Gold Electrodes Modified with Calix[4]arene for Electrochemical Determination of Dopamine in the Presence of Selected Neurotransmitters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
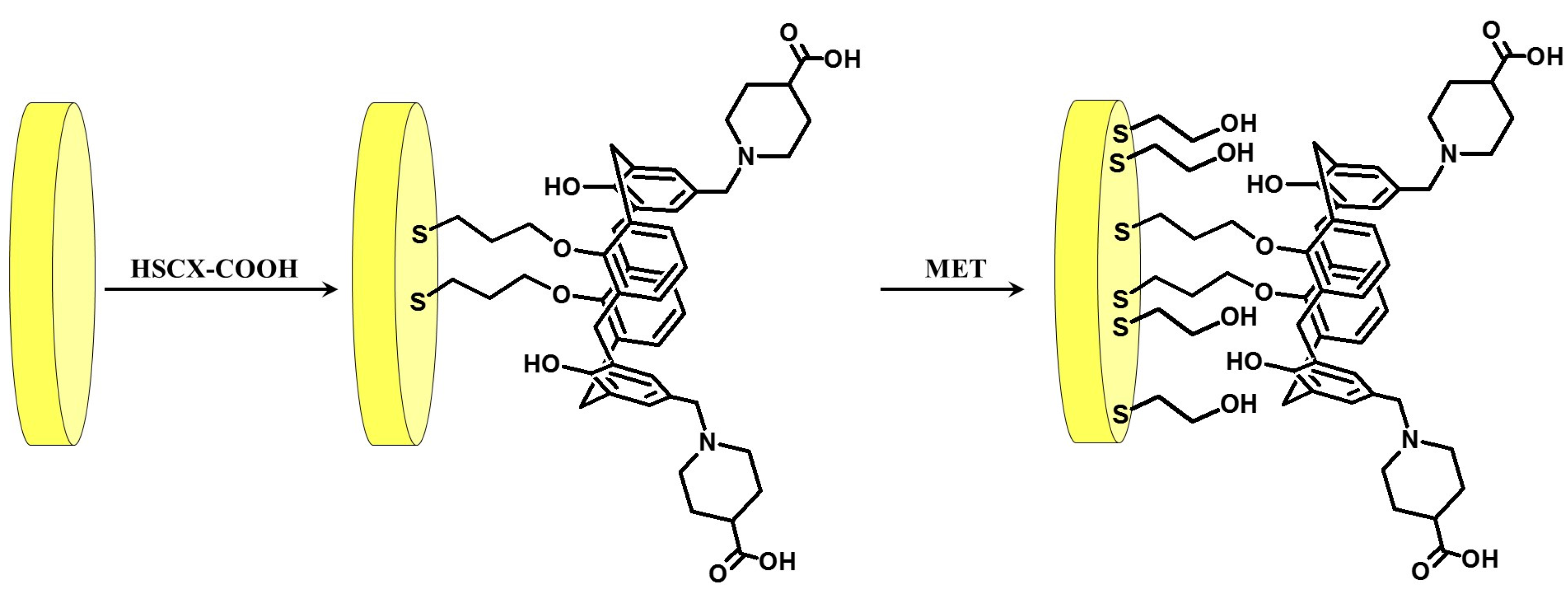
2.2. Electrode Preparation
2.3. Electrochemical Measurements
3. Results and Discussion
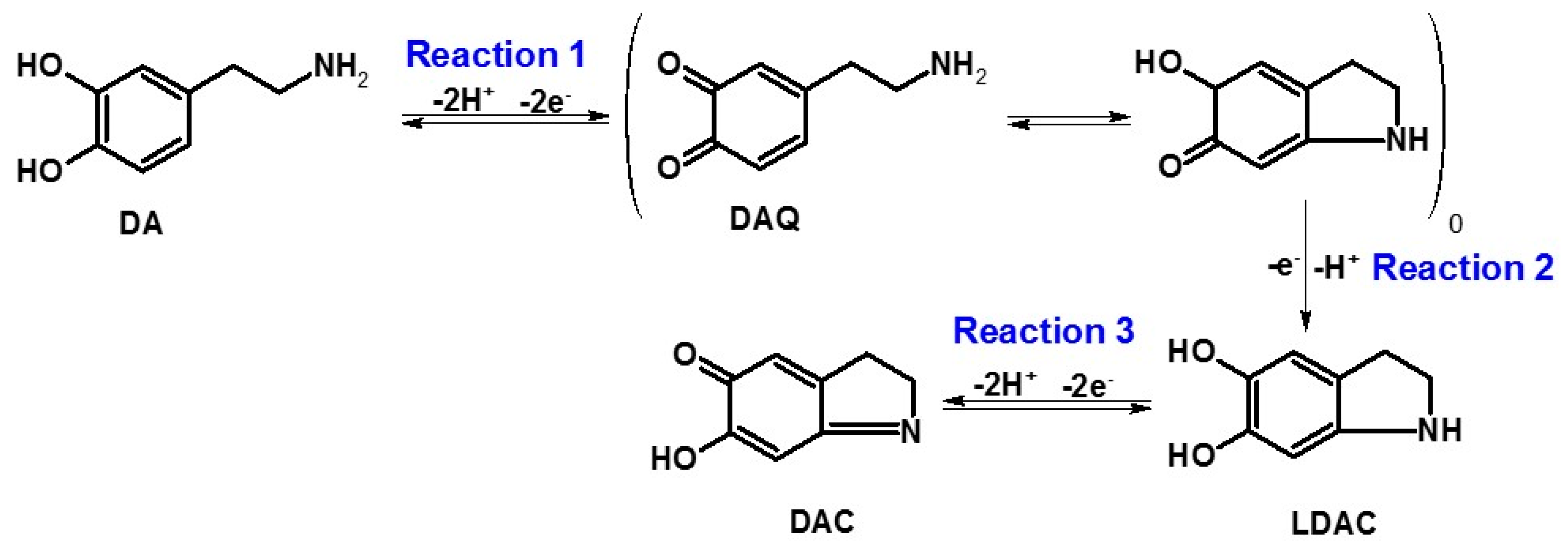
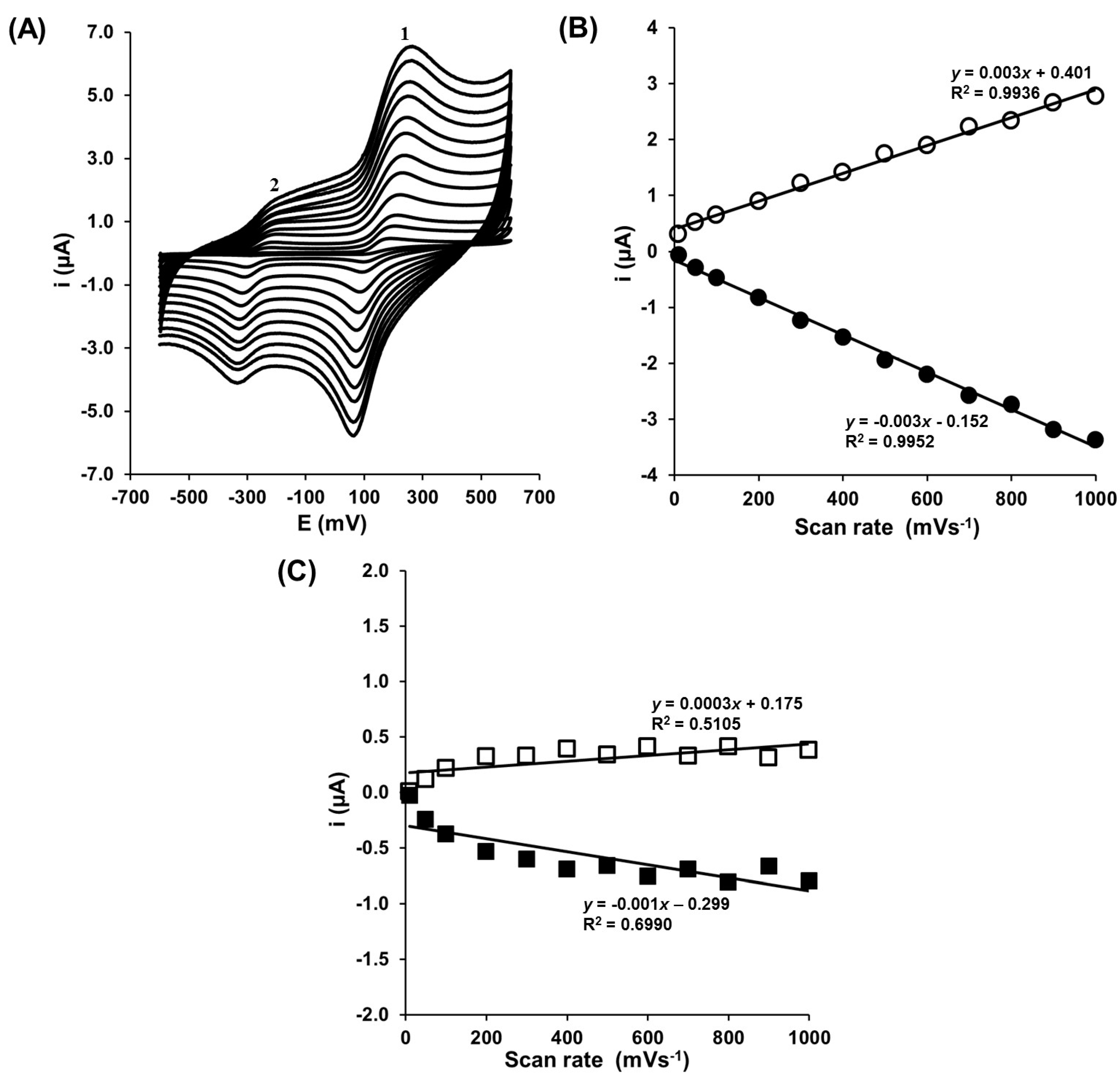
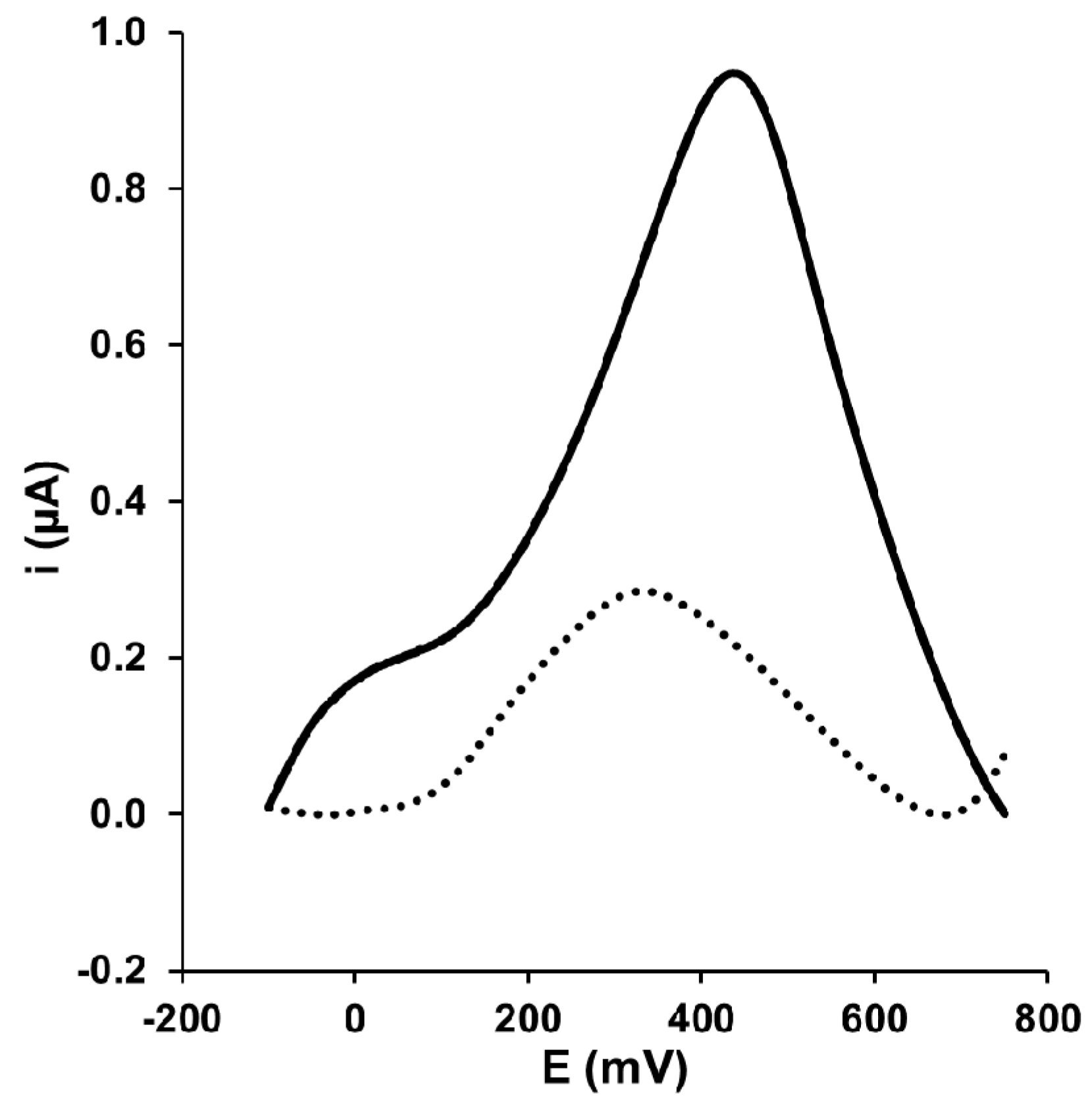
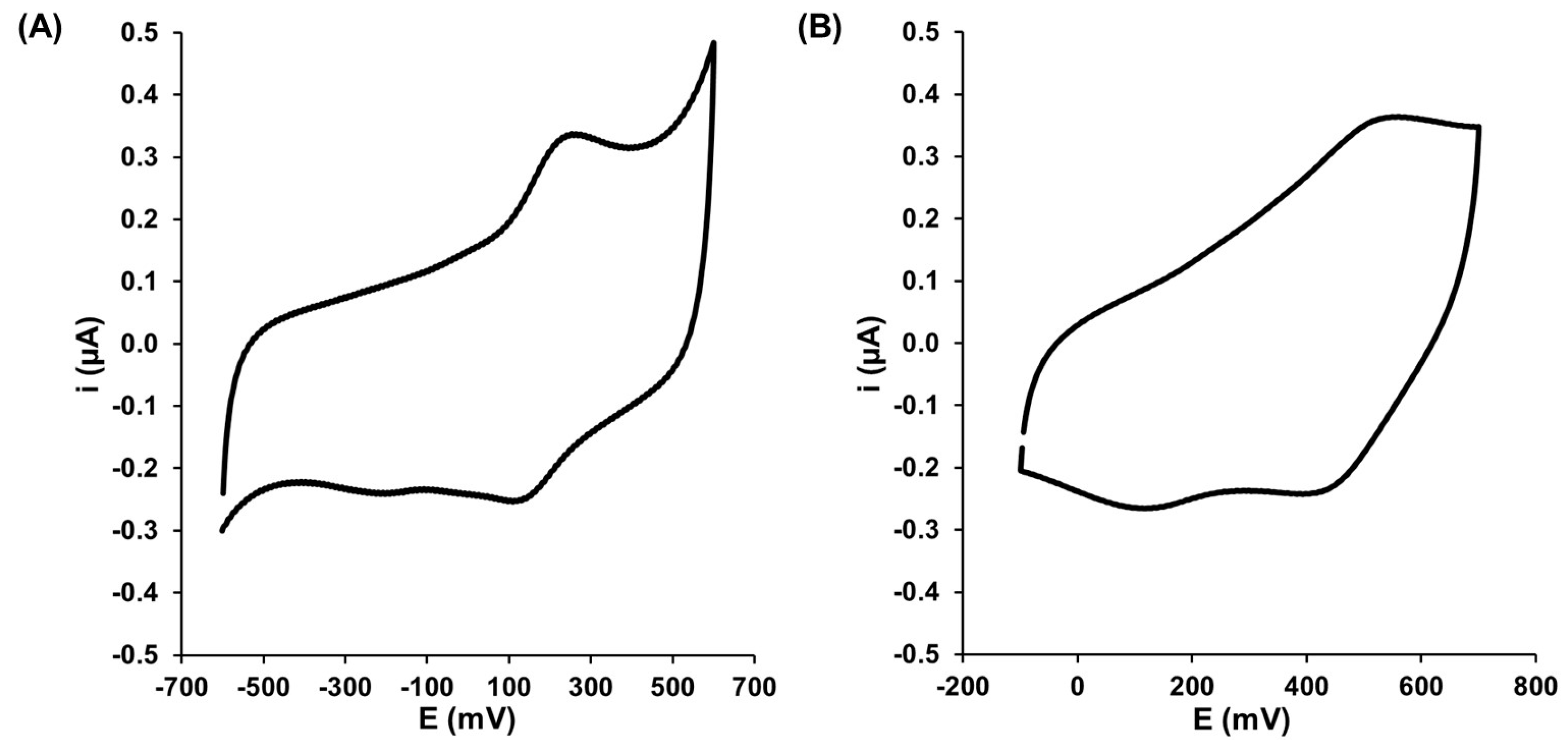
3.1. Electrochemical Characterization of Gold Electrode Modified with HS-CXCOOH in the Presence of Dopamine
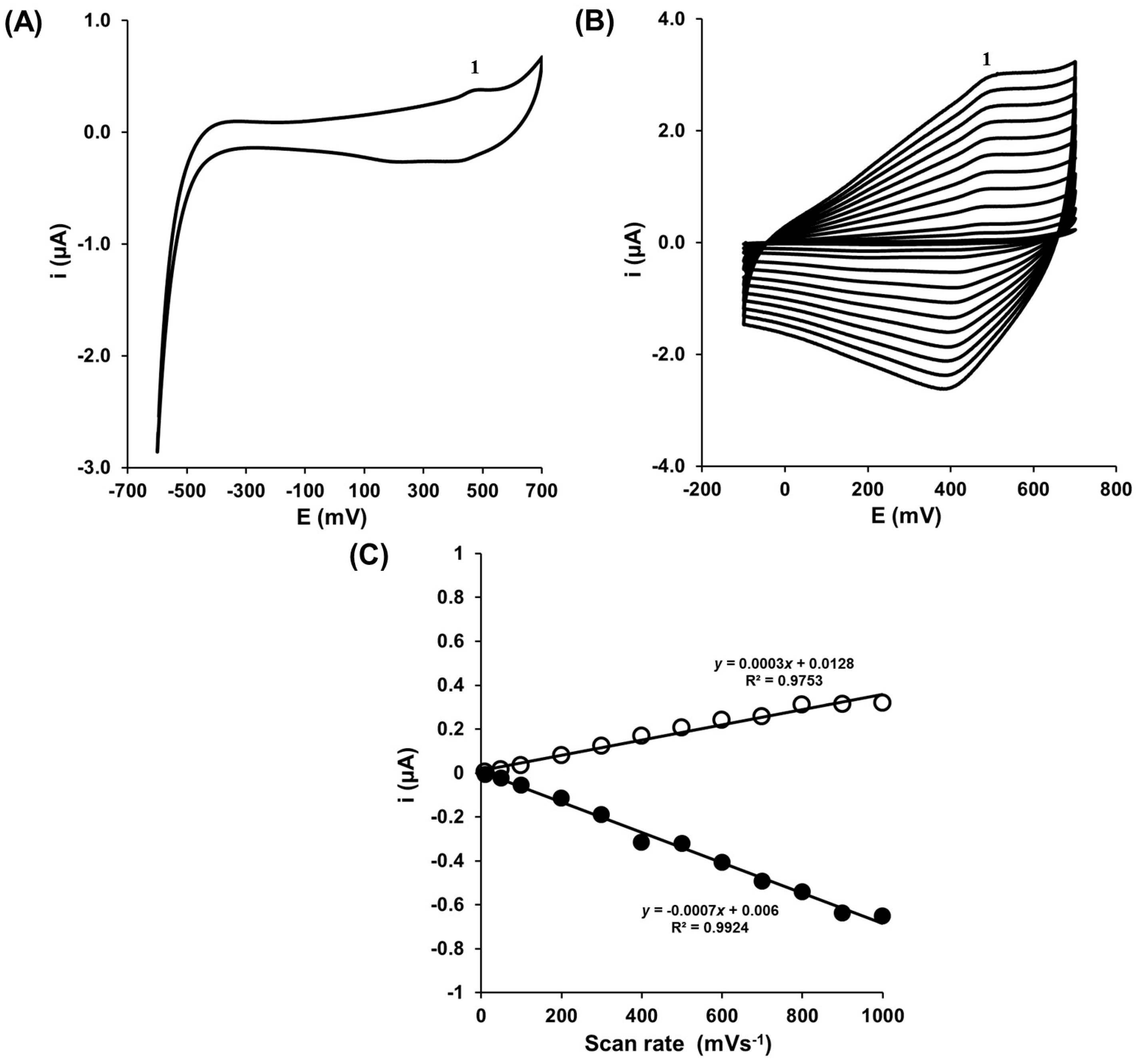
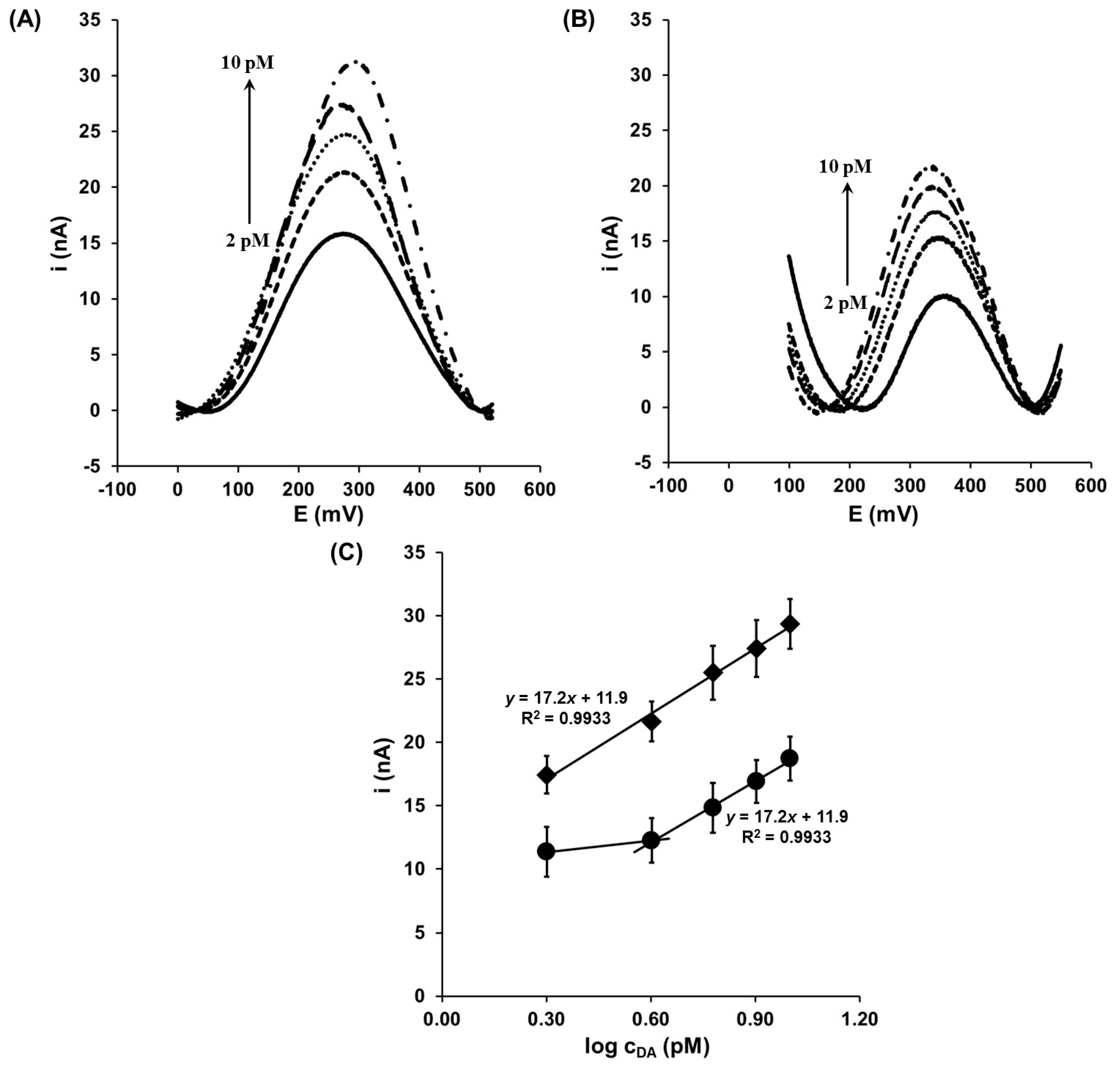
3.2. Determination of Dopamine in the Presence of Buffer Solution
3.3. Determination of Dopamine in the Presence of Interferents
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Álvarez-Martos, I.; Ferapontova, E.E. Electrochemical label-free aptasensor for specific analysis of dopamine in serum in the presence of structurally related neurotransmitters. Anal. Chem. 2016, 88, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Grace, A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience 1991, 41, 1–24. [Google Scholar] [CrossRef]
- Obeso, J.; Rodriguez-Oroz, M.C.; Geotz, C.G.; Marin, C.; Kordower, J.H.; Rodriguez, M.; Hirsch, E.C.; Farrer, M.; Schapira, A.H.; Haliday, G. Missing pieces in the Parkinson's disease puzzle. Nat. Med. 2010, 16, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Hornykiewicz, O.; Kish, S.J. Inverse relationship between brain noradrenaline level and dopamine loss in Parkinson Disease—A possible neuroprotective role for noradrenaline. Arch. Neurol. 