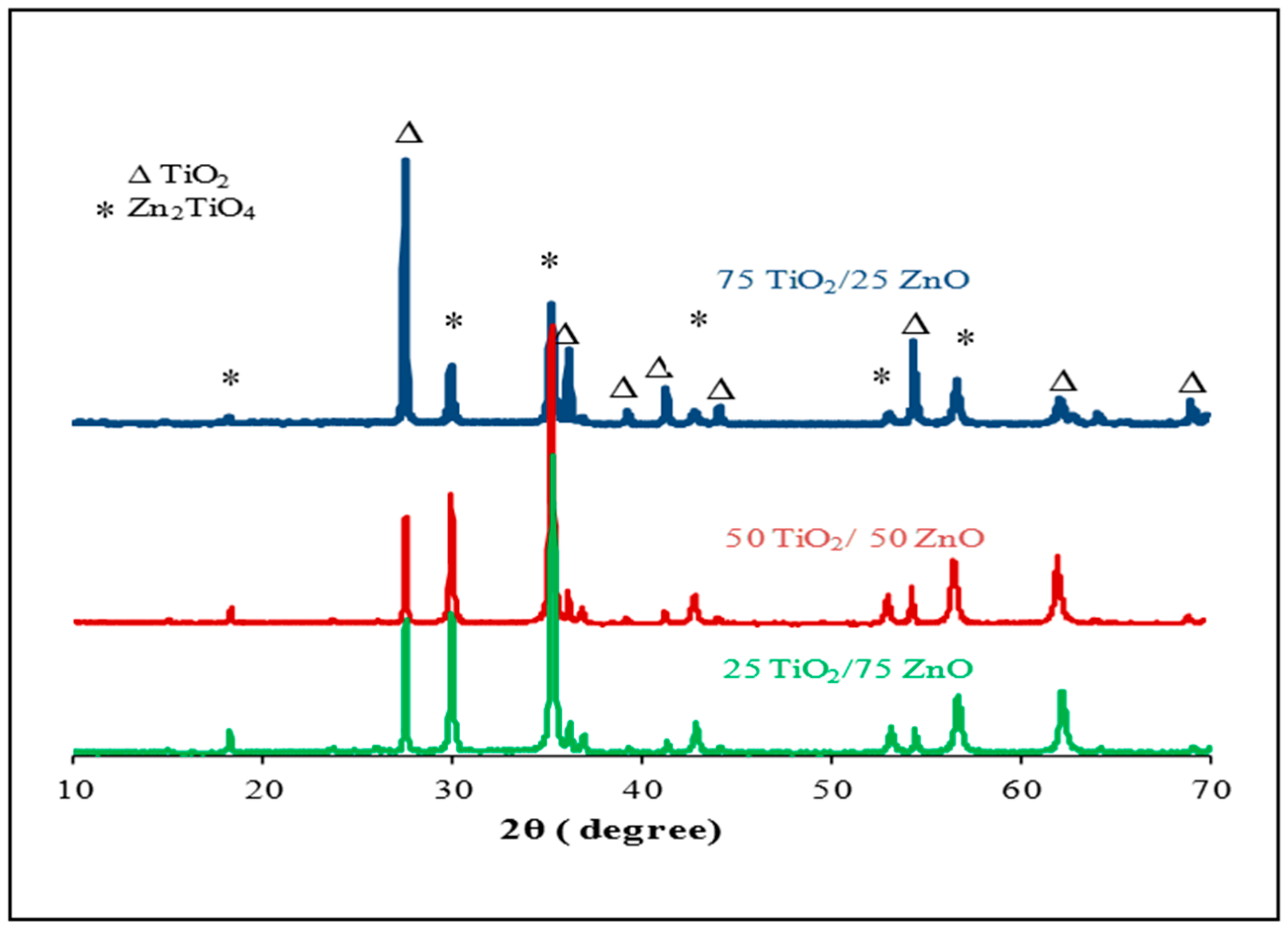
3.1. SEM and XRD Results
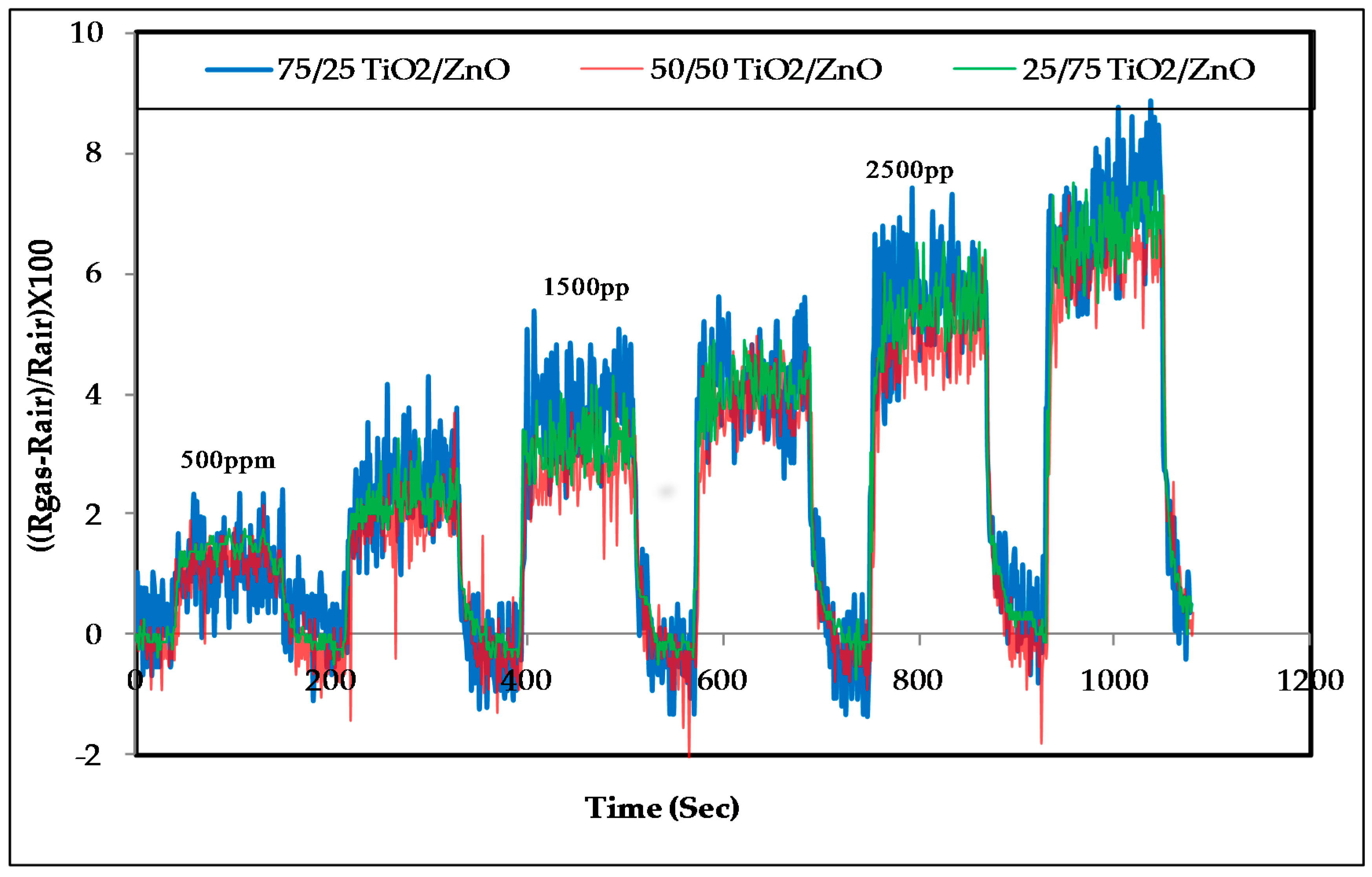
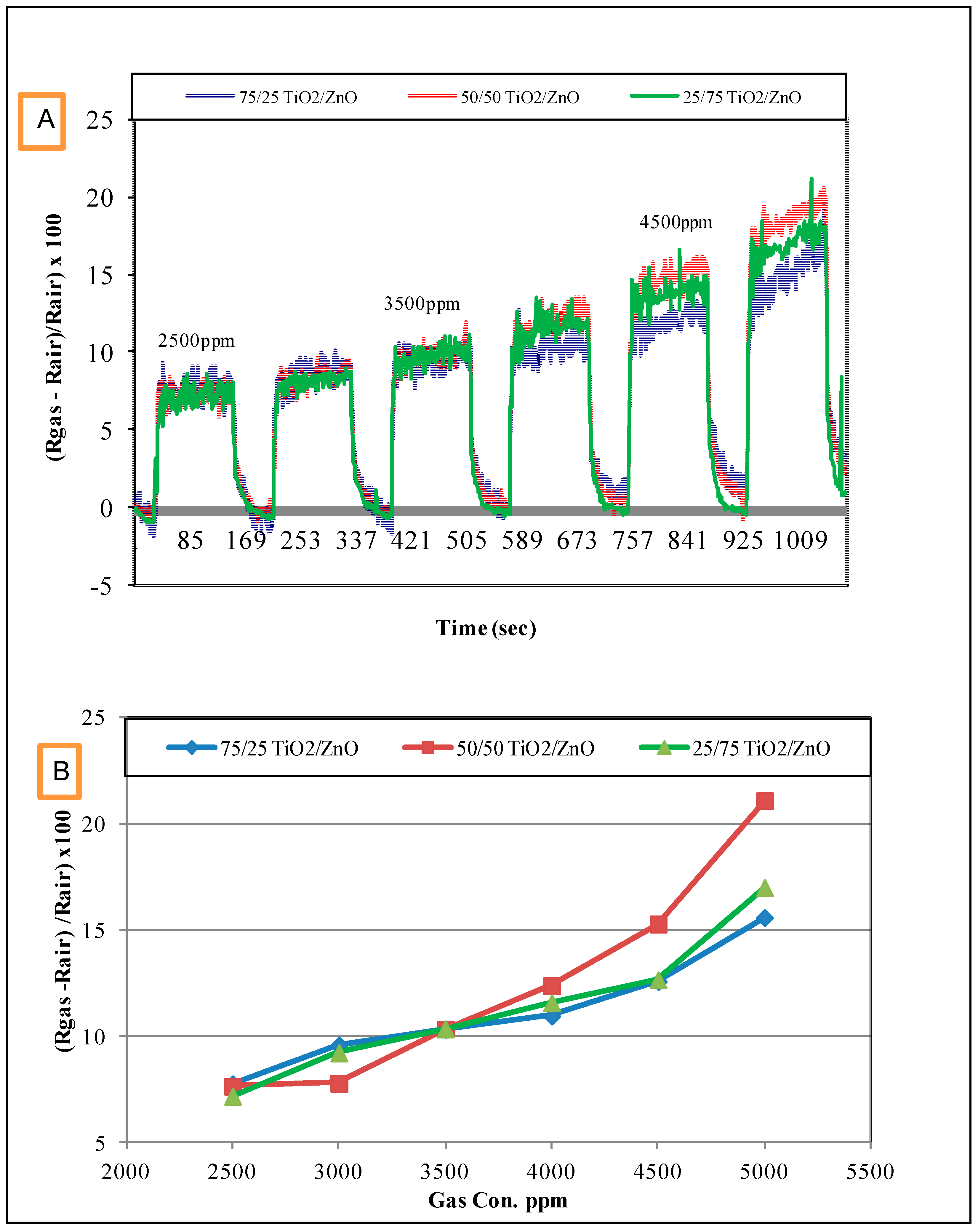
3.2. Response of the Sensors to Propanol
- The response of the sensors increased as the gas concentration increased;
- However, no significant difference was observed in the response of the three sensors at low concentrations; sensor 2 (50/50 TiO2/ZnO) had the highest sensitivity for propanol, followed by sensor 3 and then sensor 1 at higher concentrations (above 4000 ppm);
- This difference in sensor response could be associated with the final composition of the sensing materials. The XRD results proved that the peaks of Zn2TiO4 were higher for Samples 2 and 3 (the highest density) than for Sample 1. The TiO2 was at the highest density in Sample 1. In addition to this, the surface morphology of the three samples was also different in terms of the number, size and shape of the pores on the surface of the films (see Figure 3);
- The signal noise was high in the lower concentration range (500–2500 ppm) compared with the response of the same sensors for the higher concentration range (2500–5000 ppm). This high noise could be attributed to the effect of a slight change in the working temperature or the effect of some other gases that were already in the air of the gas testing chamber.
