Rapid ELISA Using a Film-Stack Reaction Field with Micropillar Arrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Film-Stack Reaction Field with a Micro-Pillars Array
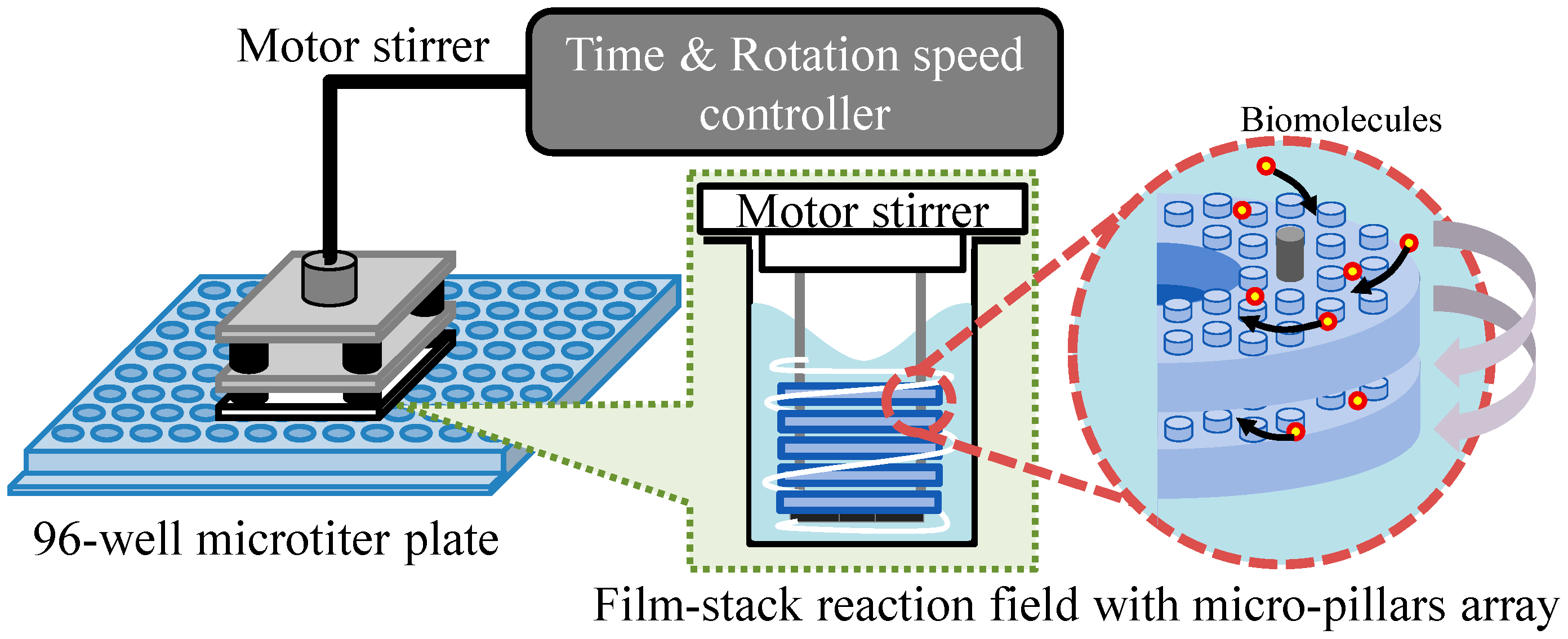
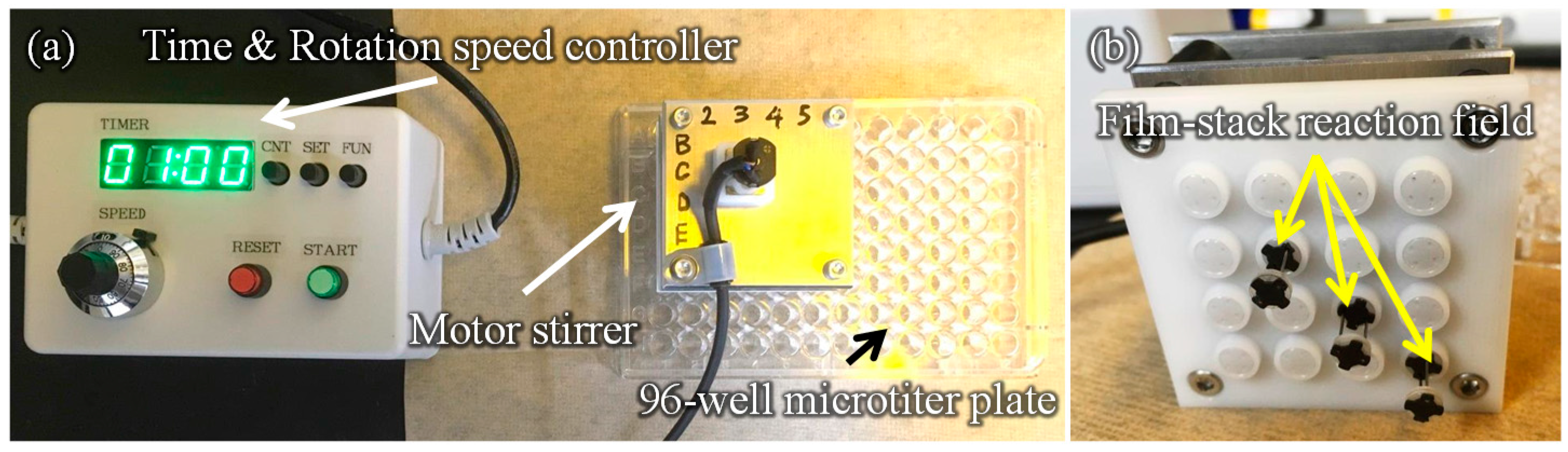
2.2. Experiment Setup
2.3. IgA ELISA
2.4. Theoretical Modeling of Physical Parameters Related with Biomolecule Transport
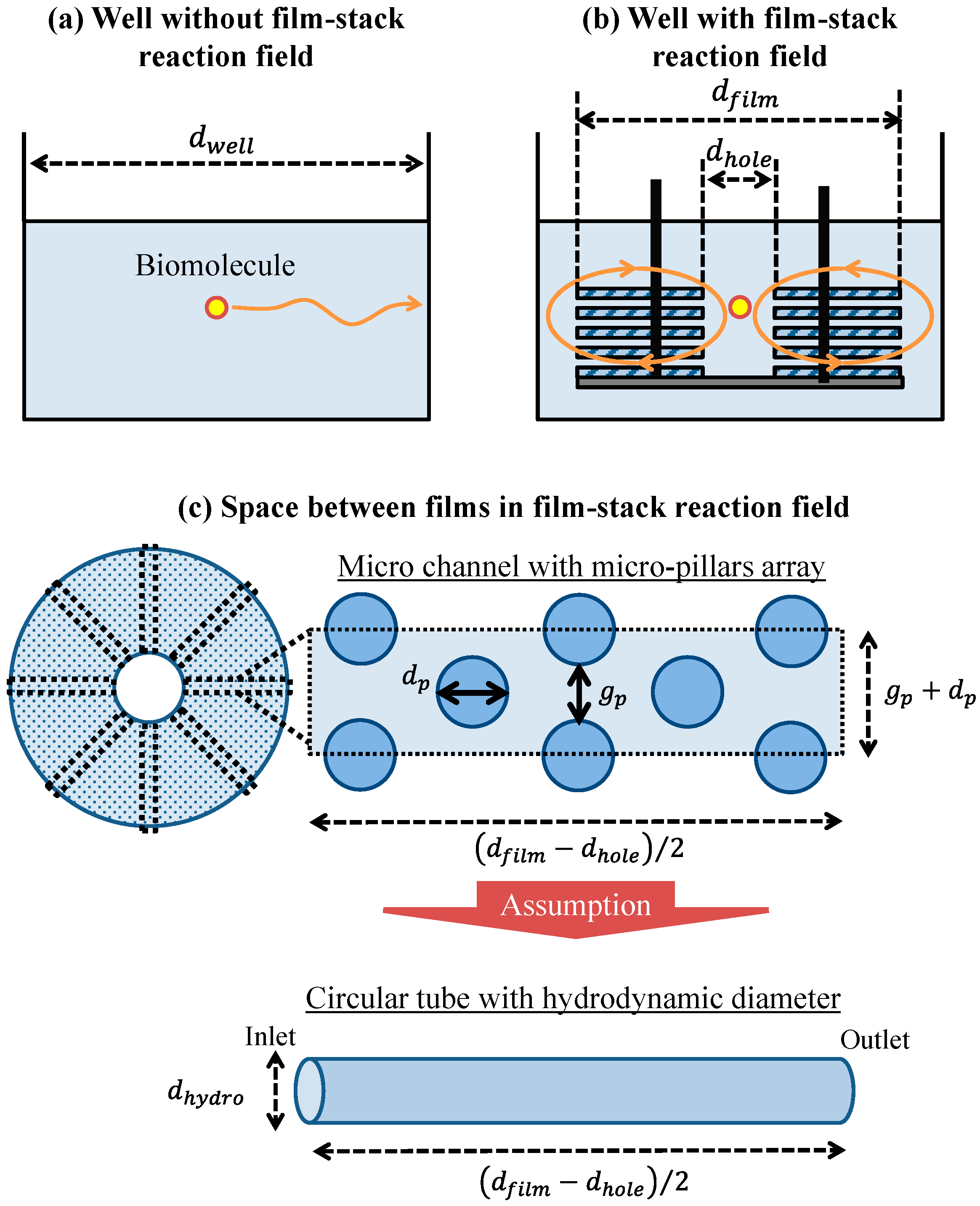
2.4.1. Geometric Model of Well and Space between Films
2.4.2. Physical Parameters in Theoretical Analysis for the Well without the Film-Stack Reaction Field
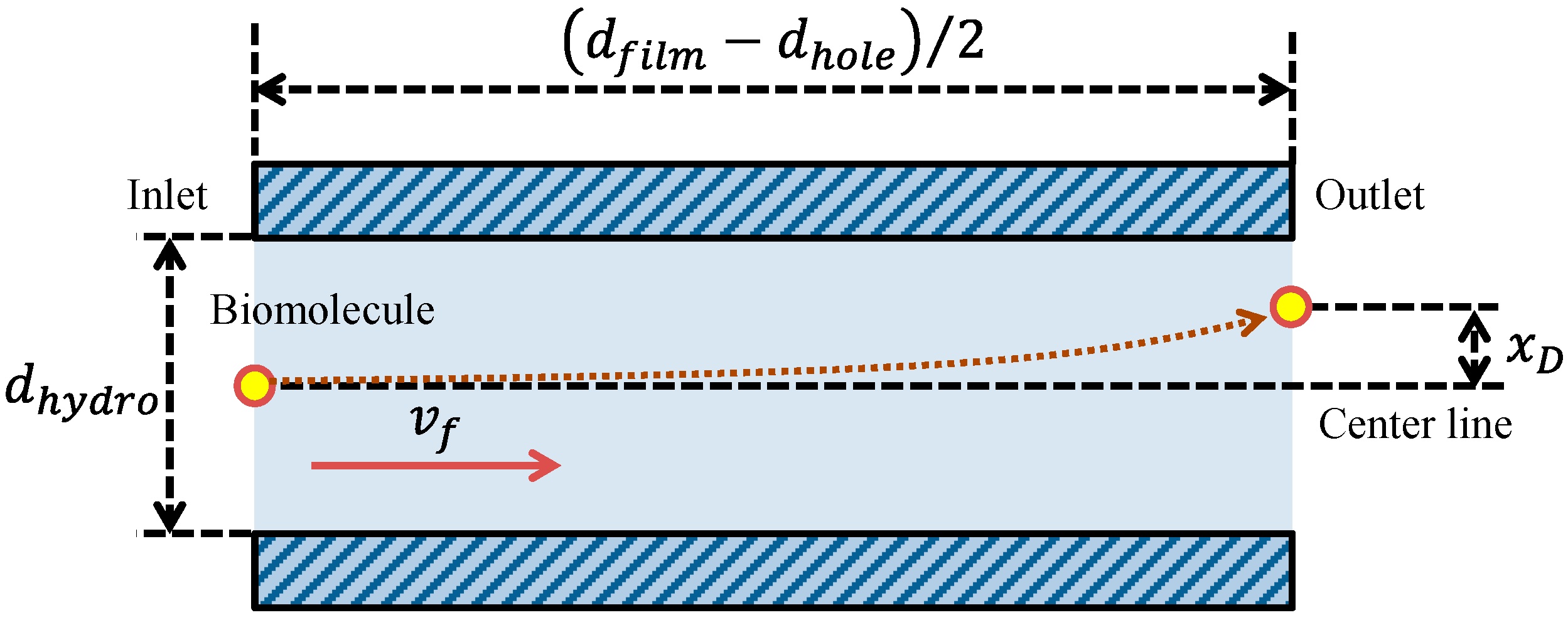
2.4.3. Physical Parameters in Theoretical Analysis for the Well with the Film-Stack Reaction Field
3. Results
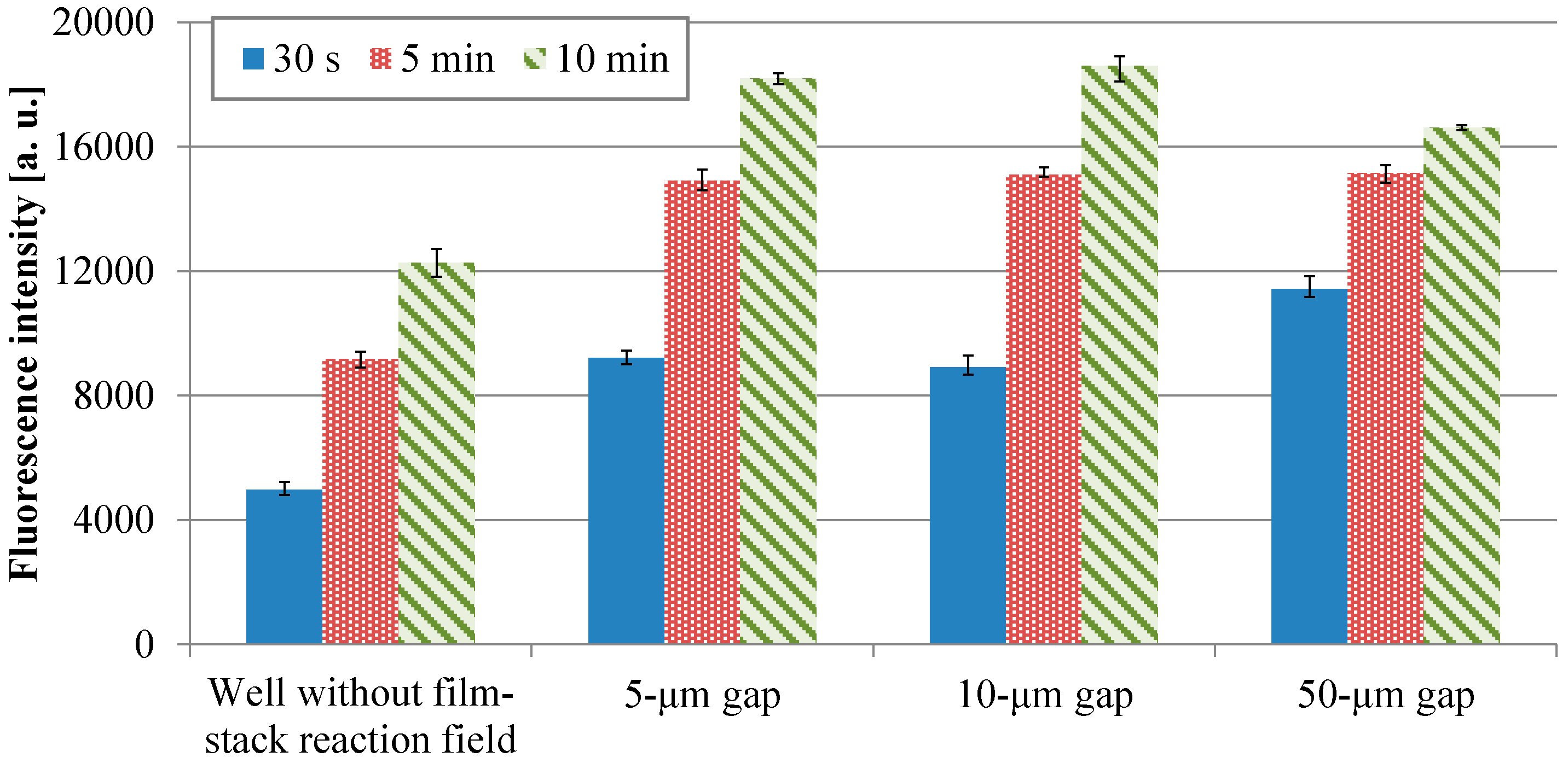
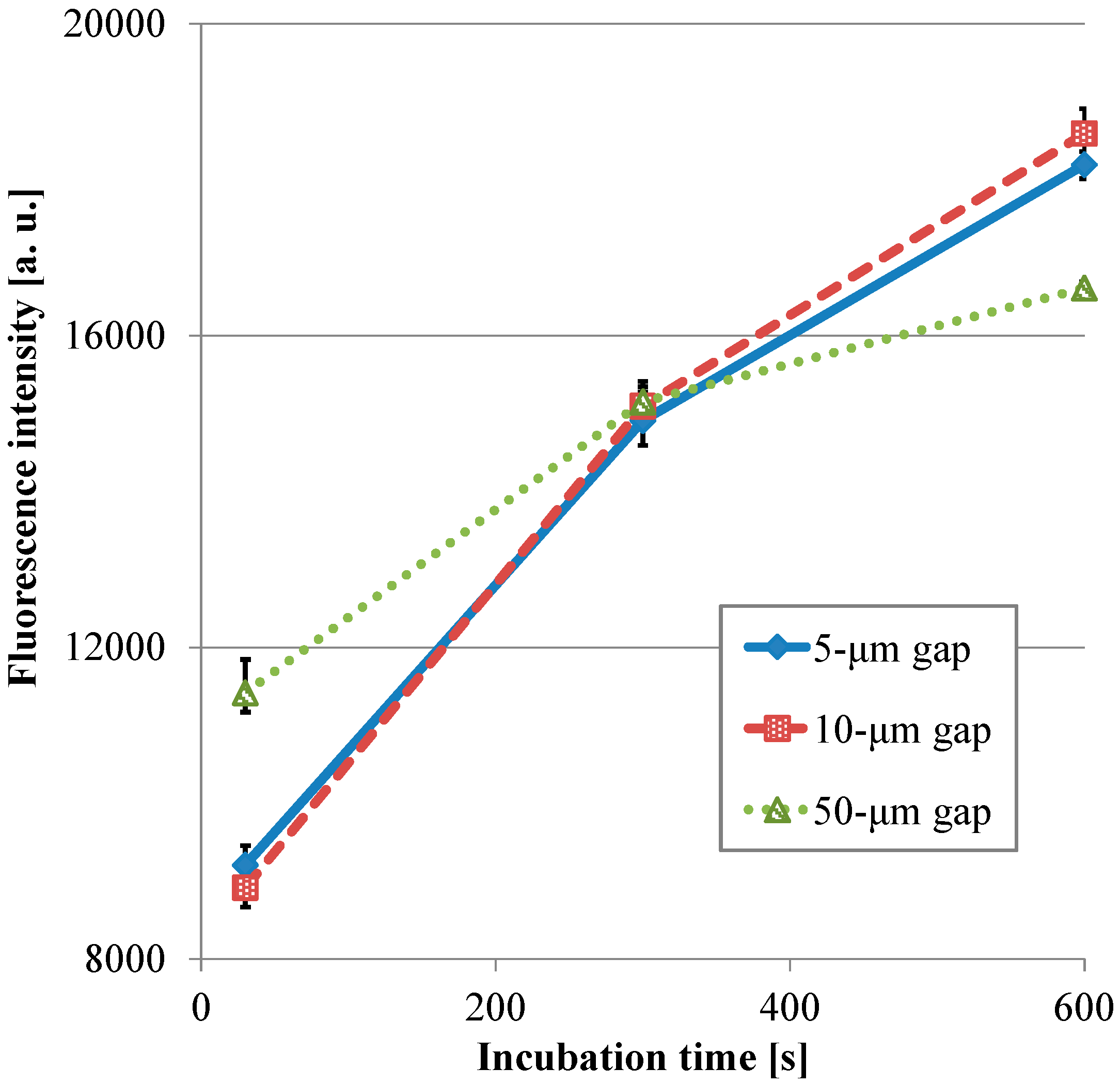