2006, 63, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Panholzer, T.J.; Beyer, J.; Lichtwald, K. Coupled-column liquid chromatographic analysis of catecholamines, serotonin, and metabolites in human urine. Clin. Chem. 1999, 45, 262–268. [Google Scholar] [PubMed]
- Chauveau, J.; Fert, V.; Morel, A.M.; Delaage, M.A. Rapid and specific enzyme immunoassay of serotonin. Clin. Chem. 1991, 37, 1178–1184. [Google Scholar] [PubMed]
- Middelkoop, C.M.; Dekker, G.A.; Kraayenbrink, A.A.; Popp-Snijders, C. Platelet-poor plasma serotonin in normal and preeclamptic pregnancy. Clin. Chem. 1993, 39, 1675–1678. [Google Scholar] [PubMed]
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, P.T.; Hart, J.B.; Adams, R.N. Voltammetry in brain tissue—A new neurophysiological measurement. Brain Res. 1973, 55, 209–213. [Google Scholar] [CrossRef]
- Rozniecka, E.; Jonsson-Niedziołka, M.; Celebanska, A.; Niedziolka-Jonsson, J.; Opallo, M. Selective electrochemical detection of dopamine in a microfluidic channel on carbon nanoparticulate electrodes. Analyst 2014, 129, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Lin, Y.; Yu, P.; Su, L.; Mao, L. Laccase-catalyzed oxidation and intramolecular cyclization of dopamine: A new method for selective determination of dopamine with laccase/carbon nanotube-based electrochemical biosensors. Electrochim. Acta 2007, 52, 4144–4152. [Google Scholar] [CrossRef]
- Du, J.; Yue, R.; Ren, F.; Yao, Z.; Jiang, F.; Yang, P.; Du, Y. Novel graphene flowers modified carbon fibers for simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2014, 53, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Viry, L.; Derré, A.; Poulin, P.; Kuhn, A. Discrimination of dopamine and ascorbic acid using carbon nanotube fiber microelectrodes. Phys. Chem. Chem. Phys. 2010, 12, 9993–9995. [Google Scholar] [CrossRef] [PubMed]
