The Electrostatically Formed Nanowire: A Novel Platform for Gas-Sensing Applications
Abstract
:1. Introduction
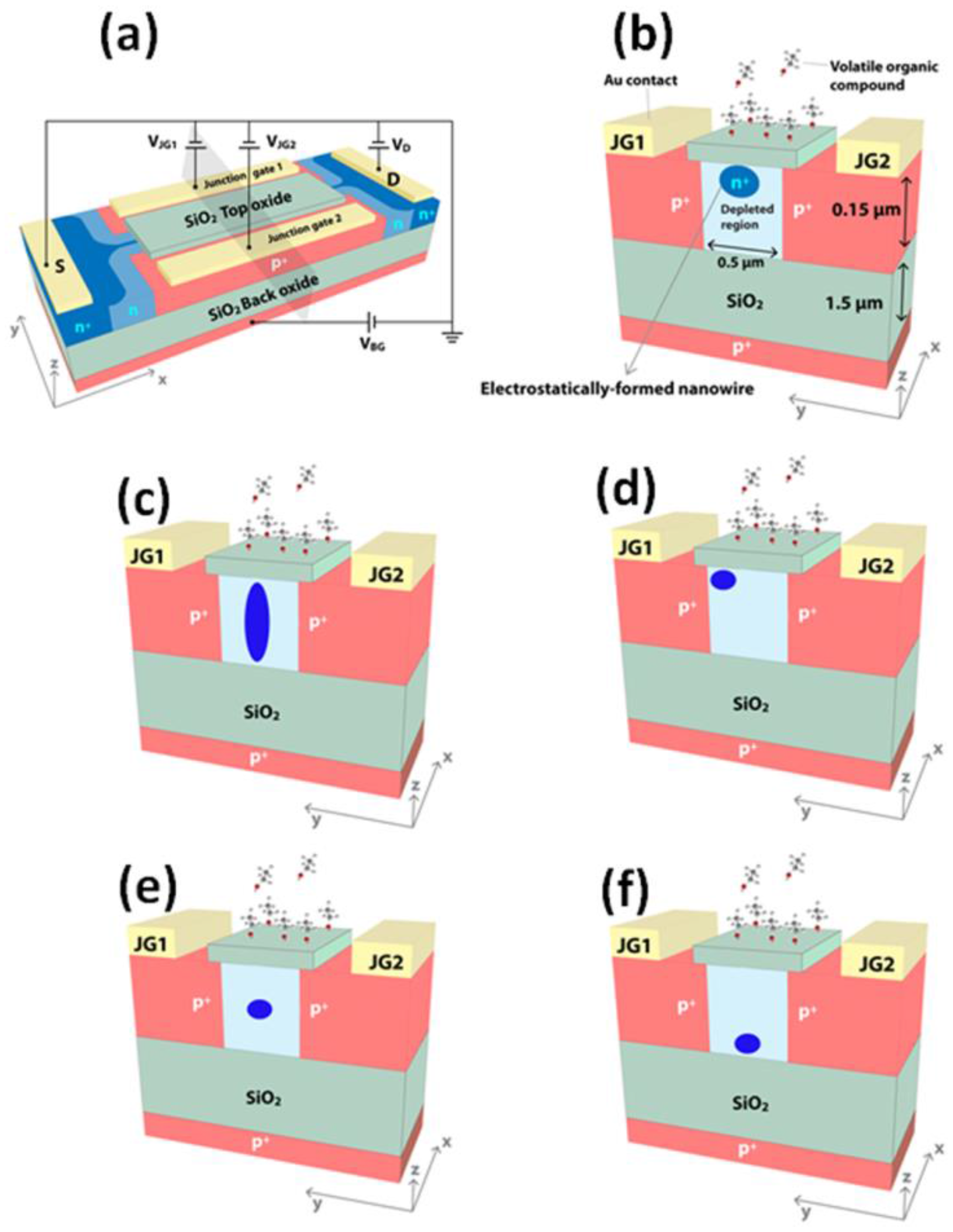
2. Design Considerations of EFN Devices
3. Fabrication of EFN Devices
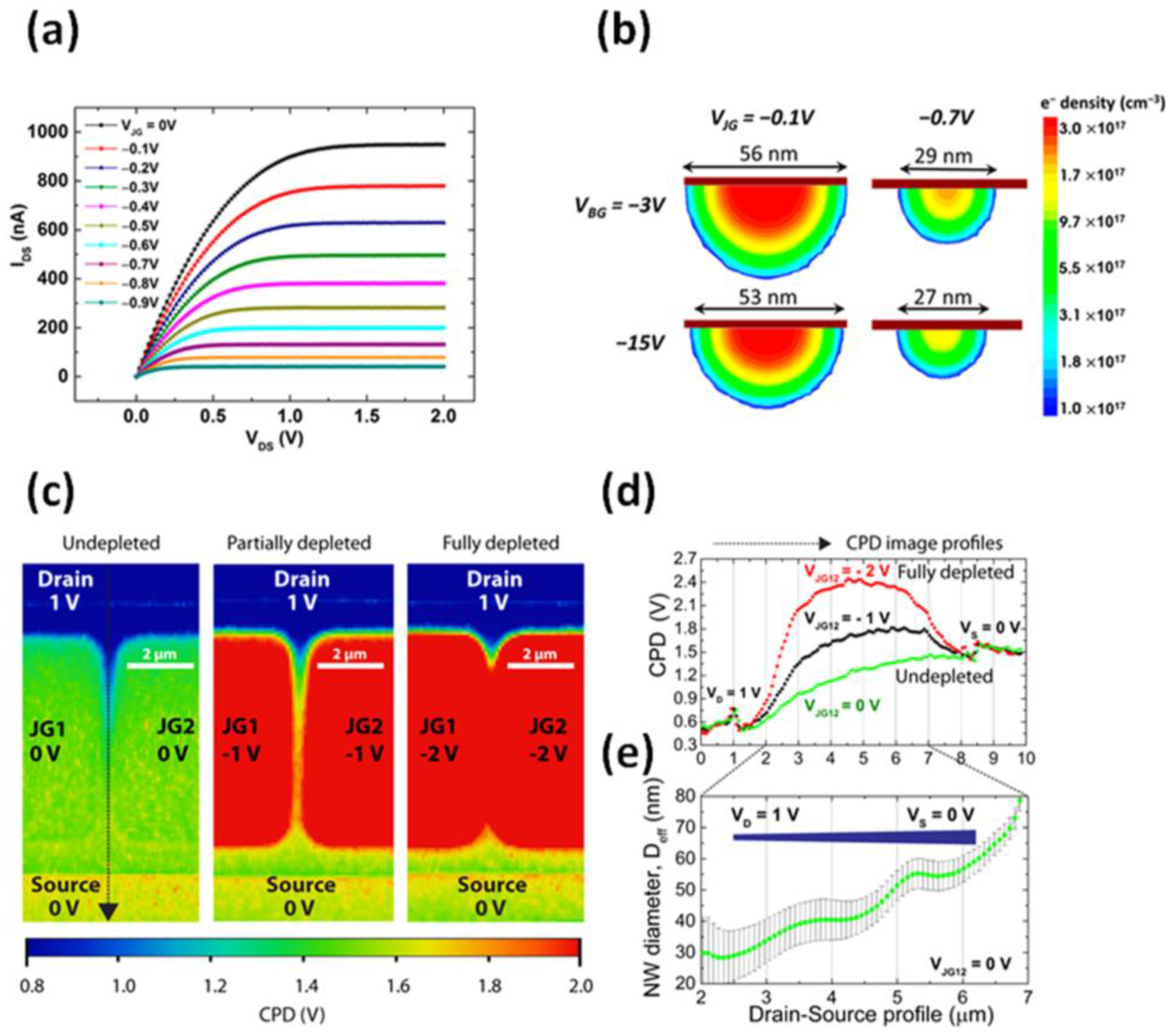
4. Electrical Performance and Characterization of EFN Devices
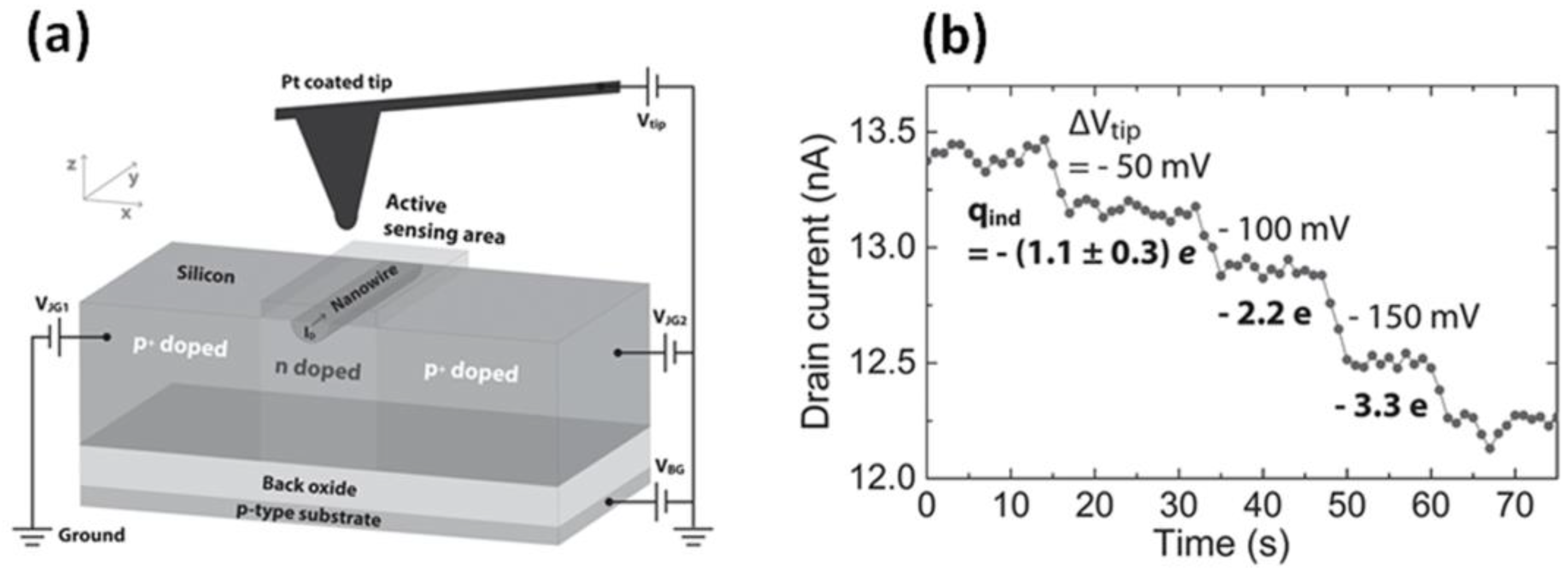
5. Gas-Sensing with an EFN Gas Sensor: Sensor Response, Tunable Sensitivity and Dynamic Range, and LOD
6. Conclusions
Conflicts of Interest
References
- Longo, G. Cancer Biomarkers: Detected Twice for Good Measure. Nat. Nanotechnol. 2014, 9, 959–960. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Singha, S.; Clemente-casares, X.; Tsai, S.; Yang, Y. Nanoparticle-Based Immunotherapy for Cancer. ACS Nano 2015, 9, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Ndieyira, J.W.; Kappeler, N.; Logan, S.; Cooper, M.A.; Abell, C.; McKendry, R.A.; Aeppli, G. Surface-Stress Sensors for Rapid and Ultrasensitive Detection of Active Free Drugs in Human Serum. Nat. Nanotechnol. 2014, 9, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lerner, M.B.; Matsunaga, F.; Han, G.H.; Hong, S.J.; Xi, J.; Crook, A.; Perez-Aguilar, J.M.; Park, Y.W.; Saven, J.G.; Liu, R.; et al. Scalable Production of Highly Sensitive Nanosensors Based on Graphene Functionalized with a Designed G Protein-Coupled Receptor. Nano Lett. 2014, 14, 2709–2714. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing Lung Cancer in Exhaled Breath Using Gold Nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.S.; Reddy, K.; Zhong, Z.; Fan, X. Graphene Nanoelectronic Heterodyne Sensor for Rapid and Sensitive Vapour Detection. Nat. Commun. 2014, 5, 4376. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xu, L.; Duan, G.; Li, T.; Zhang, H.; Li, Y.; Wang, Y.; Wang, Y.; Cai, W. Fast-Response, Sensitivitive and Low-Powered Chemosensors by Fusing Nanostructured Porous Thin Film and IDEs-Microheater Chip. Sci. Rep. 2013, 3, 1669. [Google Scholar] [CrossRef] [PubMed]
