A DFT Calculation of Fluoride-Doped TiO2 Nanotubes for Detecting SF6 Decomposition Components
Abstract
:1. Introduction
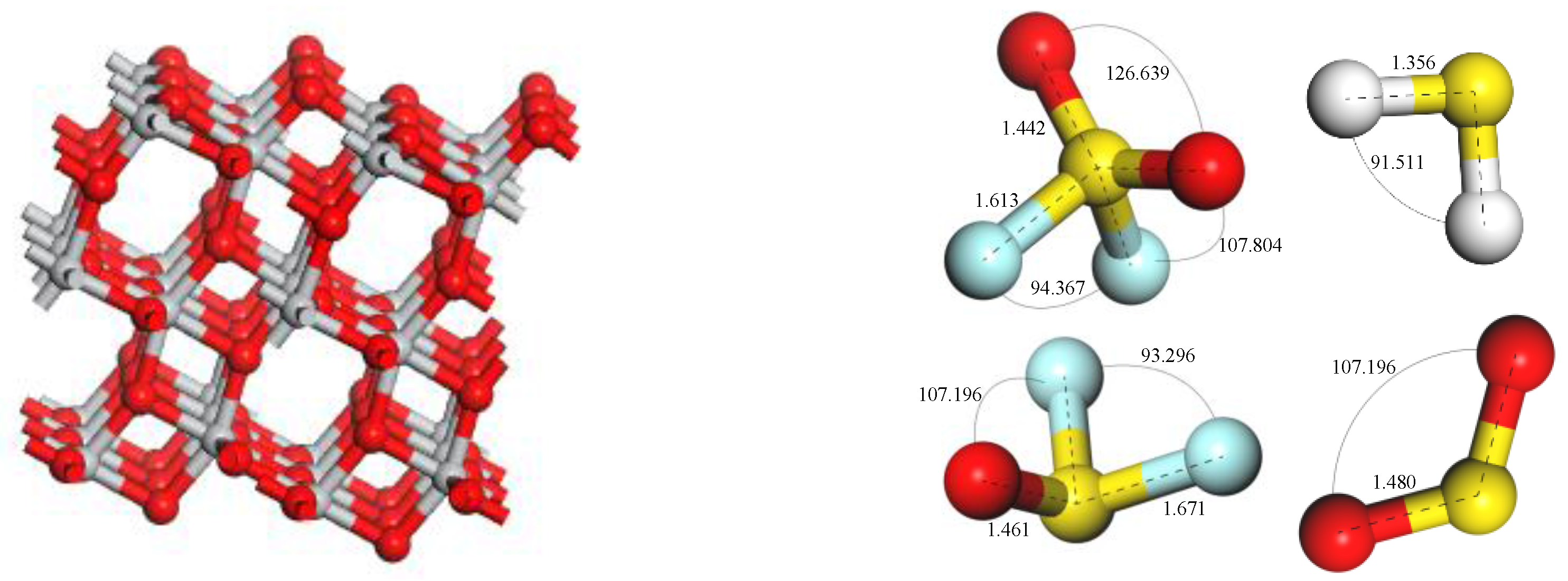
2. Calculation Parameters and Methods
3. Simulation Results and Analysis
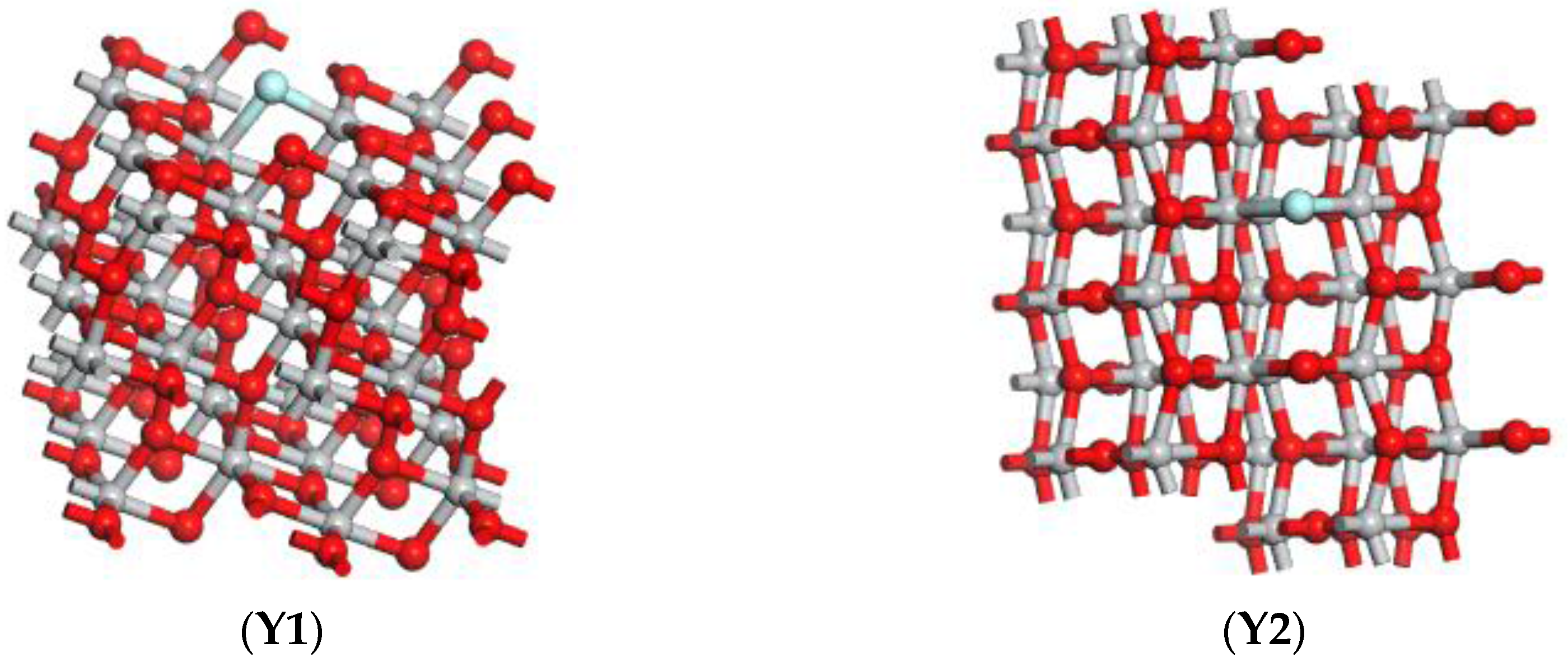
3.1. Establishment of F-Doped TiO2 Model
3.2. Parameter Calculation of Different Adsorption Systems
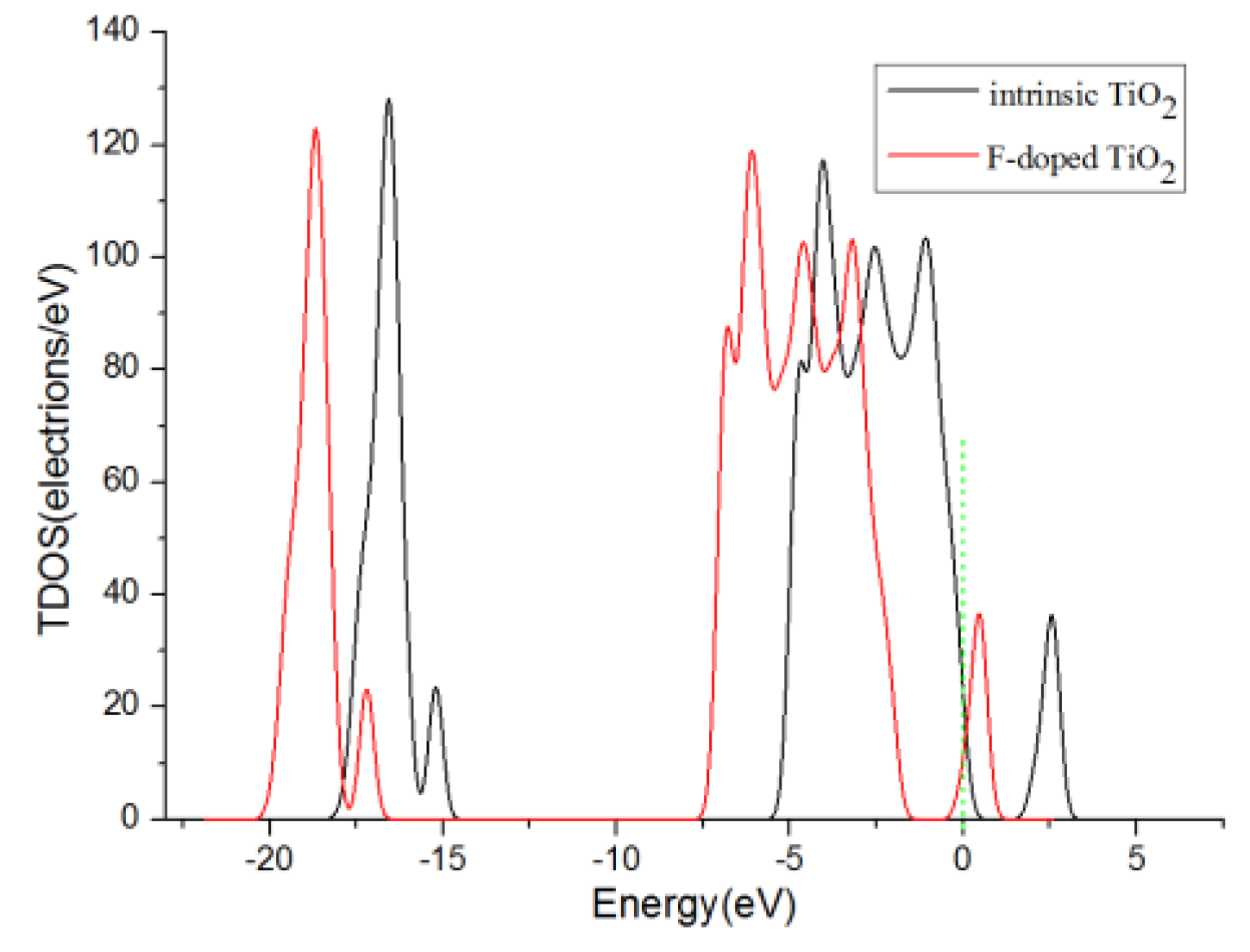
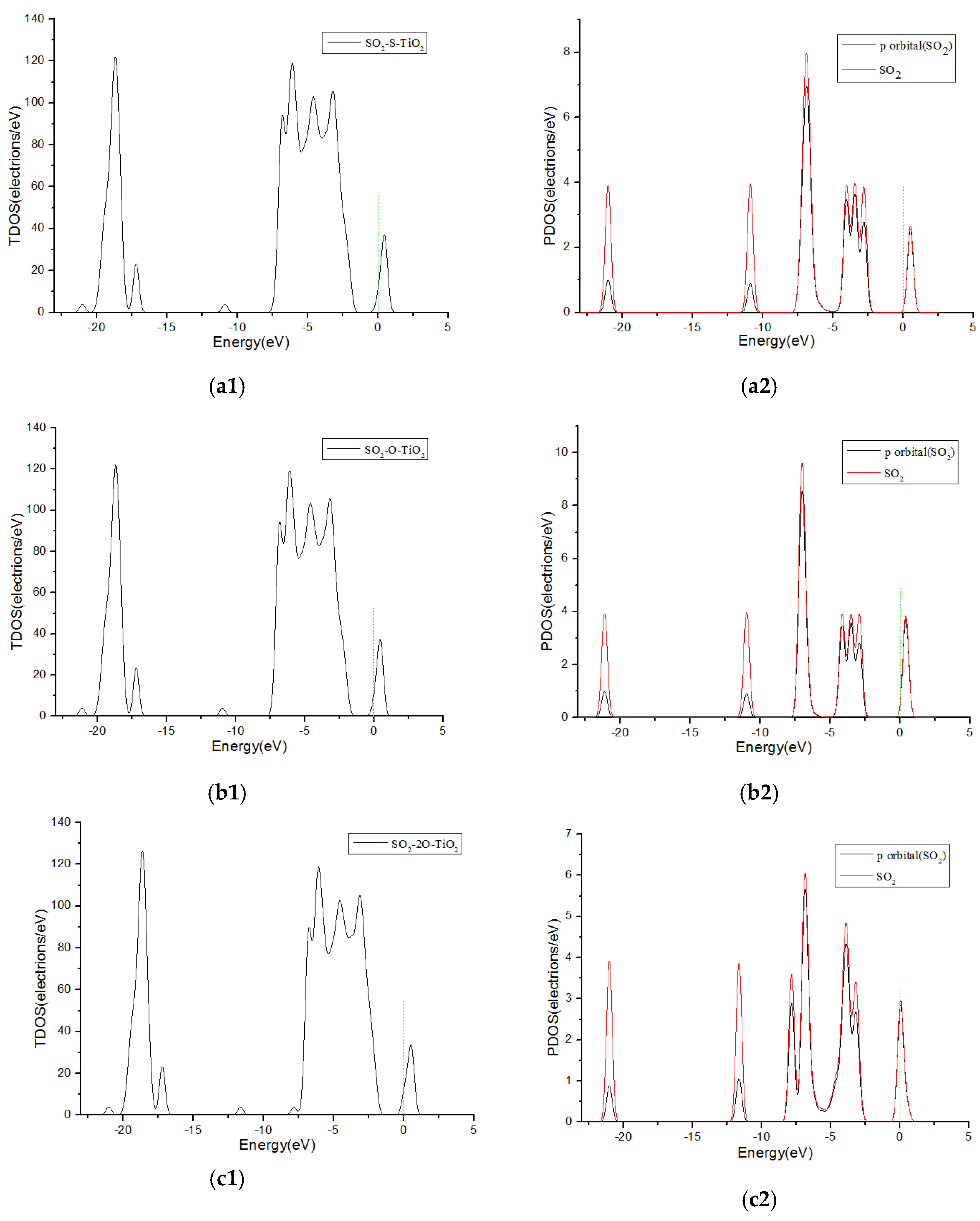
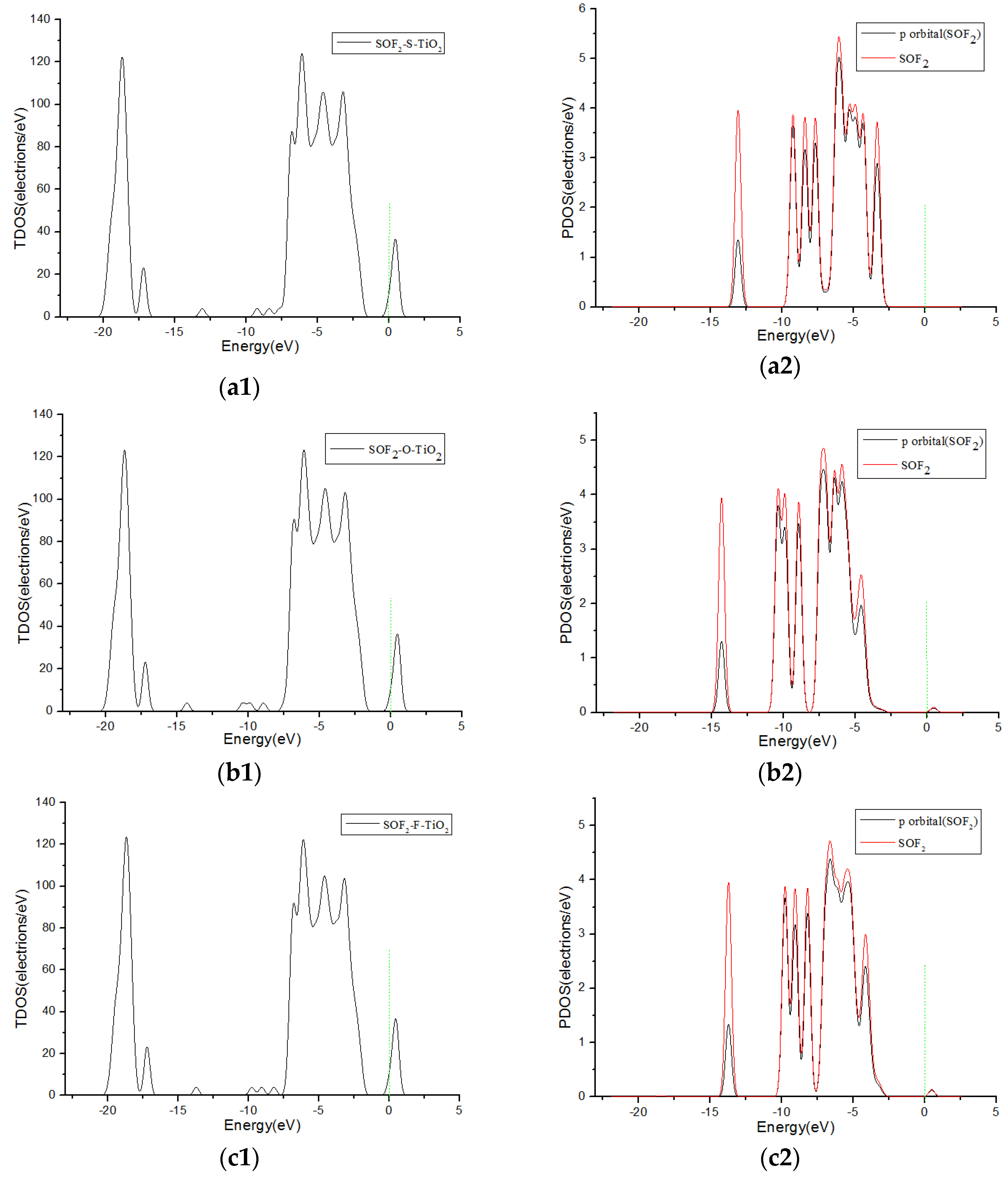
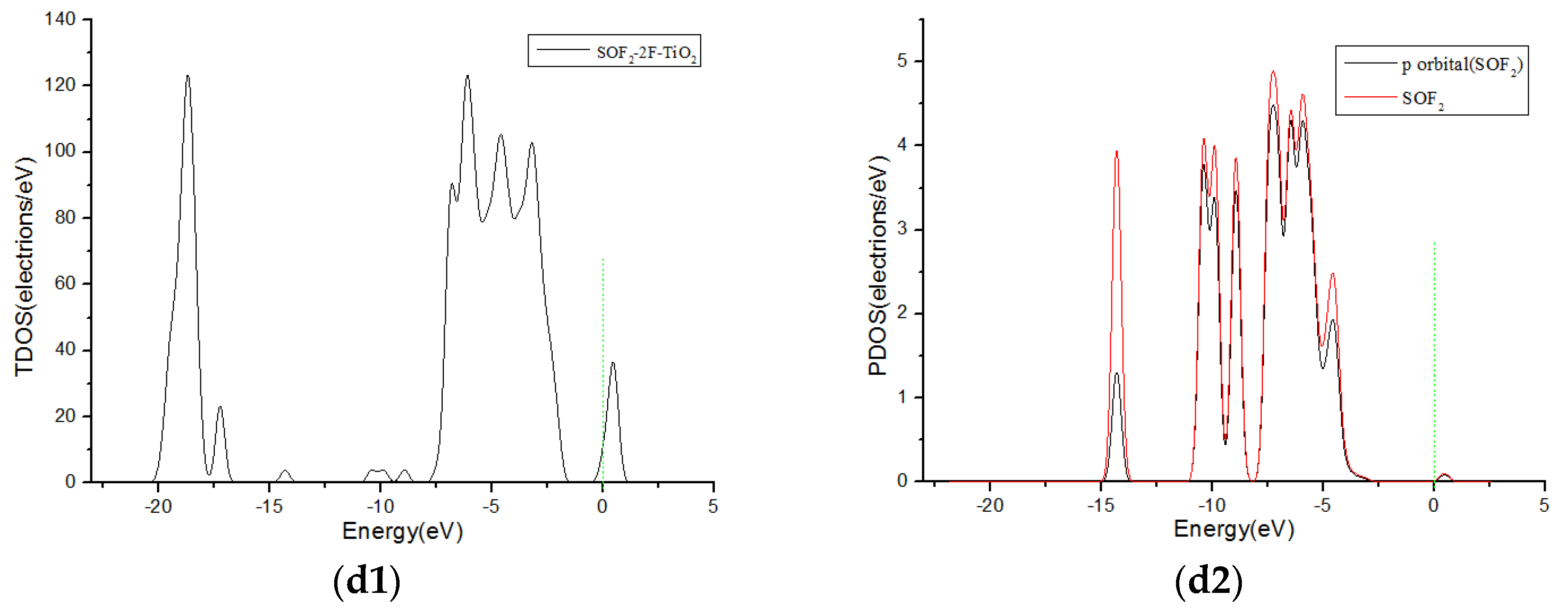
3.3. Analysis of Density of States
3.4. Comparison of Adsorption Results under Different Doping Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeng, F.; Tang, J.; Fan, Q.; Pan, J.; Zhang, X.; Yao, Q.; He, J. Decomposition characteristics of SF6 under thermal fault for temperatures below 400 °C. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 995–1004. [Google Scholar] [CrossRef]