- Ferapontova, E.E.; Castillo, J.; Gorton, L. Bioelectrocatalytic properties of lignin peroxidase from Phanerochaete chrysosporium in reactions with phenols, catechols and lignin-model compounds. Biochim. Biophys. Acta 2006, 1760, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Farjami, E.; Campos, R.; Nielsen, J.S.; Gothelf, K.V.; Kjems, J.; Ferapontova, E.E. RNA aptamer-based electrochemical biosensor for selective and label-free analysis of dopamine. Anal. Chem. 2013, 85, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martos, I.; Camposa, R.; Ferapontova, E.E. Surface state of the dopamine RNA aptamer affects specific recognition and binding of dopamine by the aptamer-modified electrodes. Analyst 2015, 140, 4089–4096. [Google Scholar] [CrossRef] [PubMed]
- Neri, P.; Sessler, J.L.; Wang, M.-X. Calixarenes and Beyond; Springer Int.: Cham, Switzerland, 2016; pp. 1–1056. [Google Scholar]
- Nimse, S.B.; Kim, T. Biological applications of functionalized calixarenes. Chem. Soc. Rev. 2013, 42, 366–386. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Chawla, H.M.; Singh, S.P. Calixarenes as sensor materials for recognition and separation of metal ions. ARKIVOC 2007, 2007, 172–200. [Google Scholar]
- Ludwig, R.; Dzung, N.T.K. Calixarene-based molecules for cation recognition. Sensors 2002, 2, 397–416. [Google Scholar] [CrossRef]