- Yamagiwa, H.; Sato, S.; Fukawa, T.; Ikehara, T.; Maeda, R.; Mihara, T.; Kimura, M. Detection of Volatile Organic Compounds by Weight-Detectable Sensors Coated with Metal-Organic Frameworks. Sci. Rep. 2014, 4, 6247. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, M.C.; Ahmad, H.; Wang, D.; Heath, J.R. Highly Ordered Nanowire Arrays on Plastic Substrates for Ultrasensitive Flexible Chemical Sensors. Nat. Mater. 2007, 6, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Engel, Y.; Elnathan, R.; Pevzner, A.; Davidi, G.; Flaxer, E.; Patolsky, F. Supersensitive Detection of Explosives by Silicon Nanowire Arrays. Angew. Chem. 2010, 49, 6830–6835. [Google Scholar] [CrossRef] [PubMed]
- Shehada, N.; Brönstrup, G.; Funka, K.; Christiansen, S.; Leja, M.; Haick, H. Ultrasensitive Silicon Nanowire for Real-World Gas Sensing: Noninvasive Diagnosis of Cancer from Breath Volatolome. Nano Lett. 2015, 15, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cancilla, J.C.; Torrecilla, J.S.; Haick, H. Artificial Sensing Intelligence with Silicon Nanowires for Ultraselective Detection in the Gas Phase. Nano Lett. 2014, 14, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.; Havivi, E.; Shacham, R.; Hahamy, E.; Leibovich, R.; Pevzner, A.; Krivitsky, V.; Davivi, G.; Presman, I.; Elnathan, R.; et al. Supersensitive Fingerprinting of Explosives by Chemically Modified Nanosensors Arrays. Nat. Commun. 2014, 5, 4195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cai, B.; Tang, Q.; Tong, Y.; Liu, Y. One-Dimensional Nanostructure Field-Effect Sensors for Gas Detection. Sensors 2014, 14, 13999–14020. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Paronyan, T.M.; Pigos, E.M.; Harutyunyan, A.R. Enhanced Gas Sensing in Pristine Carbon Nanotubes under Continuous Ultraviolet Light Illumination. Sci. Rep. 2012, 2, 343. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Sorescu, D.C.; Star, A. Photoinduced Charge Transfer and Acetone Sensitivity of Single-Walled Carbon Nanotube—Titanium Dioxide Hybrids. J. Am. Chem. Soc. 2013, 135, 9015–9022. [Google Scholar] [CrossRef] [PubMed]
