Label-Free Detection of Cancer Biomarkers Using an In-Line Taper Fiber-Optic Interferometer and a Fiber Bragg Grating
Abstract
:1. Introduction
2. In-Line Taper Fiber-Optic Interferometer and FBG
2.1. Principle and Analysis
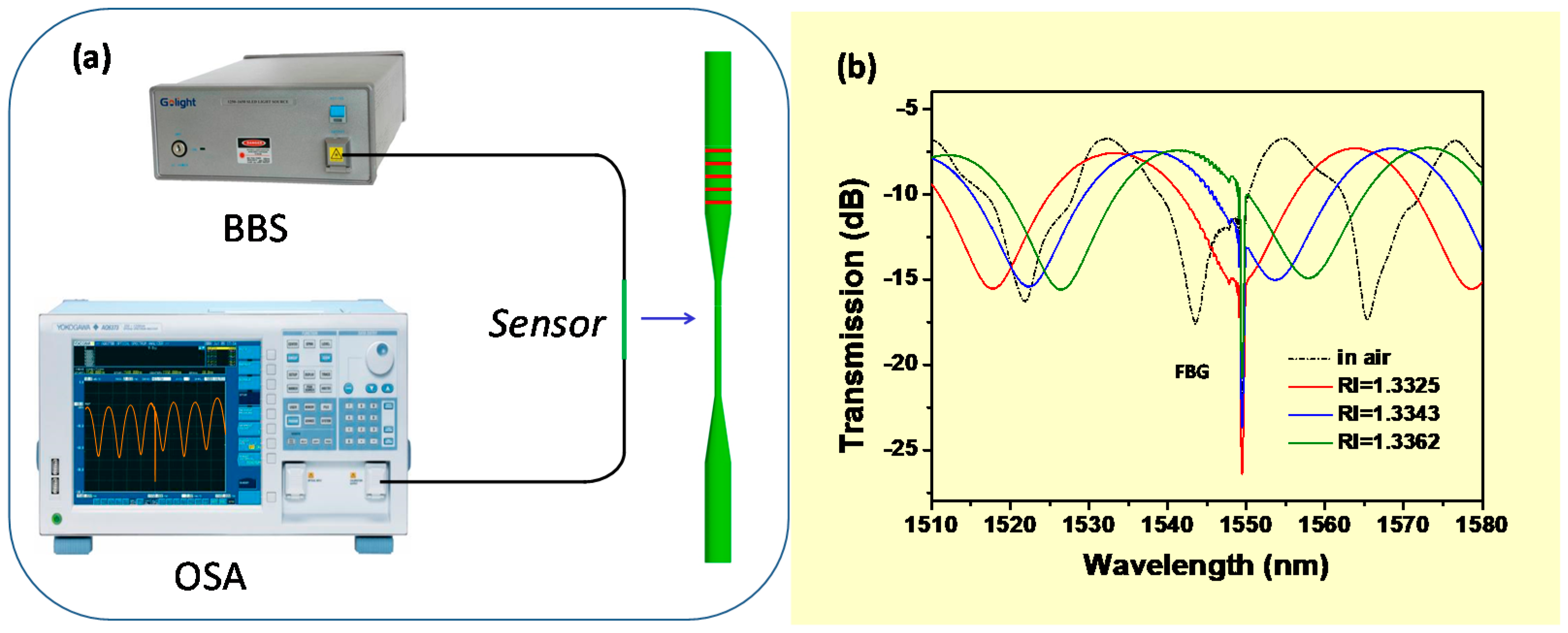
2.2. Sensor Fabrication and Characterization
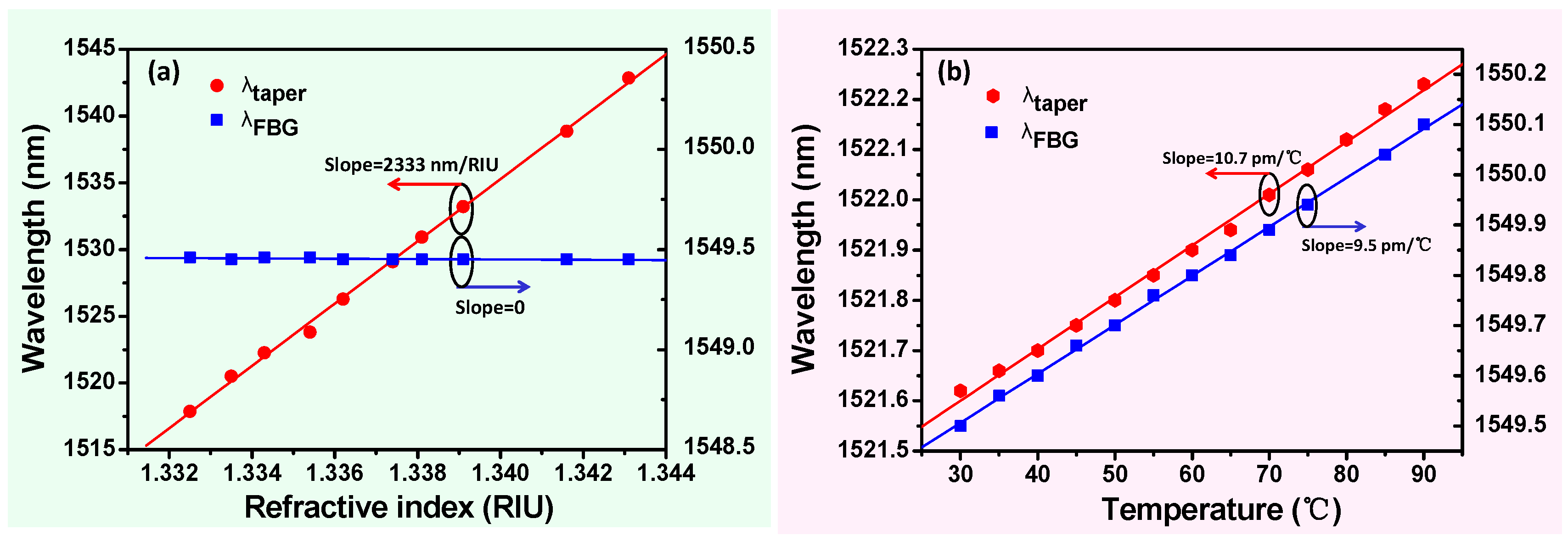
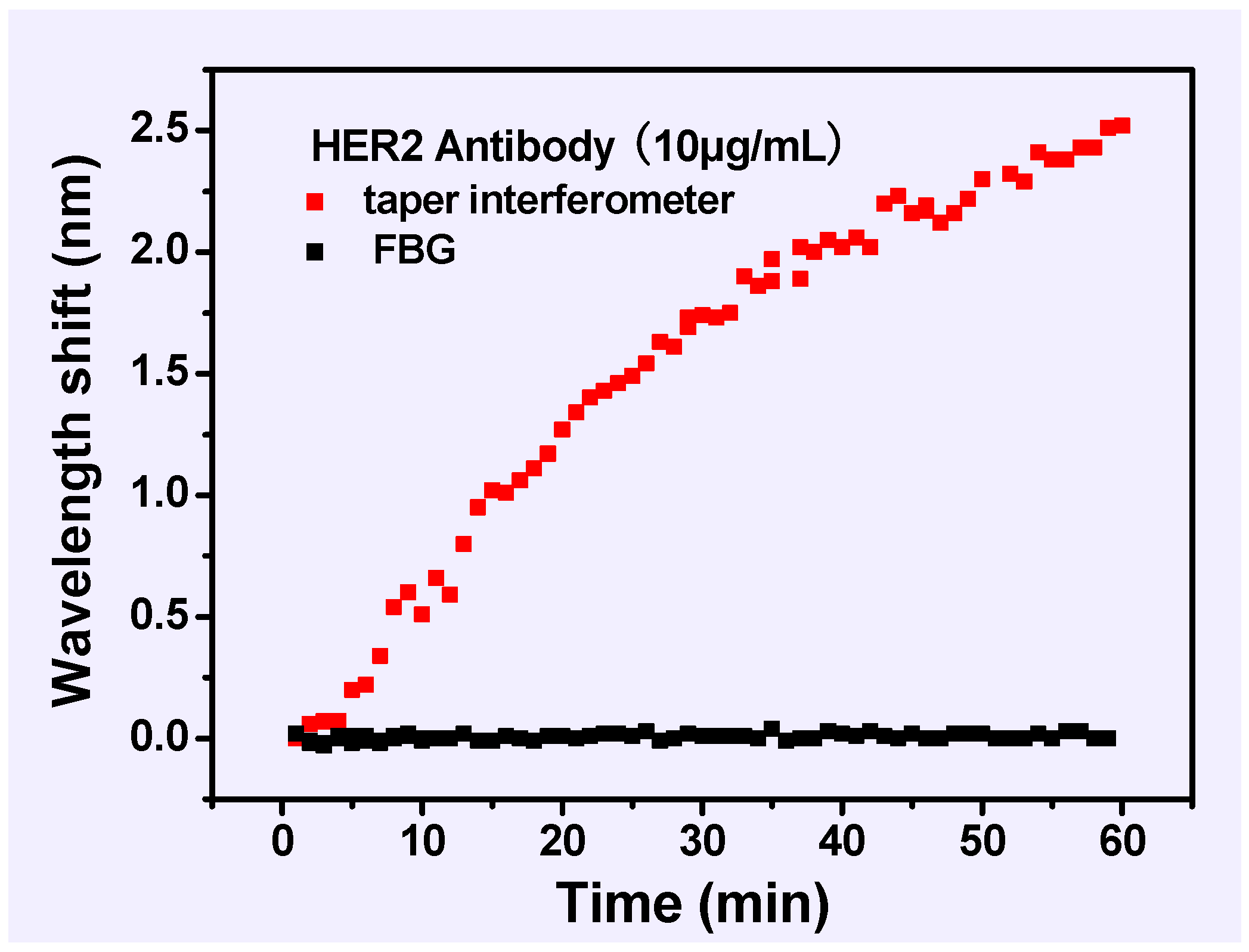
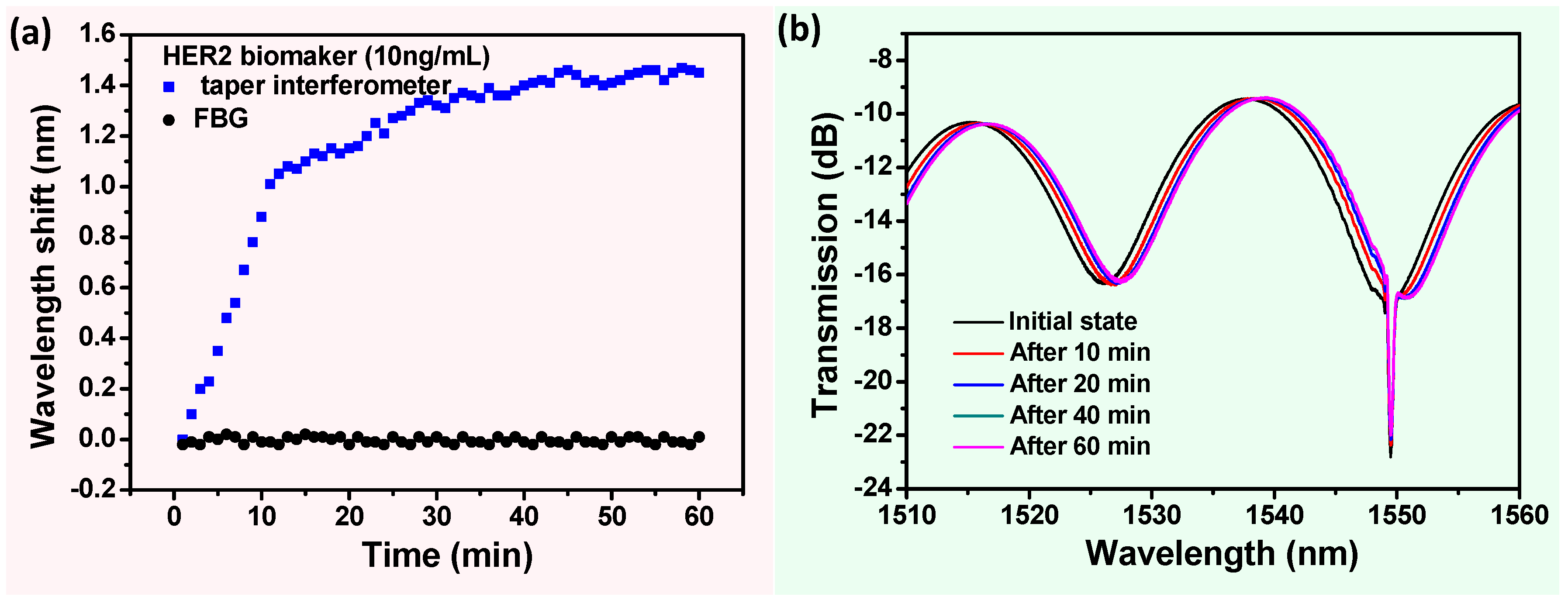
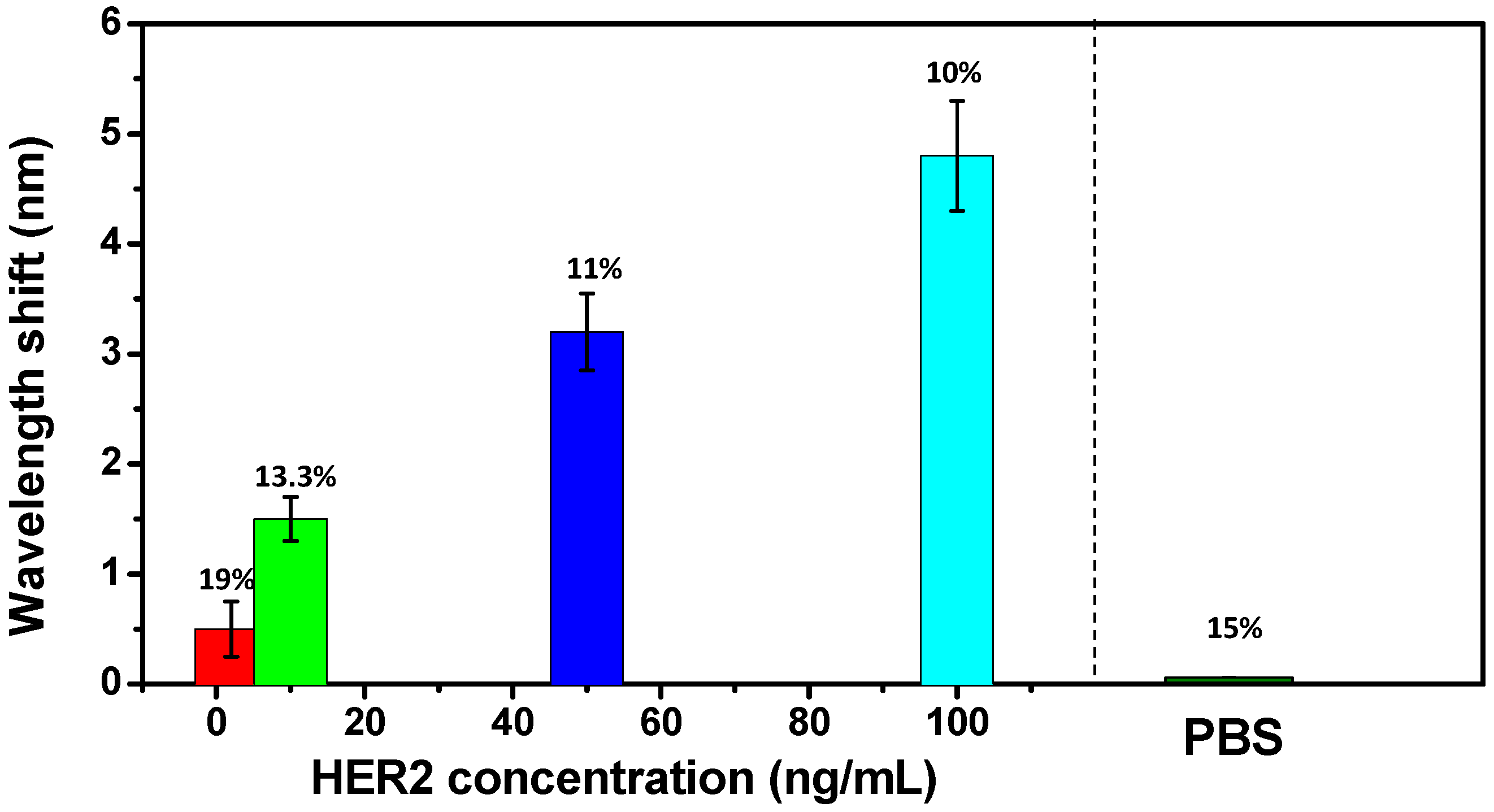