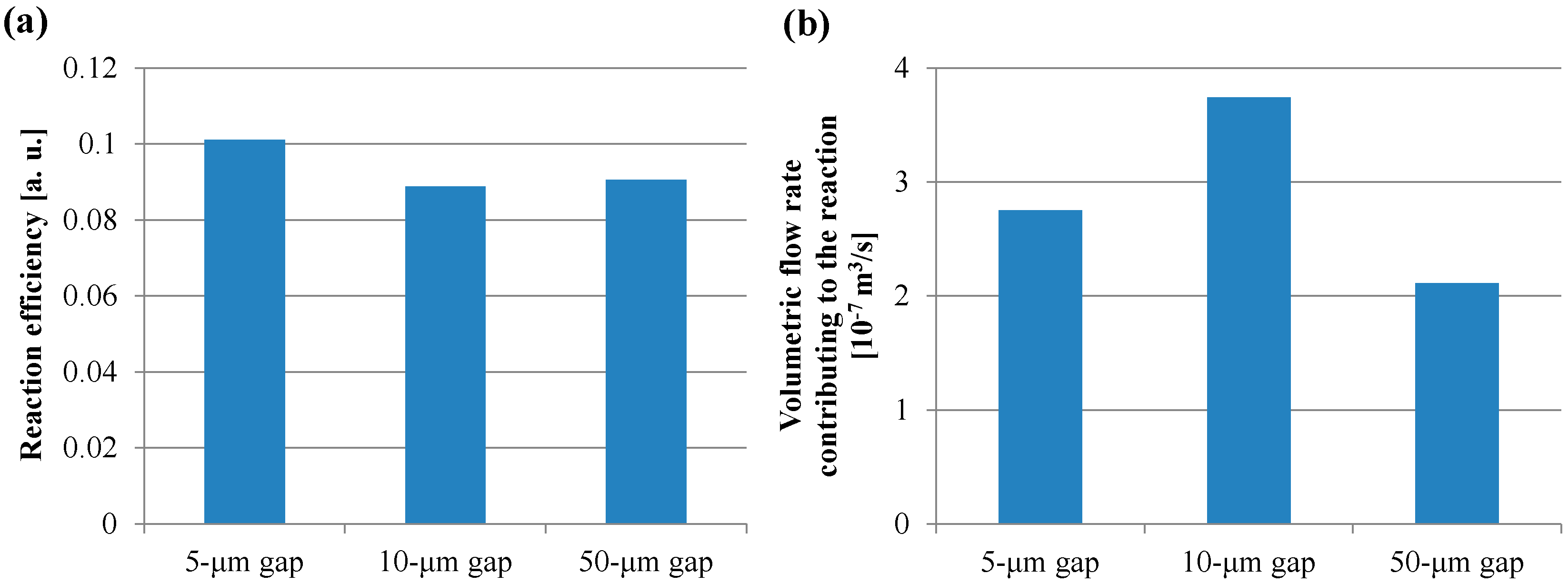
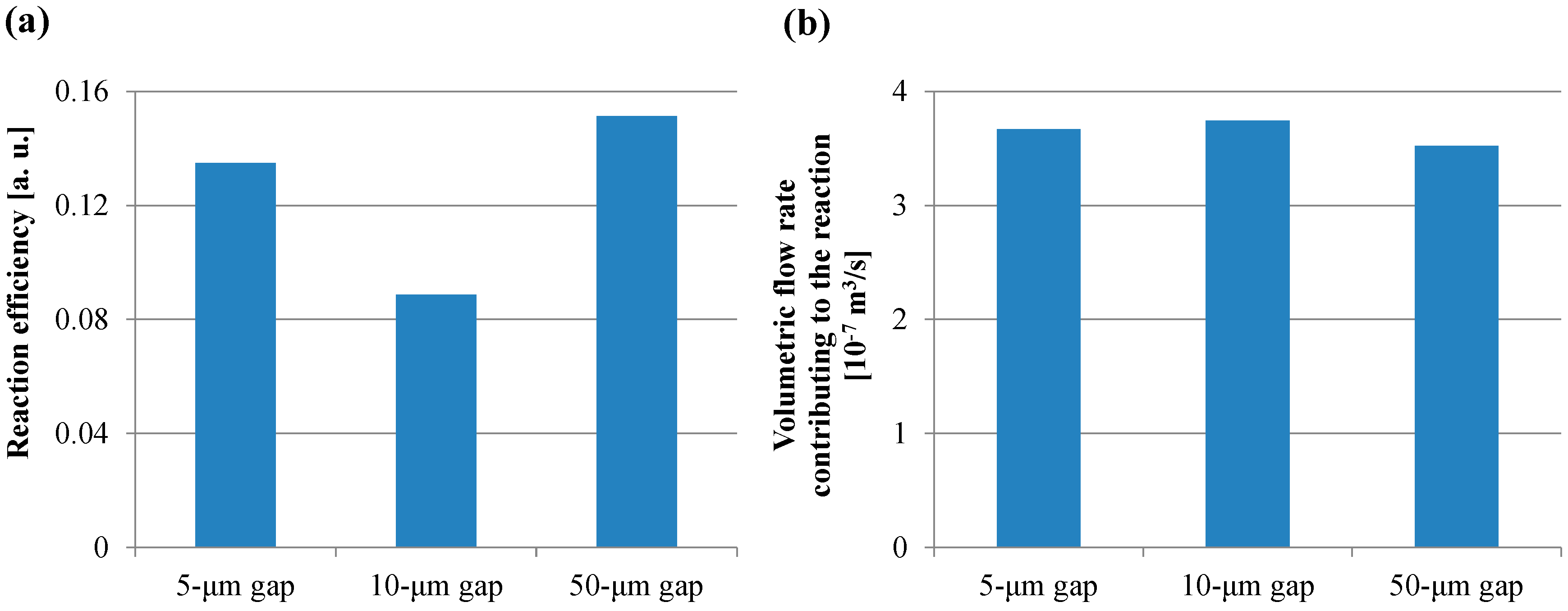
3.1. Fluorescence Intensity in IgA ELISA
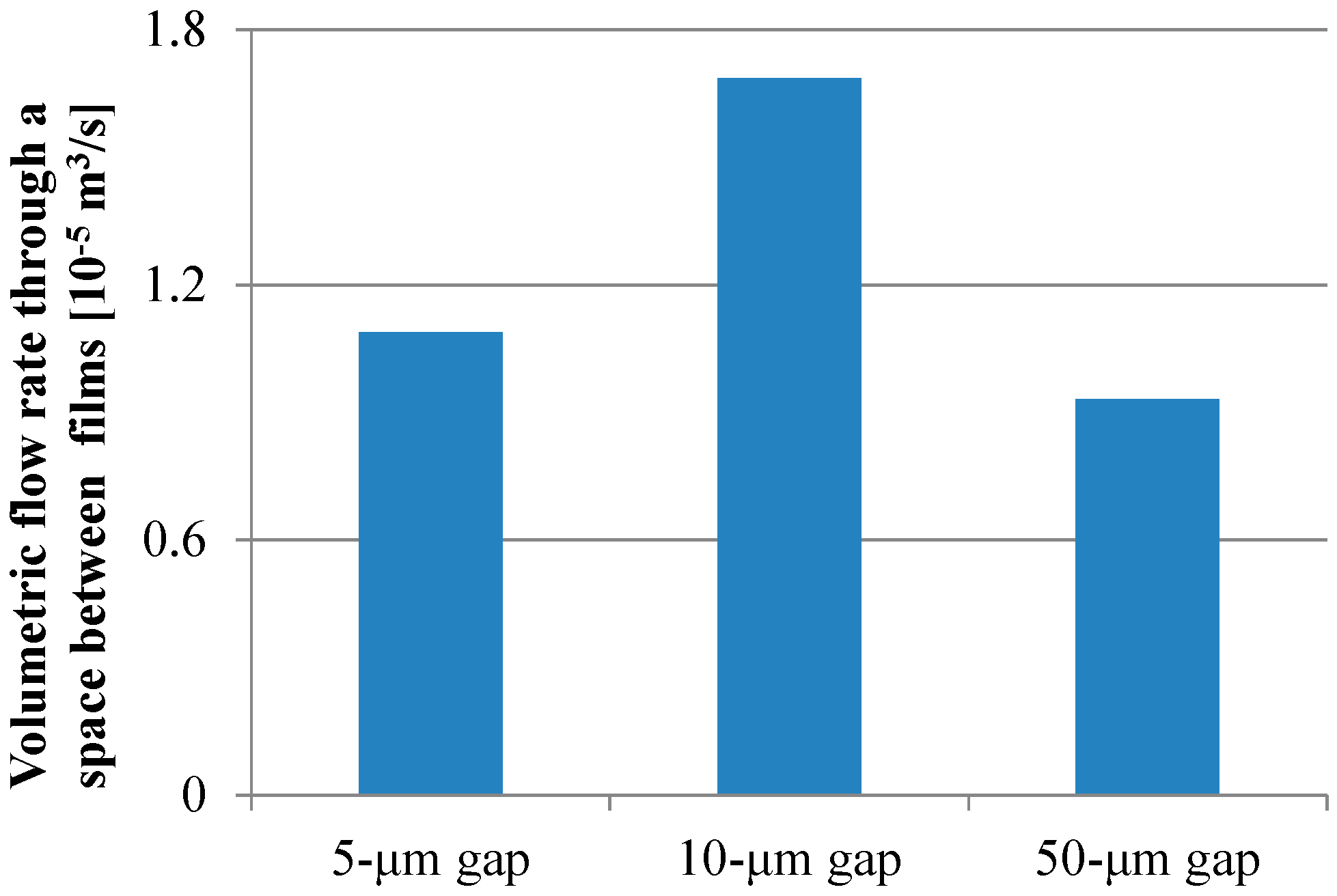
3.2. Theoretical Analysis of Physical Paramters Related with Biomolecule Transport
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Engvall, E. The ELISA, Enzyme-Linked Immunosorbent Assay. Clin. Chem. 2010, 56, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed]
- Voller, A.; Bartlett, A.; Bidwell, D.E. Enzyme immunoassays with special reference to ELISA techniques. J. Clin. Pathol. 1978, 31, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, M.; Kostov, Y.; Rassooly, A. ELISA-LOC: Lab-on-a-chip for enzyme-linked immunodetection. Lab Chip 2010, 16, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Laksanasopin, T.; Cheung, Y.K.; Steinmiller, D.; Linder, V.; Parsa, H.; Wang, J.; Moore, H.; Rouse, R; Umviligihozo, G.; et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, J.; Pang, Y.; Wang, J.; Li, W.; Ge, Z.; Huang, Y. High-throughput immunoassay through in-channel microfluidic patterning. Lab Chip 2012, 12, 2487–2490. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.G.; McQuary, P.; Mongan, A.; Gangadharan, R.; Brignac, S.; Eggers, M. High-throughput microarray-based Enzyme-Linked Immunosorbent Assay (ELISA). Biotechniques 1999, 27, 778–788. [Google Scholar] [PubMed]
- Geschke, O.; Klank, H.; Telleman, P. Microsystem Engineering of Lab-on-a-Chip Devices; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B. Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Voldman, J.; Gray, M.L.; Schmidt, M.A. Microfabrication in biology and medicine. Annu. Rev. Biomed. Eng. 1999, 1, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Zeng, H.; Yang, J.; Nakajima, H.; Uchiyama, K. A compact immunoassay platform based on a multicapillary glass plate. Sensors 2014, 14, 9132–9144. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Nishiwaki, M.; Kudo, Y.; Seino, N.; Nakagama, T.; Uchiyama, K. Preparation of two-dimensionally ordered microbeads structure dispensed with an ink-jet and its application to ELISA. Anal. Sci. 2009, 25, 235–239. [Google Scholar] [CrossRef]
- Suzuki, Y.; Pabjańczyk-Wlazło, E.; Onoda, J.; Shimizu, T.; Yang, M. Fabrication and evaluation of micro-structured reaction field with vertically aligned carbon nanotubes for micro bio-analysis device. Mech. Eng. J. 2016, 3, 15-00567. [Google Scholar] [CrossRef]