- Kurzątkowska, K.; Sayin, S.; Yilmaz, M.; Radecka, H.; Radecki, J. Calix[4]arene derivatives as dopamine hosts in electrochemical sensors. Sens. Actuators 2015, 218, 111–121. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, M.; Liu, X.; Zhou, J.; Xie, J.; Diao, G. Self-assembled thiolated calix[n]arene (n = 4, 6, 8) films on gold electrodes and application for electrochemical determination dopamine. Electrochim. Acta 2014, 136, 301–309. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Lu, K.; Ye, B. A simple but highly sensitive and selective calixarene-based voltammetric sensor for serotonin. Electrochim. Acta 2013, 87, 756–762. [Google Scholar] [CrossRef]
- Umezawa, Y.; Aoki, H. Ion channel sensors based on artificial receptors. Anal. Chem. 2004, 76, 321A–326A. [Google Scholar] [PubMed]
- Radecka, H.; Szymańska, I.; Pietraszkiewicz, M.; Pietraszkiewicz, O.; Aoki, H.; Umezawa, Y. Intramolecular ion-channel sensors using gold electrodes immobilized with macrocyclic polyamines for voltammetric detection of adenine nucleotides. Anal. Chem. 2005, 50, 85–102. [Google Scholar]
- Radecki, J.; Szymańska, I.; Bulgariu, L.; Pietraszkiewicz, M. Covalent and embedment immobilization of macrocyclic polyamines on gold electrodes and their voltammetric responses towards ethene dicarboxylic acids. Electrochim. Acta 2006, 51, 2289–2297. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, M.H.; Mutihac, L.; Vicens, J.; Kim, J.S. Host-guest sensing by calixarenes on the surfaces. Chem. Soc. Rev. 2012, 41, 1173–1190. [Google Scholar] [CrossRef] [PubMed]
- Poduval, R.; Kurzątkowska, K.; Stobiecka, M.; Dehaen, W.F.A.; Dehaen, W.; Radecka, H.; Radecki, J. Systematic study of interaction of the neutral form of anilines with undecylcalix[4]resorcinarene derivatives by means of potentiometry. Supramol. Chem. 2010, 22, 413–419. [Google Scholar] [CrossRef]