- Zanolli, Z.; Leghrib, R.; Felten, A.; Pireaux, J.J.; Llobet, E.; Charlier, J.C. Gas Sensing with Au-Decorated Carbon Nanotubes. ACS Nano 2011, 5, 4592–4599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Chaturvedi, P.; Saho, P.; Jha, P. Effect of Single Wall Carbon Nanotube Networks on Gas Sensor Response and Detection Limit. Sens. Actuators B Chem. 2017, 240, 1134–1140. [Google Scholar] [CrossRef]
- Yeow, J.T.W.; Wang, Y. A Review of Carbon Nanotubes-Based Gas Sensors. J. Sens. 2009, 2009, 493904. [Google Scholar]
- Cho, S.-Y.; Yoo, H.-W.; Kim, J.Y.; Jung, W.-B.; Jin, M.L.; Kim, J.-S.; Jeon, H.-J.; Jung, H.-T. High-Resolution P-Type Metal Oxide Semiconductor Nanowire Array as an Ultrasensitive Sensor for Volatile Organic Compounds. Nano Lett. 2016, 16, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Katwal, G.; Paulose, M.; Rusakova, I.A.; Martinez, J.E.; Varghese, O.K. Rapid Growth of Zinc Oxide Nanotube-Nanowire Hybrid Architectures and Their Use in Breast Cancer Related Volatile Organics Detection. Nano Lett. 2016, 16, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhu, J.; Yao, P.; Li, J.; Bi, H.; Wang, X. Synthesis of ZnO-Ag Hybrids and Their Gas-Sensing Performance toward Ethanol. Ind. Eng. Chem. Res. 2015, 54, 8947–8953. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Meglen, R.M.; Peterson, D.; Sluiter, J. Metal Oxide Sensor Arrays for the Detection, Differentiation, and Quantification of Volatile Organic Compounds at Sub-Parts-per-Million Concentration Levels. Sens. Actuators B Chem. 2006, 115, 322–329. [Google Scholar] [CrossRef]
