A Light-Up Probe for Detection of Adenosine in Urine Samples by a Combination of an AIE Molecule and an Aptamer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
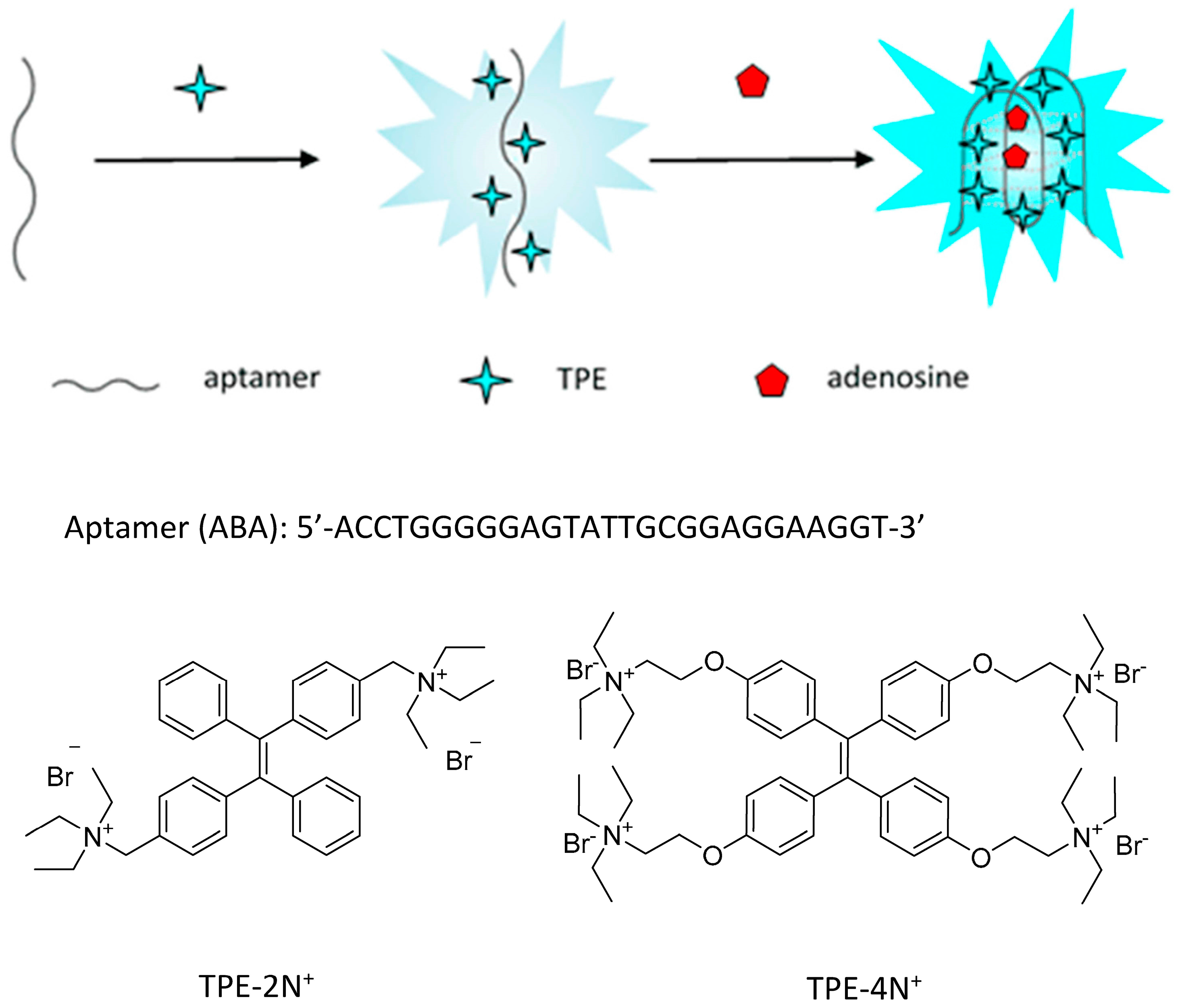
2.3. The Emission Behavior of (TPE-2N+) in Tris Buffer
2.4. The Viability of (TPE-2N+) + ABA Probe
- (1)
- 10 mM Tris-HCl;
- (2)
- 10 mM Tris-HCl + 10 μM (TPE-2N+);
- (3)
- 10 mM Tris-HCl + 10 μM (TPE-2N+) + 0.1 μM ABA;
- (4)
- 10 mM Tris-HCl + 10 μM (TPE-2N+) + 0.1 μM A;
- (5)
- 10 mM Tris-HCl + 10 μM (TPE-2N+) + 0.1 μM ABA + 0.1 μM A.