- Doyle, R.; Breslin, C.B.; Rooney, A.D. A Simple But highly selective electrochemical sensor for dopamine. Chem. Biochem. Eng. Q. 2009, 23, 93–98. [Google Scholar]
- Wang, F.; Chia, C.L.; Yua, B.; Ye, B. Simultaneous voltammetric determination of dopamine and uric acid based on Langmuir-Blodgett fil of calixarene modified glassy carbon electrode. Sens. Actuators 2015, 221, 1586–1593. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Ma, H.; Li, B.; Ye, B. A simple but highly sensitive and selective electrochemical sensor for determination of dopamine based on Langmuir-Blodgett film of calix[4]resorcinarene. J. Electrochem. Soc. 2016, 163, H367–H372. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Li, X.; Li, C.; Qin, L.; Sun, J.; Kang, S.-Z. Preparation of per-hydroxylated pillar[5]arene decorated graphene and its electrochemical behavior. Electrochim. Acta 2016, 210, 720–728. [Google Scholar] [CrossRef]
- Muguruma, H.; Inoue, Y.; Inoue, H.; Ohsawa, T. Electrochemical study of dopamine at electrode fabricated by cellulose-assisted aqueous dispersion of long-length carbon nanotube. J. Phys. Chem. 2016, 120, 12284–12292. [Google Scholar] [CrossRef]
- Hawley, M.D.; Tatawawadi, S.V.; Piekarski, S.; Adams, R.N. Electrochemical studies of the oxidation pathways of catecholamine. J. Am. Chem. Soc. 1967, 89, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.E.; Kristensen, E.W.; May, L.J.M.; Wiedemann, D.J.; Wigthman, R.M. Fast-scan voltammetry of biogenic amines. Anal. Chem. 1988, 60, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Jiang, G.; Jiang, G.; Chen, T.; Zhan, W.; Li, X.; Wu, S.; Tian, S. Detection