- Derdouri, A.; Casanovas, J.; Hergli, R.; Grob, R.; Mathieu, J. Study of the decomposition of wet SF6, subjected to 50-Hz ac corona discharges. J. Appl. Phys. 1989, 65, 1852–1857. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.; Pan, J.; Zhang, X.; Yao, Q.; He, J.; Hou, X. Correlation analysis between formation process of SF6 decomposed components and partial discharge qualities. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 864–875. [Google Scholar] [CrossRef]
- Zeng, F.; Tang, J.; Zhang, X.; Xie, Y.; Yao, Q.; Miao, Y.; Zhang, C. Reconstructing and extracting information on SF6 decomposition characteristic components induced by partial overthermal fault in GIE. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 183–193. [Google Scholar] [CrossRef]
- Casanovas, A.M.; Casanovas, J.; Lagarde, F.; Belarbi, A. Study of the decomposition of SF6 under dc negative polarity corona discharges (point-to-plane geometry): Influence of the metal constituting the plane electrode. Evolution 1992, 72, 3344–3354. [Google Scholar]
- Beyer, C.; Jenett, H.; Klockow, D. Influence of reactive SFx, gases on electrode surfaces after electrical discharges under SF6 atmosphere. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 234–240. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Wei, L.; Liang, N.; Wu, X. Theoretical calculation of the gas-sensing properties of Pt-decorated carbon nanotubes. Sensors 2013, 13, 15159–15171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gui, Y.; Dai, Z. A simulation of Pd-doped SWCNTs used to detect SF6, decomposition components under partial discharge. Appl. Surf. Sci. 2014, 315, 196–202. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Chen, Q.; Tang, J. A DFT study of SO2 and H2S gas adsorption on Au-doped single-walled carbon nanotubes. Phys. Scr. 2014, 89, 065803. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, B.; Wang, X.; Luo, C. Effect of Plasma Treatment on Multi-Walled Carbon Nanotubes for the Detection of H2S and SO2. Sensors 2012, 12, 9375–9385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Yu, L.; Zhou, J. Highly sensitive and selective polyaniline thin-film sensors for detecting SF6, decomposition products at room temperature. Synth. Met. 2015, 200, 74–79. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 612. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.K.; Gong, D.; Paulose, M.; Ong, K.G.; Grimes, C.A. Hydrogen sensing using titania nanotubes. Sens. Actuators B Chem. 2003, 93, 338–344. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.; Paulose, M.; Mukherjee, N.; Grimes, C. Fabrication of tapered, conical-shaped titania nanotubes. J. Mater. Res. 2003, 18, 2588–2593. [Google Scholar] [CrossRef]
- Nakamura, R.; Tomoaki, T.; Nakato, Y. Mechanism for Visible Light Responses in Anodic Photocurrents at N-Doped TiO2 Film Electrodes. J. Phys. Chem. B 2004, 108, 10617–10620. [Google Scholar] [CrossRef]
- Valentin, C.D.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B 2005, 109, 11414. [Google Scholar] [CrossRef] [PubMed]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S. N-Doped TiO2 Nanoparticle Based Visible Light Photocatalyst by Modified Peroxide Sol−Gel Method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energ. Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Ho, W.; Yu, J.C.; Lee, S. Synthesis of hierarchical nanoporous F-doped TiO2 spheres with visible light photocatalytic activity. Chem. Commun. 2006, 111, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Fan, Q.; Xie, Y.; Chen, J.; Shu, Q.; Yu, J.C. Sonochemical fabrication of novel square-shaped F doped TiO2 nanocrystals with enhanced performance in photocatalytic degradation of phenol. J. Hazard. Mater. 2012, 237–238, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.G.; Liao, S.J.; Dang, Z. Preparation, characterization and photocatalytic performance of anatase F doped TiO2 sol. Acta Chim. Sin. 2006, 64, 1805–1811. [Google Scholar]
- Guo, M.; Zhang, X.; Liang, C.; Jia, G. Mechanism of Visible Photoactivity of F-Doped TiO2. Chin. Phys. Lett. 2010, 27, 204–207. [Google Scholar]
- Li, D.; Haneda, H.; Labhsetwar, N.K.; Hishita, S.; Ohashi, N. Visible-light-driven photocatalysis on fluorine-doped TiO2, powders by the creation of surface oxygen vacancies. Chem. Phys. Lett. 2005, 401, 579–584. [Google Scholar] [CrossRef]