3. Results and Discussion
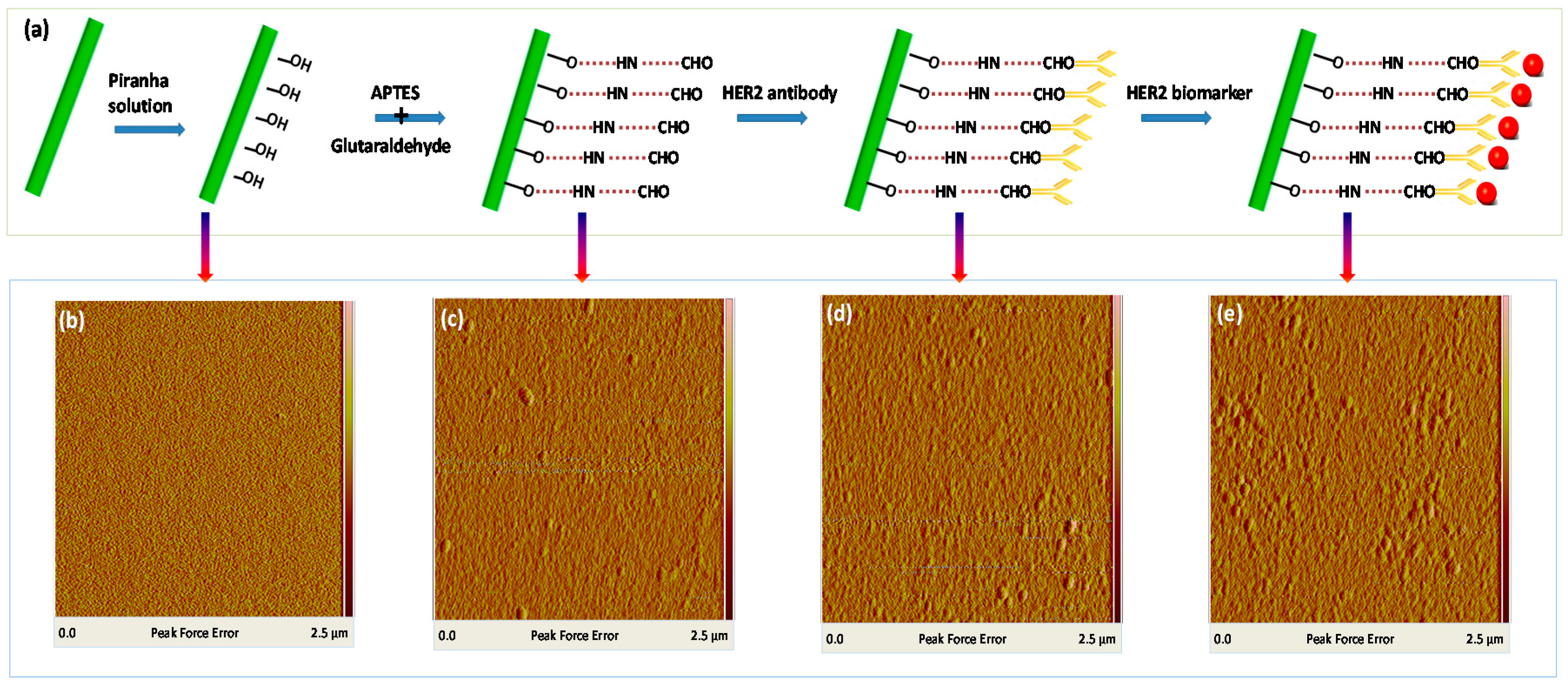
- (1)
- Treatment with a freshly prepared piranha solution (H2SO4/H2O2 = 70:30, v/v), which is processed for 30 min, rinsed with de-ionized (DI) water, and dried with nitrogen
- (2)
- Reaction of the fiber surface 5% 3-aminopropyl-triethoxysilane (APTES) in ethyl alcohol, which is processed for 15 min, thoroughly rinsed with DI water, and dried.
- (3)
- Crosslinking reaction of 2.5% glutaraldehyde solution for 30 min, which is thoroughly rinsed with DI water and dried.
- (4)
- Immobilization of the HER2 antibody with a concentration of 10 µg/mL, which is processed for 1 h and thoroughly rinsed with DI water.