of dopamine on a mercapto-terminated hexanuclear Fe(III) cluster modified gold electrode. Talanta 2015, 137, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.E.; Brett, C.M.A. Simple and efficient epinephrine sensor based on carbon nanotube modified carbon film electrodes. Anal. Lett. 2013, 46, 1379–1393. [Google Scholar] [CrossRef]
- Hu, M.; Fritsch, I. Application of electrochemical redox cycling: Toward differentiation of dopamine and norepinephrine. Anal. Chem. 2016, 88, 5574–5578. [Google Scholar] [CrossRef] [PubMed]
- Jagiło, A.; Grabowska, I.; Radecka, H.; Sulima, M.; Marszałek, I.; Wysłouch-Cieszyńska, A.; Dehaen, W.; Radecki, J. Redox active dipyrromethene—Cu(II) monolayer for oriented immobilization of His-tagged RAGE domains—The base of electrochemical biosensor for determination of Aβ16–23. Electroanalysis 2013, 25, 1185–1193. [Google Scholar] [CrossRef]
- Malecka, K.; Grabowska, I.; Radecki, J.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Radecka, H. Voltammetric detection of a specific DNA Ssequence of Avian Influenza Virus H5N1 using HS-ssDNA probe deposited onto gold electrode. Electroanalysis 2012, 24, 439–446. [Google Scholar] [CrossRef]
- Patel, A.N.; Tan, S.-Y.; Miller, T.S.; Macpherson, J.V.; Unwin, P.R. Comparison and reappraisal of carbon electrodes for the voltammetric detection of dopamine. Anal. Chem. 2013, 85, 11755–11764. [Google Scholar] [CrossRef] [PubMed]