- Fatoyinbo, H.O.; Labeed, F.H. Microfluidics in Detection Science; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Jomeh, S.; Hoorfar, M. Numerical modeling of mass transport in microfluidic biomolecule-capturing devices equipped with reactive surfaces. Chem. Eng. J. 2010, 165, 668–677. [Google Scholar] [CrossRef]
- Singh, H.; Morita, T.; Suzuki, Y.; Shimojima, M.; Van, A.L.; Sugamata, M.; Yang, M. High sensitivity, high surface area Enzyme-linked Immunosorbent Assay (ELISA). Biomed. Mater. Eng. 2015, 26, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Morioka, K.; Shimizu, T.; Nakajima, H.; Uchiyama, K.; Yang, M. Influence of structural dimensions of micro-pillar array in reaction field on sensitivity of Enzyme-linked Immunosorbent Assay (ELISA). Biotechnol. Biotechnol. Equip. 2017. [Google Scholar] [CrossRef]
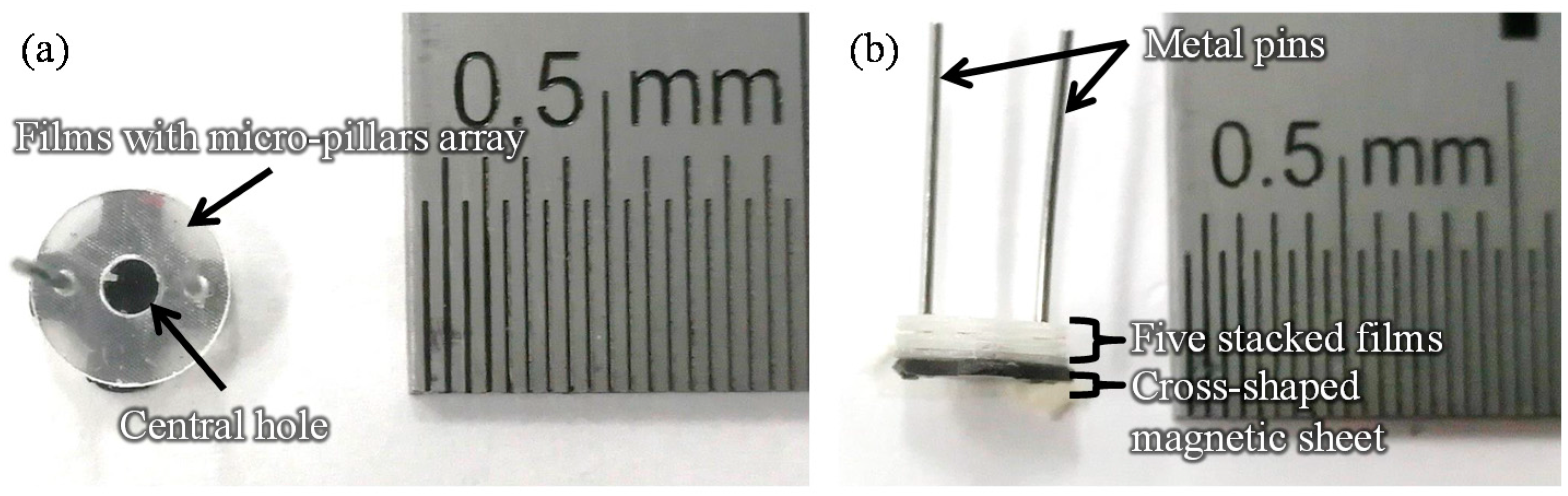
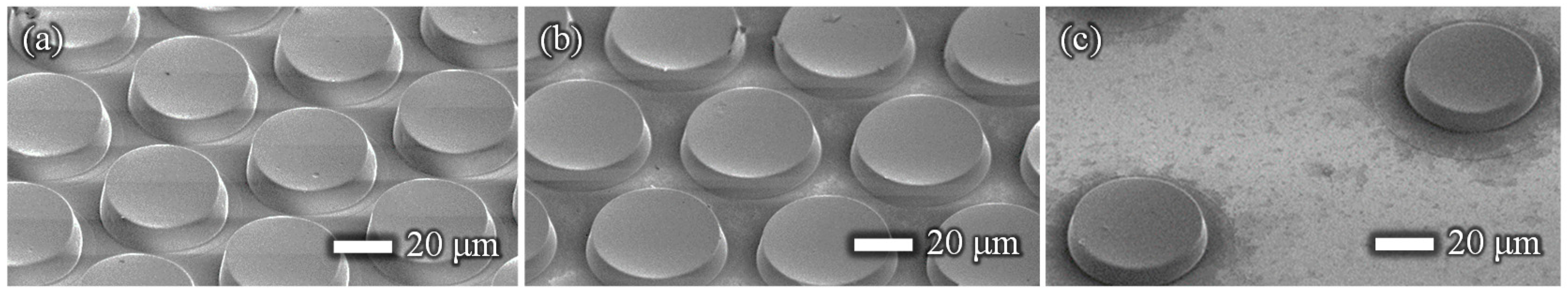

| Diameter [µm] | Height [µm] | Gap [µm] | |
|---|---|---|---|
| (a) | 50 | 10 | 5 |
| (b) | 50 | 10 | 10 |
| (c) | 50 | 10 | 50 |
| Characteristic Parameters for Physical Parameters | Values |
|---|---|
| Gap between micro pillars gp (µm) | 5, 10, 50 |
| Pillar diameter dp (µm) | 50 |
| Pillar height hp (µm) | 10 |
| Well diameter dwell (mm) | 7 |
| Outside diameter of a film dfilm (µm) | 5 |
| Central-hole diameter of a film dhole (µm) | 2 |
| Surface area of a well Sw (mm2) | 93 |
| Surface area of a film Sf (mm2) | 4.18 (5-µm gap), 4.00 (10-µm gap), 5.08 (50-µm gap) |
| Number of films per film-stack reaction field nf | 5 |
| Solution volume used in ELISA procedure VL (µL) | 100 |