- Leidinger, M.; Sauerwald, T.; Conrad, T.; Reimringer, W.; Ventura, G.; Schütze, A. Selective Detection of Hazardous Indoor VOCs Using Metal Oxide Gas Sensors. Procedia Eng. 2014, 87, 1449–1452. [Google Scholar] [CrossRef]
- Koren, E.; Elias, G.; Boag, A.; Hemesath, E.R.; Lauhon, L.J.; Rosenwaks, Y. Direct Measurement of Individual Deep Traps in Single Silicon Nanowires. Nano Lett. 2011, 11, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Shalev, G.; Landman, G.; Amit, I.; Rosenwaks, Y.; Levy, I. Specific and Label-Free Femtomolar Biomarker Detection with an Electrostatically Formed Nanowire Biosensor. NPG Asia Mater. 2013, 5, e41. [Google Scholar] [CrossRef]
- Akarvardar, K.; Cristoloveanu, S.; Gentil, P. Analytical Modeling of the Two-Dimensional Potential Distribution and Threshold Voltage of the SOI Four-Gate Transistor. IEEE Trans. Electron. Devices 2006, 53, 2569–2577. [Google Scholar] [CrossRef]
- Akarvardar, K.; Cristoloveanu, S.; Gentil, P.; Schrimpf, R.D.; Blalock, B.J. Depletion-All-Around Operation of the SOI Four-Gate Transistor. IEEE Trans. Electron. Devices 2007, 54, 323–331. [Google Scholar] [CrossRef]
- Dufrene, B.; Akarvardar, K.; Cristoloveanu, S.; Blalock, B.J.; Gentil, P.; Kolawa, E.; Mojarradi, M.M. Investigation of the Four-Gate Action in G-FETs. IEEE Trans. Electron. Devices 2004, 51, 1931–1935. [Google Scholar] [CrossRef]
- Henning, A.; Swaminathan, N.; Godkin, A.; Shalev, G.; Amit, I.; Rosenwaks, Y. Tunable Diameter Electrostatically-Formed Nanowire for High Sensitivity Gas Sensing. Nano Res. 2015, 8, 600–610. [Google Scholar] [CrossRef]
- Vandamme, L.K.J.; Li, X.; Rigaud, D. 1/F Noise in MOS Devices, Mobility or Number Fluctuations? IEEE Trans. Electron. Devices 1994, 41, 1936–1945. [Google Scholar] [CrossRef]