- Hattori, A.; Tada, H. High Photocatalytic Activity of F-Doped TiO2 Film on Glass. J. Sol-Gel Sci. Technol. 2001, 22, 47–52. [Google Scholar] [CrossRef]
- Hazra, S.K.; Kim, N.K.; Park, J.; Choi, B.; Lee, S. Gettering by cf4-ar plasma-treated titanium within anodically bonded glass-silicon microcavities. Sens. Mater. 2009, 21, 37–51. [Google Scholar]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244. [Google Scholar] [CrossRef]
- Li, D.; Ohashi, N.; Hishita, S.; Kolodiazhnyi, T.; Haneda, H. Origin of visible-light-driven photocatalysis: A comparative study on N/F-doped and N-F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J. Solid State Chem. 2005, 178, 3293–3302. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N.; Labhsetwar, N.K. Fluorine-doped TiO2, powders prepared by spray pyrolysis and their improved photocatalytic activity for decomposition of gas-phase acetaldehyde. J. Fluorine Chem. 2005, 126, 69–77. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Hu, W.; Zhang, J. A DFT study of SF6 decomposed gas adsorption on an anatase (1 0 1) surface. Appl. Surf. Sci. 2013, 286, 47–53. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Cui, H.; Zhang, J. A first principle simulation of competitive adsorption of SF6 decomposition components on nitrogen-doped anatase TiO2 (101) surface. Appl. Surf. Sci. 2017, 422, 331–338. [Google Scholar] [CrossRef]
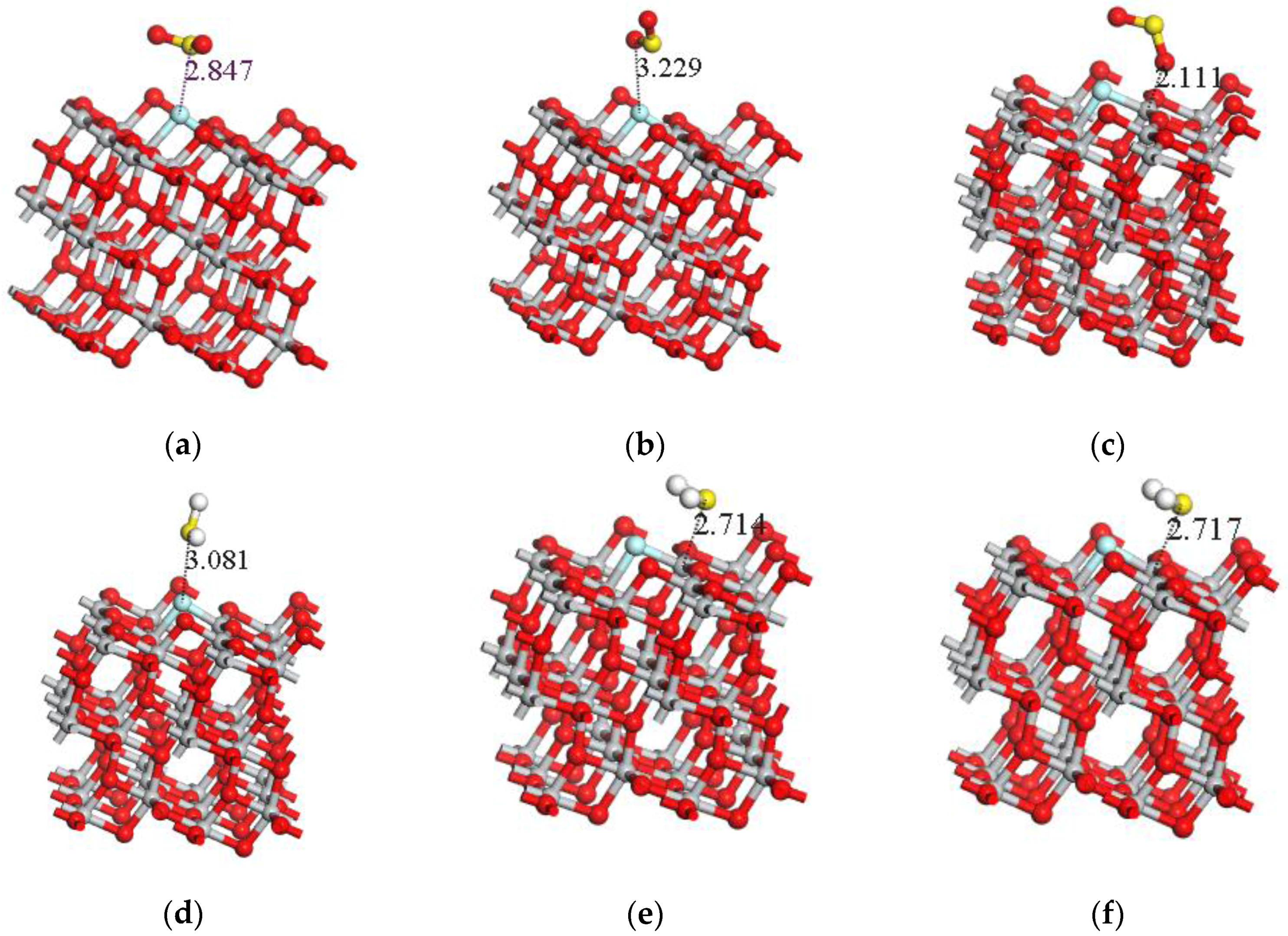
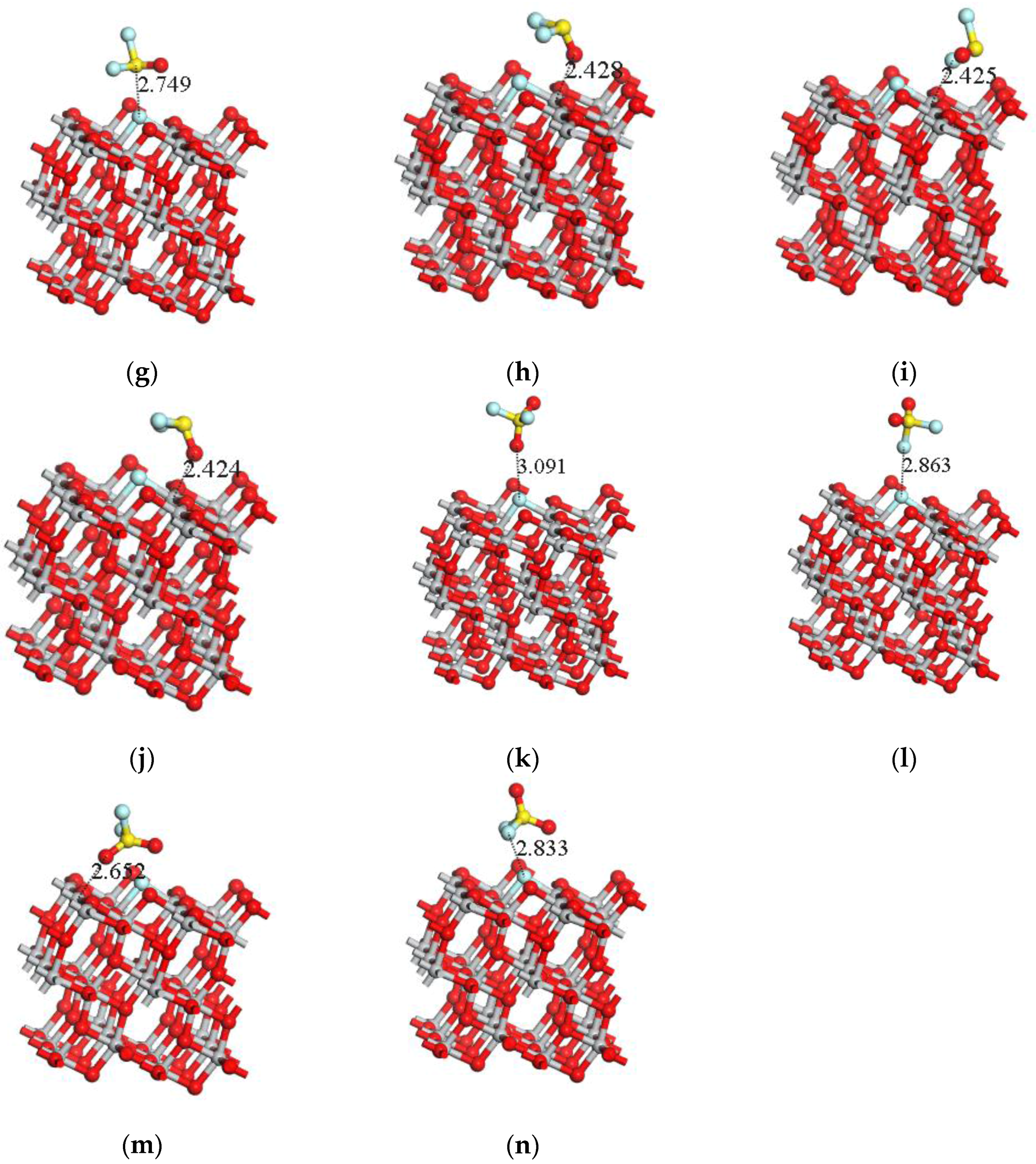

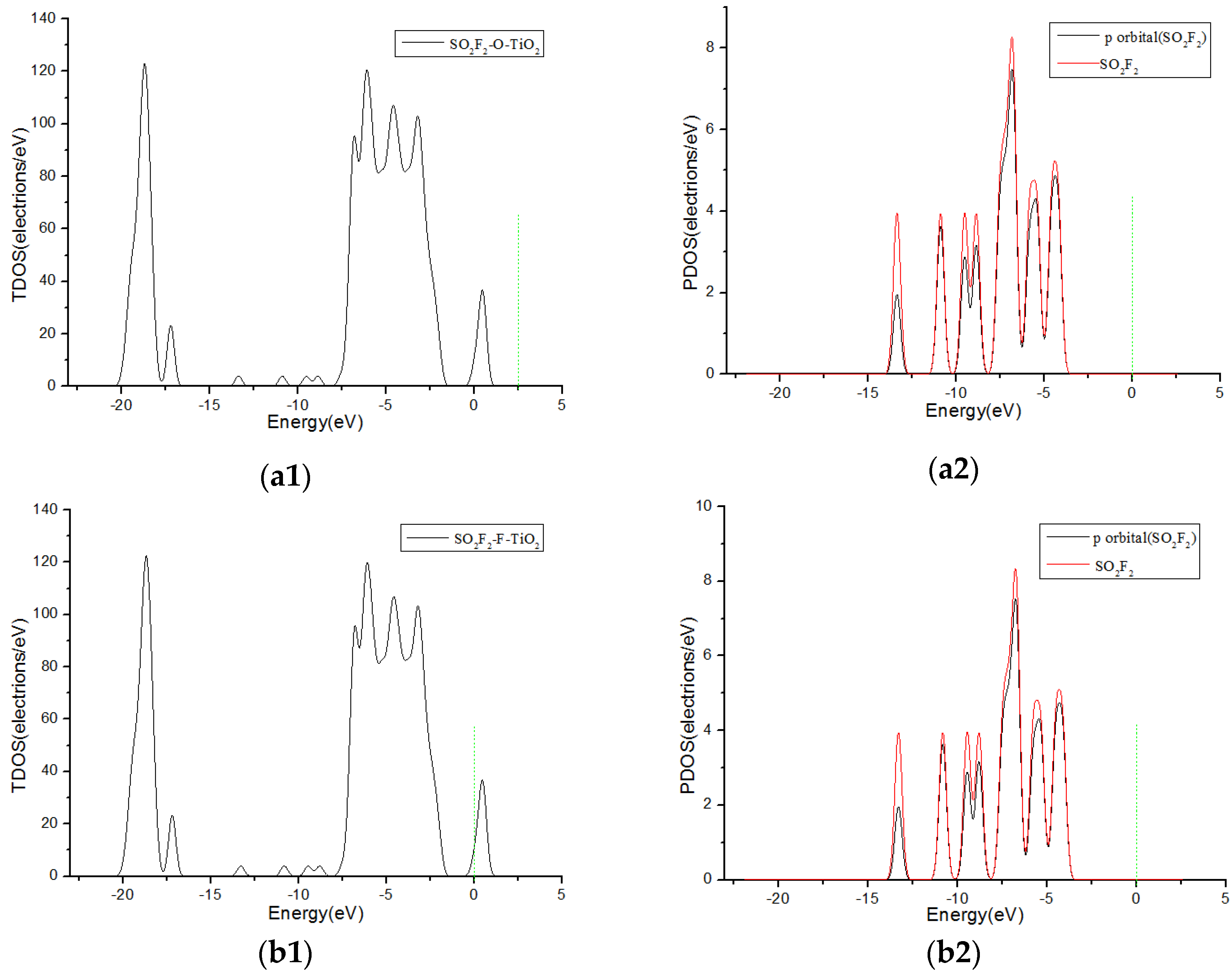
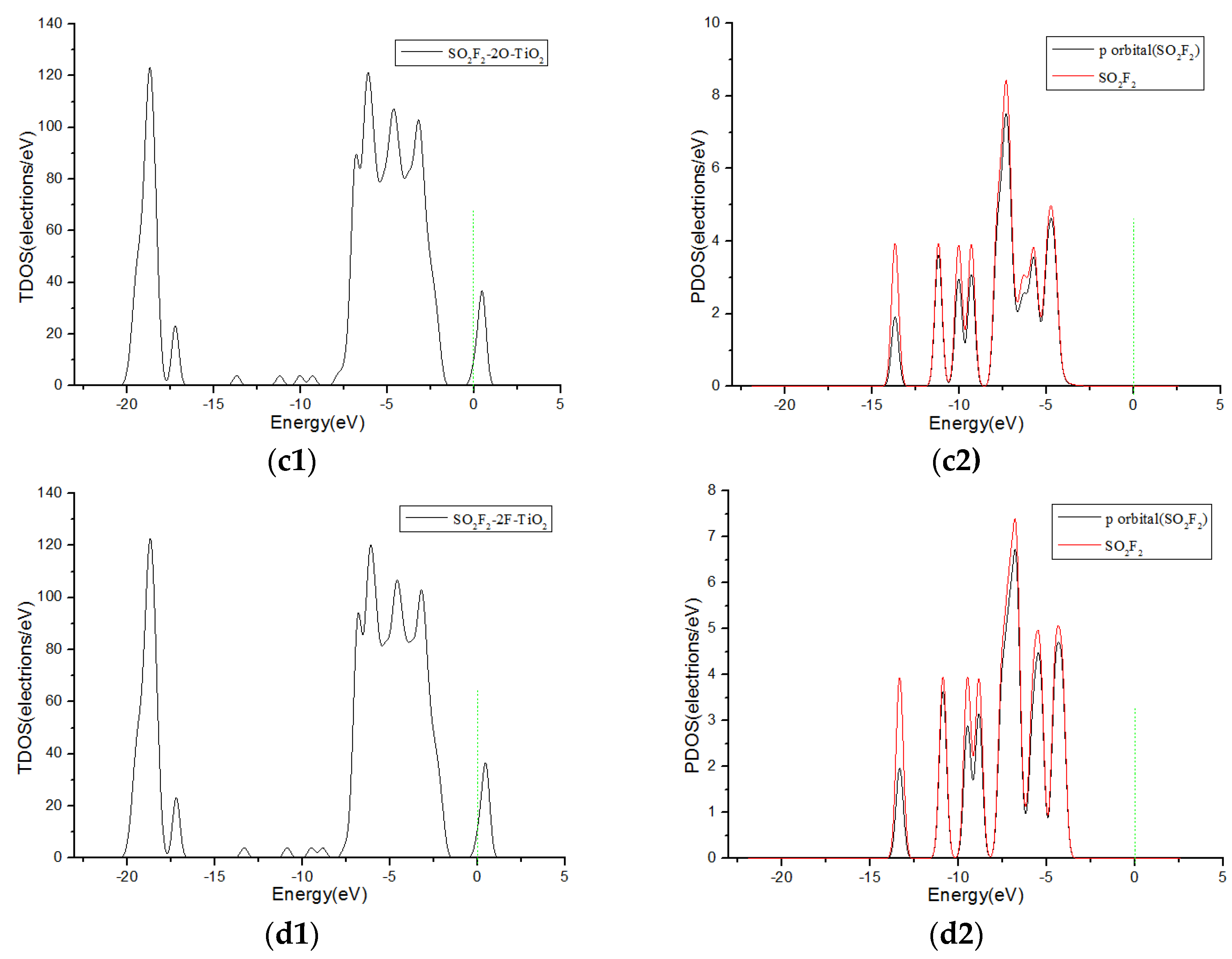
| Adsorption System | Adsorption Structure | Adsorption Energy Ea (eV) | Charge transfer Amount Qt (e) | Adsorption Distance (Å) |
|---|---|---|---|---|
| SO2-S-TiO2 | a | −0.173 | −0.013 | 2.847 |
| SO2-O-TiO2 | b | −0.132 | 0 | 3.229 |
| SO2-2O-TiO2 | c | −0.617 | −0.12 | 2.111 |
| H2S-S-TiO2 | d | −0.209 | 0.008 | 2.835 |
| H2S-H-TiO2 | e | −0.837 | 0.267 | 2.714 |
| H2S-2H-TiO2 | f | −0.836 | 0.266 | 2.717 |
| SOF2-S-TiO2 | g | −0.254 | −0.017 | 2.749 |
| SOF2-O-TiO2 | h | −0.412 | 0.098 | 2.438 |
| SOF2-F-TiO2 | i | −0.534 | 0.038 | 2.425 |
| SOF2-2F-TiO2 | j | −0.423 | 0.098 | 2.424 |