4. Conclusions
- They had good sensitivity to propanol vapor at room temperature;
- The response of the sensors monotonically increased as the gas concentration increased;
- XRD measurements of the screen-printed sensing materials illustrated that the final compositions (Zn2TiO4 to TiO2) depended on the ratio of TiO2 to ZnO used in preparing the mixtures before being heated;
- SEM images of the films showed granular microstructure ranging from 2–5 microns;
- Using copper as an electrode material makes sensors cost effective compared to sensors with Pt and Au electrodes;
- The simple structure of the sensor with copper electrodes makes it easy to interface with electrical systems;
- The developed sensors can be used in various applications at room temperature, including as gas safety monitors;
- Further detailed studies are required to better understand the interaction mechanism between the sensing material and the tested gas.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takada, T. Temperature drop of semiconductor gas sensor when exposed to reducing gases—Simultaneous measurement of changes in sensor temperature and in resistance. Sens. Actuators B Chem. 2000, 66, 1–3. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Comini, E.; Faglia, G.; Atashbar, M.Z.; Wlodarski, W. Titanium dioxide thin films prepared for alcohol microsensor application. Sens. Actuators B Chem. 2000, 66, 139–141. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wu, X. Preparation and gas-sensing properties of perovskite-type SrFeO3 oxide. Mater. Lett. 2001, 49, 361–364. [Google Scholar] [CrossRef]
- Chu, X.; Liu, X.; Meng, G. Effects of CdO dopant on the gas sensitivity properties of ZnFe2O4 semiconductors. Sens. Actuators B Chem. 2000, 65, 1–3. [Google Scholar]
- Santhaveesuk, T.; Gardchareon, A.; Wongratanaphisan, D.; Choopun, S. Ethanol sensing properties of Zn2TiO4 particles. Ceram. Int. 2015, 41, S809–S813. [Google Scholar] [CrossRef]
- Mabrook, M.; Hawkins, P. A rapidly-responding sensor for benzene, methanol and ethanol vapours based on films of titanium dioxide dispersed in a polymer operating at room temperature. Sens. Actuators B Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Carotta, M.C.; Ferroni, M.; Gnani, D.; Guidi, V.; Merli, M.; Martinelli, G.; Casale, M.C.; Notaro, M. Nanostructured pure and Nb-doped TiO2 as thick film gas sensors for environmental monitoring. Sens. Actuators B Chem. 1999, 58, 310–317. [Google Scholar] [CrossRef]
- Yamada, Y.; Seno, Y.; Masuoka, Y.; Nakamura, T.; Yamashita, K. NO2 sensing characteristics of Nb doped TiO2 thin films and their electronic properties. Sens. Actuators B Chem. 2000, 66, 164–166. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, P.; Xu, T.; Zheng, D.; Li, X. ZnO-nanowire size effect induced ultra-high sensing response to ppb-level H2S. Sens. Actuators B Chem. 2017, 240, 264–272. [Google Scholar] [CrossRef]
- Xie, T.S.; Steffens, N.; Wen, K.; Liu, B.; Debnath, G.; Davydov, R.; Gomez, A.; Motayed, A. UV-assisted room-temperature chemiresistive NO2 sensor based on TiO2 thin film. J. Alloys Compd. 2015, 653, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Catto, A.C.; da Silva, L.F.; Ribeiro, C.; Bernardini, S.; Aguir, K.; Longo, E.; Mastelaro, V.R. An easy method of preparing ozone gas sensors based on ZnO nanorods. RSC Adv. 2015, 5, 19528–19533. [Google Scholar] [CrossRef]