- Šnejdárková, M.; Poturnayová, A.; Rybár, P.; Lhoták, P.; Himl, M.; Flídrová, K.; Hianki, T. High sensitive calixarene-based sensor for detection of dopamine by electrochemical and acoustic methods. Bioelectrochemistry 2010, 80, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-Q.; Zhang, Q.-L.; Zheng, J.-N.; Lv, Z.-Y.; Wei, J.; Wang, A.-J.; Feng, J.-J. Simultaneous determination of dopamine and uric acid in the presence of ascorbic acid using Pt nanoparticles supported on reduced graphene oxide. Electrochem. Acta 2014, 115, 109–115. [Google Scholar] [CrossRef]
- Tan, S.; Radi, S.R.; Gaudier, F.; Evans, R.A.; Rivera, A.; Kirk, K.A.; Parks, D.A. Physiologic levels of uric acid inhibit xanthine oxidase in human plasma. Pediatr. Res. 1993, 34, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- AlZahra’a Alatraktchi, F.; Bakmand, T.; Dimaki, M.; Svendsen, W.E. Novel membrane-based electrochemical sensor for real-time bio-applications. Sensors 2014, 14, 22128–22139. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.-S.; Zhang, H.-L.; Jina, C.-M. Electrocatalysis and voltammetric determination of dopamine at a calix[4]arene crown-4 ether modified glassy carbon electrode. Electroanalysis 2007, 19, 496–501. [Google Scholar] [CrossRef]

| Electrode Modification | Measuring Technique | Interferences | Calibration Range (M) | Detection Limit (M) | Reference |
|---|---|---|---|---|---|
| Au/Cys/Dopa_RNA aptamer | CA | CH, NEP, EP, L-DOPA, DOPAC, Tyr, HMP | 0.1–5.0 × 10−6 | 1.0 × 10−7 | [15] |
| Au/Cys/Dopa_RNA aptamer | CV, CA | EP, NEP, L-DOPA, DOPAC, CH, Tyr, HMP | 0.1–2.0 × 10−6 | 1.0 × 10−7 | [16] |
| Au-Cys/HS-Dopa_RNA aptamer | CA | CT, NEP, L-DOPA | 0.1–1.0 × 10−6 | 6.2 × 10−8 | [1] |
| Au-PEI membrane | CA | None | 3.1 × 10−12–1.7 × 10−2 | 3.1 × 10−12 | [46] |
| GCE-CACE | CV | AA | 0.2 × 10−6–1.0 × 10−3 | 3.4 ×10−6 | [47] |
| Au/TC8A | DPV | None | 1.0 × 10−6–1.0 × 10−3 | 5.0 × 10−7 | [22] |