| SO2F2-O-TiO2 | k | −0.044 | 0 | 3.091 |
| SO2F2-F-TiO2 | l | −0.051 | −0.002 | 2.863 |
| SO2F2-2O-TiO2 | m | −0.398 | 0.046 | 2.652 |
| SO2F2-2F-TiO2 | n | −0.198 | −0.003 | 2.833 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, J.; Dong, X.; Cui, H. A DFT Calculation of Fluoride-Doped TiO2 Nanotubes for Detecting SF6 Decomposition Components. Sensors 2017, 17, 1907. https://doi.org/10.3390/s17081907
Zhang X, Zhang J, Dong X, Cui H. A DFT Calculation of Fluoride-Doped TiO2 Nanotubes for Detecting SF6 Decomposition Components. Sensors. 2017; 17(8):1907. https://doi.org/10.3390/s17081907
Chicago/Turabian StyleZhang, Xiaoxing, Jun Zhang, Xingchen Dong, and Hao Cui. 2017. "A DFT Calculation of Fluoride-Doped TiO2 Nanotubes for Detecting SF6 Decomposition Components" Sensors 17, no. 8: 1907. https://doi.org/10.3390/s17081907
APA StyleZhang, X., Zhang, J., Dong, X., & Cui, H. (2017). A DFT Calculation of Fluoride-Doped TiO2 Nanotubes for Detecting SF6 Decomposition Components. Sensors, 17(8), 1907. https://doi.org/10.3390/s17081907