- Santhaveesuk, T.; Wongratanaphisan, D.; Choopun, S. Enhancement of Ethanol Sensing Properties by Alloying. IEEE Sens. J. 2010, 10, 39–43. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Xie, C.; Wu, J.; Zeng, D.; Liao, Y. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceram. Int. 2012, 38, 503–509. [Google Scholar] [CrossRef]
- Mun, Y.; Park, S.; An, S.; Lee, C.; Kim, H.W. NO2 gas sensing properties of Au-functionalized porous ZnO nanosheets enhanced by UV irradiation. Ceram. Int. 2013, 39, 8615–8622. [Google Scholar] [CrossRef]
- Perillo, P.M.; Rodrígue, D.F. A room temperature chloroform sensor using TiO2 nanotubes. Sens. Actuators B Chem. 2014, 193, 263–266. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Guo, B.; Chen, J. MCo2O4 (M = Ni, Cu, Zn) nanotubes: Template synthesis and application in gas sensors. Sens. Actuators B Chem. 2006, 114, 402–409. [Google Scholar] [CrossRef]
- Sun, F.; Li, X.; Liu, L.; Wang, J. Novel Zn–M–O (M = Sn, Co) sensing electrodes for selective mixed potential CO/C3H8 sensors. Sens. Actuators B Chem. 2013, 184, 220–227. [Google Scholar] [CrossRef]
- Gawande, K.B.; Gawande, S.B.; Thakare, S.R.; Mate, V.R.; Kadam, S.R.; Kale, B.B.; Kulkarni, M.V. Effect of zinc: Cobalt composition in ZnCo2O4 spinels for highly selective liquefied petroleum gas sensing at low and high temperatures. RSC Adv. 2015, 5, 40429–40436. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Jadhav, H.; M’Peko, J.C.; Torres, B.B.M.; Aguir, K.; Mastelaroa, V.R.; Oliveira, O.N., Jr. One-step approach for preparing ozone gas sensors based on hierarchical NiCo2O4 structures. RSC Adv. 2016, 6, 92655–92662. [Google Scholar] [CrossRef]
- Schaller, E.; Bosset, J.O.; Escher, F. ‘Electronic noses’ and their application to food. LWT-Food Sci. Technol. 1998, 31, 305–316. [Google Scholar] [CrossRef]
- Arshak, K.; Gaidan, I.; Moore, E.G.; Cunniffe, C. The effect of the addition of carbon black and the increase in film thickness on the sensing layers of ZnO/ZnFe2O4 in polymer thick film gas sensors. Superlattices Microstruct. 2007, 42, 348–356. [Google Scholar] [CrossRef]
- Arshak, K.; Gaidan, I. NiO/Fe2O3 polymer thick films as room temperature gas sensors. Thin Solid Films 2006, 495, 286–291. [Google Scholar] [CrossRef]
- Arshak, K.; Gaidan, I. Effects of NiO/TiO2 addition in ZnFe2O4-based gas sensors in the form of polymer thick films. Thin Solid Films 2006, 495, 292–298. [Google Scholar] [CrossRef]
- Da Silva, L.F.; M’Peko, J.C.; Catto, A.C.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Ribeiro, C.; Longo, E. UV-enhanced ozone gas sensing response of ZnO-SnO2 heterojunctions at room temperature. Sens. Actuators B Chem. 2017, 240, 573–579. [Google Scholar] [CrossRef]
- Hongsith, N.; Choopun, S. Enhancement of ethanol sensing properties by impregnating platinum on surface of zno tetrapods. IEEE Sensors J. 2010, 10, 34–38. [Google Scholar] [CrossRef]
- Roessler, A.L.; Pratsinis, A.; Sahm, S.E.; Gurlo, T.; Barsan, A.; Weimar, N. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sens. Actuators B Chem. 2006, 114, 283–295. [Google Scholar]
- Xu, H.; Liu, X.; Cui, D.; Li, M.; Jiang, M. A novel method for improving the performance of ZnO gas sensors. Sens. Actuators B Chem. 2006, 114, 301–307. [Google Scholar] [CrossRef]
- Kersen, U.; Holappa, L. H2S-sensing properties of SnO2 produced by ball milling and different chemical reactions. Anal. Chim. Acta 2006, 562, 110–114. [Google Scholar] [CrossRef]
- Viricelle, J.; Pauly, J.P.; Mazet, A.; Brunet, L.; Bouvet, J.; Varenne, M.; Pijolat, C. Selectivity improvement of semi-conducting gas sensors by selective filter for atmospheric pollutants detection. Mater. Sci. Eng. 2006, 26, 186–195. [Google Scholar] [CrossRef]