- Swaminathan, N.; Henning, A.; Vaknin, Y.; Shimanovich, K.; Godkin, A.; Shalev, G.; Rosenwaks, Y. Dynamic Range Enhancement Using the Electrostatically Formed Nanowire Sensor. ACS Sens. 2016, 1, 688–695. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; O’Boyle, M.P.; Wickramasinghe, H.K. Kelvin Probe Force Microscopy. Appl. Phys. Lett. 1991, 58, 2921. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Hu, J.; Tian, C.; Shen, Y.R. Structure of the Submonolayer of Ethanol Adsorption on a Vapor/fused Silica Interface Studied with Sum Frequency Vibrational Spectroscopy. J. Phys. Chem. A 2015, 119, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Pan, V.H.; Tao, T.; Zhou, J.; Maciel, G.E. Hydrogen Bonding between Acetone and Silica Gel, as Studied by NMR. J. Phys. Chem. B 1999, 103, 6930–6943. [Google Scholar] [CrossRef]
- Jeziorowski, H.; Knözinger, H.; Meye, W.; Muller, D. H-Bonding on Solid Oxide Surfaces. Infra-red spectroscopic study of the adsorption of alcohols on silica and alumina. J. Chem. Soc. Faraday Trans. 1973, 69, 1744–1758. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Fry, B. Nanotechnology-Enabled Sensors; Springer: New York, NY, USA, 2008; p. 17. [Google Scholar]
- Henning, A.; Molotskii, M.; Swaminathan, N.; Vaknin, Y.; Godkin, A.; Shalev, G.; Rosenwaks, Y. Electrostatic Limit of Detection of Nanowire-Based Sensors. Small 2015, 11, 4931–4937. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalev, G. The Electrostatically Formed Nanowire: A Novel Platform for Gas-Sensing Applications. Sensors 2017, 17, 471. https://doi.org/10.3390/s17030471
Shalev G. The Electrostatically Formed Nanowire: A Novel Platform for Gas-Sensing Applications. Sensors. 2017; 17(3):471. https://doi.org/10.3390/s17030471
Chicago/Turabian StyleShalev, Gil. 2017. "The Electrostatically Formed Nanowire: A Novel Platform for Gas-Sensing Applications" Sensors 17, no. 3: 471. https://doi.org/10.3390/s17030471
APA StyleShalev, G. (2017). The Electrostatically Formed Nanowire: A Novel Platform for Gas-Sensing Applications. Sensors, 17(3), 471. https://doi.org/10.3390/s17030471