- (5)
- Immersion in 1% bovine serum albumin (BSA) solution in phosphate buffer saline (PBS) buffer for 5 min to block the non-reacted sites to minimize nonspecific adsorption.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bohunicky, B.; Mousa, S.A. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1–10. [Google Scholar] [PubMed]
- Ribaut, C.; Loyez, M.; Larrieu, J.-C.; Chevineau, S.; Lambert, P.; Remmelink, M.; Wattiez, R.; Caucheteur, C. Cancer biomarker sensing using packaged plasmonic optical fiber gratings: Towards in vivo diagnosis. Biosens. Bioelectron. 2016, 92, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W., Jr.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, G.; Dandekar, S.; Rong, H.M.; Ramos, L.; Peng, H.J.; Seshadri, R.; Slamon, D.J. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: A direct comparison of fluorescence in situ hybridization and immunohistochemistry. J. Clin. Oncol. 2000, 18, 3651–3664. [Google Scholar] [CrossRef] [PubMed]
- Owens, M.A.; Horten, B.C.; Da Silva, M.M. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin. Breast Cancer 2004, 5, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hempen, C.; Karst, U. Labeling strategies for bioassays. Anal. Bioanal. Chem. 2006, 384, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Huang, Y.; Zhou, J.; Guo, T.; Guan, B.O. Understanding the pH-dependent interaction between graphene oxide and single-stranded DNA through a fiber-optic interferometer. Phys. Chem. Chem. Phys. 2016, 18, 32266–32271. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Ding, M.F.; Guo, T.; Hu, D.J.; Cao, Y.Y.; Jin, L.; Guan, B.-O. A fiber-optic sensor for neurotransmitters with ultralow concentration: Near-infrared plasmonic electromagnetic field enhancement using raspberry-like meso-SiO2 nanospheres. Nanoscale 2017, 9, 14929–14936. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, D.N.; Yang, M.; Cheng, J.; Li, J. In-line Mach-Zehnder Interferometer and FBG with Pd film for simultaneous hydrogen and temperature detection. Sens. Actuators B Chem. 2014, 202, 893–896. [Google Scholar] [CrossRef]
- Kersey, A.D.; Davis, M.A.; Patrick, H.J.; LeBlanc, M.; Koo, K.P.; Askins, C.G.; Putnam, M.A.; Friebele, E.J. Fiber grating sensors. J. Lightware Technol. 1997, 15, 1442–1463. [Google Scholar] [CrossRef]
- Sun, L.P.; Li, J.; Tan, Y.Z.; Gao, S.; Jin, L.; Guan, B.O. Bending effect on modal interference in a fiber taper and sensitivity enhancement for refractive index measurement. Opt. Express 2013, 21, 26714–26720. [Google Scholar] [CrossRef] [PubMed]
- Dandridge, A. Fiber optic sensors based on the Mach–Zehnder and Michelson interferometers. In Fiber Optic Sensors: An Introduction for Engineers and Scientists; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- Tan, Y.Z.; Sun, L.P.; Jin, L.; Li, J.; Guan, B.O. Microfiber Mach-Zehnder interferometer based on long period grating for sensing applications. Opt. Express 2013, 21, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.P.; Jin, L.; Sun, L.P.; Li, J.; Ran, Y.; Guan, B.O. Highly Sensitive Fiber Taper Interferometric Hydrogen Sensors. IEEE Photon. J. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Sun, D.; Guo, T.; Ran, Y.; Huang, Y.; Guan, B.O. In-situ DNA hybridization detection with a reflective microfiber grating biosensor. Biosens. Bioelectron. 2014, 61, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Tiwari, U.; Kumar, P.; Mishra, V.; Jain, S.C.; Singh, N.; Bharadwaj, L.M. Immobilization of enzyme on long period grating fibers for sensitive glucose detection. Biosens. Bioelectron. 2012, 33, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Li, K.W.; Liu, G.G.; Wu, Y.H.; Hao, P.; Zhou, W.C.; Zhang, Z.Q. Gold nanoparticle amplified optical microfiber evanescent wave absorption biosensor for cancer biomarker detection in serum. Talanta 2014, 120, 419–424. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Ran, Y.; Wang, G. Label-Free Detection of Cancer Biomarkers Using an In-Line Taper Fiber-Optic Interferometer and a Fiber Bragg Grating. Sensors 2017, 17, 2559. https://doi.org/10.3390/s17112559
Sun D, Ran Y, Wang G. Label-Free Detection of Cancer Biomarkers Using an In-Line Taper Fiber-Optic Interferometer and a Fiber Bragg Grating. Sensors. 2017; 17(11):2559. https://doi.org/10.3390/s17112559
Chicago/Turabian StyleSun, Dandan, Yang Ran, and Guanjun Wang. 2017. "Label-Free Detection of Cancer Biomarkers Using an In-Line Taper Fiber-Optic Interferometer and a Fiber Bragg Grating" Sensors 17, no. 11: 2559. https://doi.org/10.3390/s17112559
APA StyleSun, D., Ran, Y., & Wang, G. (2017). Label-Free Detection of Cancer Biomarkers Using an In-Line Taper Fiber-Optic Interferometer and a Fiber Bragg Grating. Sensors, 17(11), 2559. https://doi.org/10.3390/s17112559