- Kamionka, M.; Breuil, P.; Pijolat, C. Atmospheric pollution measurement with a multi-materials sensing device. Mater. Sci. Eng. 2006, 26, 290–296. [Google Scholar] [CrossRef]
- Jain, K.; Pant, R.P.; Lakshmikumar, S.T. Effect of Ni doping on thick film SnO2 gas sensor. Sens. Actuators B Chem. 2006, 113, 823–829. [Google Scholar] [CrossRef]
- Lampe, U.; Simon, E.; Pohle, R.; Fleischer, M.; Meixner, H.; Frerichs, H.P.; Kiss, G. GasFET for the detection of reducing gases. Sens. Actuators B Chem. 2005, 111, 106–110. [Google Scholar] [CrossRef]
- Pijolat, C.; Viricelle, J.P.; Tournier, G.; Montmeat, P. Application of membranes and filtering films for gas sensors improvements. Thin Solid Films 2005, 490, 7–16. [Google Scholar] [CrossRef]
- Saukko, S.; Lassi, U.; Lantto, V.; Kroneld, M.; Novikov, S.; Kuivalainen, P.; Mizsei, J. Experimental studies of O2-SnO2 surface interaction using powder, thick films and monocrystalline thin films. Thin Solid Films 2005, 490, 48–53. [Google Scholar] [CrossRef]
- Kanda, K.; Maekawa, T. Development of a WO3 thick-film-based sensor for the detection of VOC. Sens. Actuators B Chem. 2005, 108, 97–101. [Google Scholar] [CrossRef]
- Ruiz, A.M.; Sakai, G.; Cornet, A.; Shimanoe, K.; Morante, J.R.; Yamazoe, N. Microstructure control of thermally stable TiO2 obtained by hydrothermal process for gas sensors. Sens. Actuators B Chem. 2004, 103, 312–317. [Google Scholar] [CrossRef]
- Kotsikau, D.; Ivanovskaya, M.; Orlik, D.; Falasconi, M. Gas-sensitive properties of thin and thick film sensors based on Fe2O3-SnO2 nanocomposites. Sens. Actuators B Chem. 2004, 101, 199–206. [Google Scholar] [CrossRef]
- Ivanov, P.; Llobet, E.; Vilanova, X.; Brezmes, J.; Hubalek, J.; Correig, X. Development of high sensitivity ethanol gas sensors based on Pt-doped SnO2 surfaces. Sens. Actuators B Chem. 2004, 99, 201–206. [Google Scholar] [CrossRef]
- Su, P.-G.; Chen, I.C. Laminating two-layer thick films structure tin oxide-based butane gas sensor operating at low temperature. Sens. Actuators B Chem. 2004, 99, 304–309. [Google Scholar] [CrossRef]
- Wagh, M.S.; Patil, L.A.; Seth, T.; Amalnerkar, D.P. Surface cupricated SnO2-ZnO thick films as a H2S gas sensor. Mater. Chem. Phys. 2004, 84, 228–233. [Google Scholar] [CrossRef]
- Choi, U.-S.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Sensing properties of SnO2-Co3O4 composites to CO and H2. Sens. Actuators B Chem. 2004, 98, 166–173. [Google Scholar] [CrossRef]
- Chu, X.; Cheng, Z. High sensitivity chlorine gas sensors using CdSnO3 thick film prepared by co-precipitation method. Sens. Actuators B Chem. 2004, 98, 215–217. [Google Scholar]
- Chu, X. Dilute CH3SH-sensing characteristics of BaSnO3 thick film sensor. Mater. Sci. Eng. B 2004, 106, 305–307. [Google Scholar] [CrossRef]
- Zhu, B.L.; Xie, C.S.; Wang, W.Y.; Huang, K.J.; Hu, J.H. Improvement in gas sensitivity of ZnO thick film to volatile organic compounds (VOCs) by adding TiO2. Mater. Lett. 2004, 58, 624–629. [Google Scholar] [CrossRef]
- Chu, X.; Zheng, C. Sulfide-sensing characteristics of MFe2O4 (M = Zn, Cd, Mg and Cu) thick film prepared by co-precipitation method. Sens. Actuators B Chem. 2003, 96, 504–508. [Google Scholar]
- Noh, W.; Shin, Y.; Kim, J.; Lee, W.; Hong, K.; Akbar, S.A.; Park, J. Effects of NiO addition in WO3-based gas sensors prepared by thick film process. Solid State Ionics 2002, 152–153, 827–832. [Google Scholar] [CrossRef]