| Biomolecule radius rb (nm) | 6.5 |
| Viscosity of buffer solution µ (Pa) | 0.001 |
| Atmosphere temperature T (K) | 293.15 |
| Average velocity of the convection flow in a circular tube with the rotation speed of 1000 rpm vf (m/s) | 1.74 (5-µm gap), 1.01 (10-µm gap), 0.35 (50-µm gap) |
| Protein incubation time (s) | 30, 300, 600 |
| Well without a Reaction Field | Average of Reaction Fields | |
|---|---|---|
| Specific surface area (m−1) | 930 | 2871 |
| Diffusion time (s) | 3.71 × 105 | 1.07 |
| Structural Dimension of a Micropillar Array | Specific Surface Area SSf (m−1) |
|---|---|
| 5-µm gap | 3010 |
| 10-µm gap | 2930 |
| 50-µm gap | 2674 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, Y.; Morioka, K.; Ohata, S.; Shimizu, T.; Nakajima, H.; Uchiyama, K.; Yang, M. Rapid ELISA Using a Film-Stack Reaction Field with Micropillar Arrays. Sensors 2017, 17, 1608. https://doi.org/10.3390/s17071608
Suzuki Y, Morioka K, Ohata S, Shimizu T, Nakajima H, Uchiyama K, Yang M. Rapid ELISA Using a Film-Stack Reaction Field with Micropillar Arrays. Sensors. 2017; 17(7):1608. https://doi.org/10.3390/s17071608
Chicago/Turabian StyleSuzuki, Yuma, Kazuhiro Morioka, Soichiro Ohata, Tetsuhide Shimizu, Hizuru Nakajima, Katsumi Uchiyama, and Ming Yang. 2017. "Rapid ELISA Using a Film-Stack Reaction Field with Micropillar Arrays" Sensors 17, no. 7: 1608. https://doi.org/10.3390/s17071608
APA StyleSuzuki, Y., Morioka, K., Ohata, S., Shimizu, T., Nakajima, H., Uchiyama, K., & Yang, M. (2017). Rapid ELISA Using a Film-Stack Reaction Field with Micropillar Arrays. Sensors, 17(7), 1608. https://doi.org/10.3390/s17071608