| Au/TC6A | 3.0 × 10−6–1.0 × 10−3 | 8.0 × 10−7 | |||
| Au/TC4A | 5.0 × 10−6–1.0 × 10−3 | 1.0 × 10−6 | |||
| GCE/CUCR-LB | DPV | AA | 0.08–6.0 × 10−6 | 2.0 × 10−8 | [30] |
| GCE/C4A-LB | DPV | UA, AA | 5.0 × 10−8–1.0 × 10−5 | 1.5 × 10−8 | [31] |
| GCE/RGO-P5A | DPV | UA, AA | 1.0–90 × 10−6 | 2.0 × 10−7 | [32] |
| Au-SCX-COOH/MET | SWV with addition of redox marker | UA, AA | 4.9–12.2 × 10−12 | 4.9 × 10−12 | [21] |
| Au-CX-COOH/MET | SWV | NEP, EP, UA, AA | 2.0–10.0 × 10−12 | 1.4 × 10−12 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzątkowska, K.; Sayin, S.; Yilmaz, M.; Radecka, H.; Radecki, J. Gold Electrodes Modified with Calix[4]arene for Electrochemical Determination of Dopamine in the Presence of Selected Neurotransmitters. Sensors 2017, 17, 1368. https://doi.org/10.3390/s17061368
Kurzątkowska K, Sayin S, Yilmaz M, Radecka H, Radecki J. Gold Electrodes Modified with Calix[4]arene for Electrochemical Determination of Dopamine in the Presence of Selected Neurotransmitters. Sensors. 2017; 17(6):1368. https://doi.org/10.3390/s17061368
Chicago/Turabian StyleKurzątkowska, Katarzyna, Serkan Sayin, Mustafa Yilmaz, Hanna Radecka, and Jerzy Radecki. 2017. "Gold Electrodes Modified with Calix[4]arene for Electrochemical Determination of Dopamine in the Presence of Selected Neurotransmitters" Sensors 17, no. 6: 1368. https://doi.org/10.3390/s17061368
APA StyleKurzątkowska, K., Sayin, S., Yilmaz, M., Radecka, H., & Radecki, J. (2017). Gold Electrodes Modified with Calix[4]arene for Electrochemical Determination of Dopamine in the Presence of Selected Neurotransmitters. Sensors, 17(6), 1368. https://doi.org/10.3390/s17061368