- Solis, J.L.; Saukko, S.; Kish, L.; Granqvist, C.G.; Lantto, V. Semiconductor gas sensors based on nanostructured tungsten oxide. Thin Solid Films 2001, 391, 255–260. [Google Scholar] [CrossRef]
- Arshak, K.; Cunniffe, C.; Moore, E.; Cavanagh, L.; Harris, J. A novel approach to electronic nose-head design, using a copper thin film electrode patterning technique. In Proceedings of the 28th International Spring Seminar on Electronics Technology, Wiener Neustadt, Austria, 19–22 May 2005; pp. 185–190. [Google Scholar]
- Girish, K.M.; Naik, R.; Prashantha, S.C.; Nagabhushana, H.; Nagaswarupa, H.P.; Raju, K.A.; Nagabhushana, B.M. Zn2TiO4: Eu3+ anophosphor: Self explosive route and its near UV excited photoluminescence properties for WLEDs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, C.; Tamaekong, N.; Phanichphant, S. Characterization of single phase Pt-doped Zn2TiO4 nanoparticles synthesized by flame spray pyrolysis. Mater. Lett. 2012, 68, 97–100. [Google Scholar] [CrossRef]
- Siriwong, C.; Phanichphant, S. Flame-made single phase Zn2TiO4 nanoparticles. Mater. Lett. 2011, 65, 2007–2009. [Google Scholar] [CrossRef]
- Arshak, K.; Gaidan, I. Development of an array of polymer/MnO2/Fe2O3 mixtures for use in gas sensing applications. Sens. Actuators B Chem. 2006, 118, 386–392. [Google Scholar] [CrossRef]
| Sensing Materials | Sensitive Gases | Year/Reference |
|---|---|---|
| ZnO | H2S | 2017 [9] |
| ZnO–SnO2 | O3 | 2016 [24] |
| TiO2 | NO2 | 2015 [10] |
| Zn2TiO4 | C2H5OH | 2015 [5] |
| ZnO | NO2 | 2013 [14] |
| Zn/Zn2SnO4 + SnO4 | CO/C3H8 | 2013[17] |
| TiO2 | Chloroform | 2014 [15] |
| TiO2 and ZnO | C2H5OH | 2012 [13] |
| TiO2-ZnO | NO2 | 2010 [12] |
| ZnO | C2H5OH | 2010 [25] |
| SnO2 and Pt/SnO2 | CO | 2006 [26] |
| ZnO | C2H5OH and Acetone | 2006 [27] |
| SnO2 | H2S and CO | 2006 [28] |
| SnO2 | O3, COand NO2 | 2006 [29] |
| SnO2 | O3 and NO2 | 2006 [30] |
| SnO2/Al/Ni | LPG gas | 2006 [31] |
| SnO2 or Ga2O3 | CO and CO2 | 2005 [32] |
| SnO2 | H2, C2H5OH CH4 | 2005 [33] |
| SnO2 | CO and CO2 | 2005 [34] |
| WO3 | Hydrocarbon gases | 2005 [35] |
| TiO2 | CO | 2004 [36] |
| Fe2O3–SnO2 | NO2 and C2H5OH | 2004 [37] |
| Pt-SnO2 | C2H5OH | 2004 [38] |
| SnO2 | C4H10 | 2004 [39] |
| SnO2–ZnO and SnO2–ZnO–CuO | H2S LPG, NOx, CO2, CO and CH4 | 2004 [40] |
| SnO2–Co3O4 | CO and H2 | 2004 [41] |
| CdSnO3 | Cl2 | 2004 [42] |
| BaSnO3 | H2S, CH3SH | 2004 [43] |
| ZnO–TiO2 | Alcohol, Acetone, Benzene, Toluene and xylene | 2004 [44] |
| MFe2O4 | H2S, CH3SH | 2003 [45] |
| NiO-doped WO3 | NO2 | 2003 [46] |
| WO3 | H2S | 2001 [47] |
| Sample | TiO2 (Mwt %) | ZnO (Mwt %) |
|---|---|---|
| Sensor 1 | 75 | 25 |
| Sensor 2 | 50 | 50 |
| Sensor 3 | 25 | 75 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaidan, I.; Brabazon, D.; Ahad, I.U. Response of a Zn2TiO4 Gas Sensor to Propanol at Room Temperature. Sensors 2017, 17, 1995. https://doi.org/10.3390/s17091995
Gaidan I, Brabazon D, Ahad IU. Response of a Zn2TiO4 Gas Sensor to Propanol at Room Temperature. Sensors. 2017; 17(9):1995. https://doi.org/10.3390/s17091995
Chicago/Turabian StyleGaidan, Ibrahim, Dermot Brabazon, and Inam Ul Ahad. 2017. "Response of a Zn2TiO4 Gas Sensor to Propanol at Room Temperature" Sensors 17, no. 9: 1995. https://doi.org/10.3390/s17091995
APA StyleGaidan, I., Brabazon, D., & Ahad, I. U. (2017). Response of a Zn2TiO4 Gas Sensor to Propanol at Room Temperature. Sensors, 17(9), 1995. https://doi.org/10.3390/s17091995