2.5. Optimization of the Concentration and the Concentration Ratio of the (TPE-2N+) and the ABA
2.6. The Calibration Curve of Adenosine Detection
2.7. Specificity of (TPE-2N+)-ABA Detection System
2.8. Analytical Application to Urine Samples
3. Results and Discussions
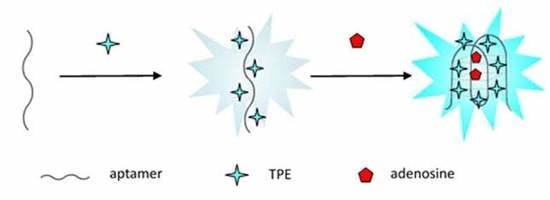
3.1. The Detection Principle
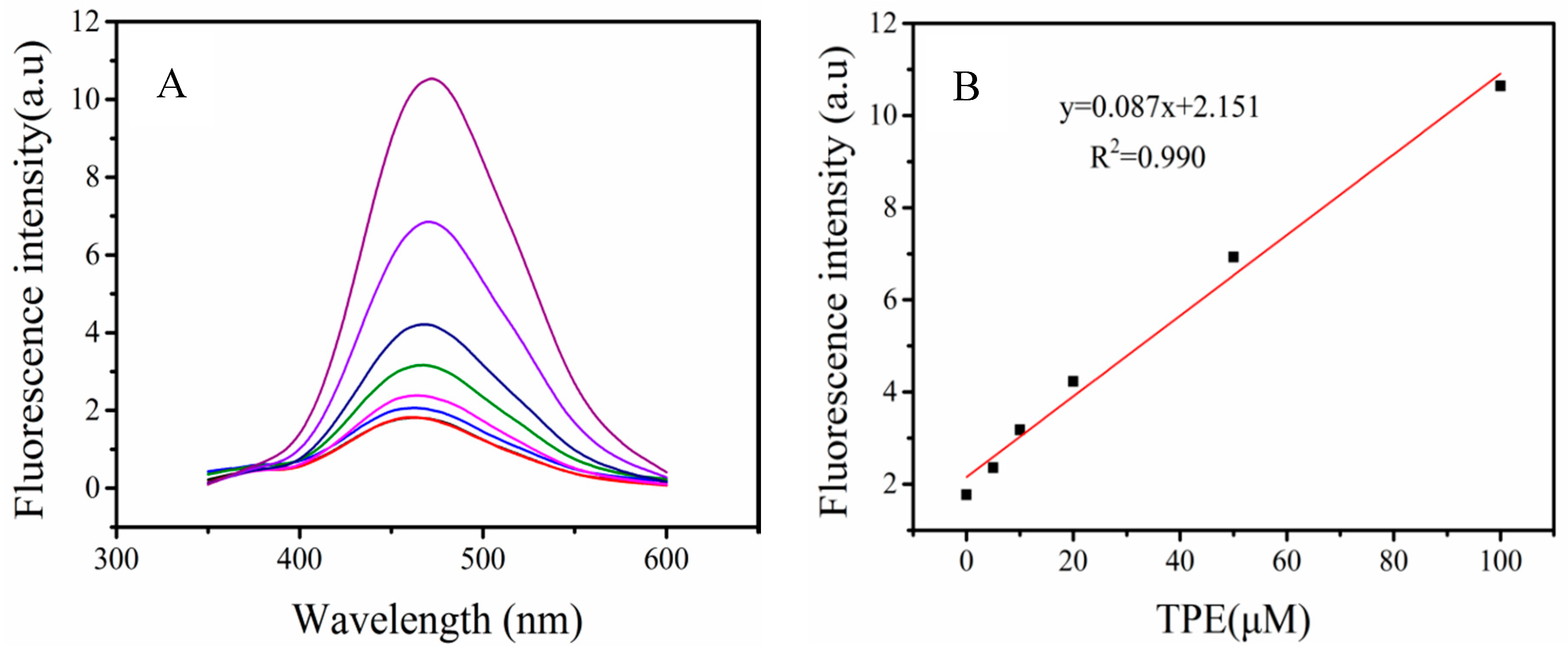
3.2. The Emission Behavior of TPE Fluorogen in Tris Buffer
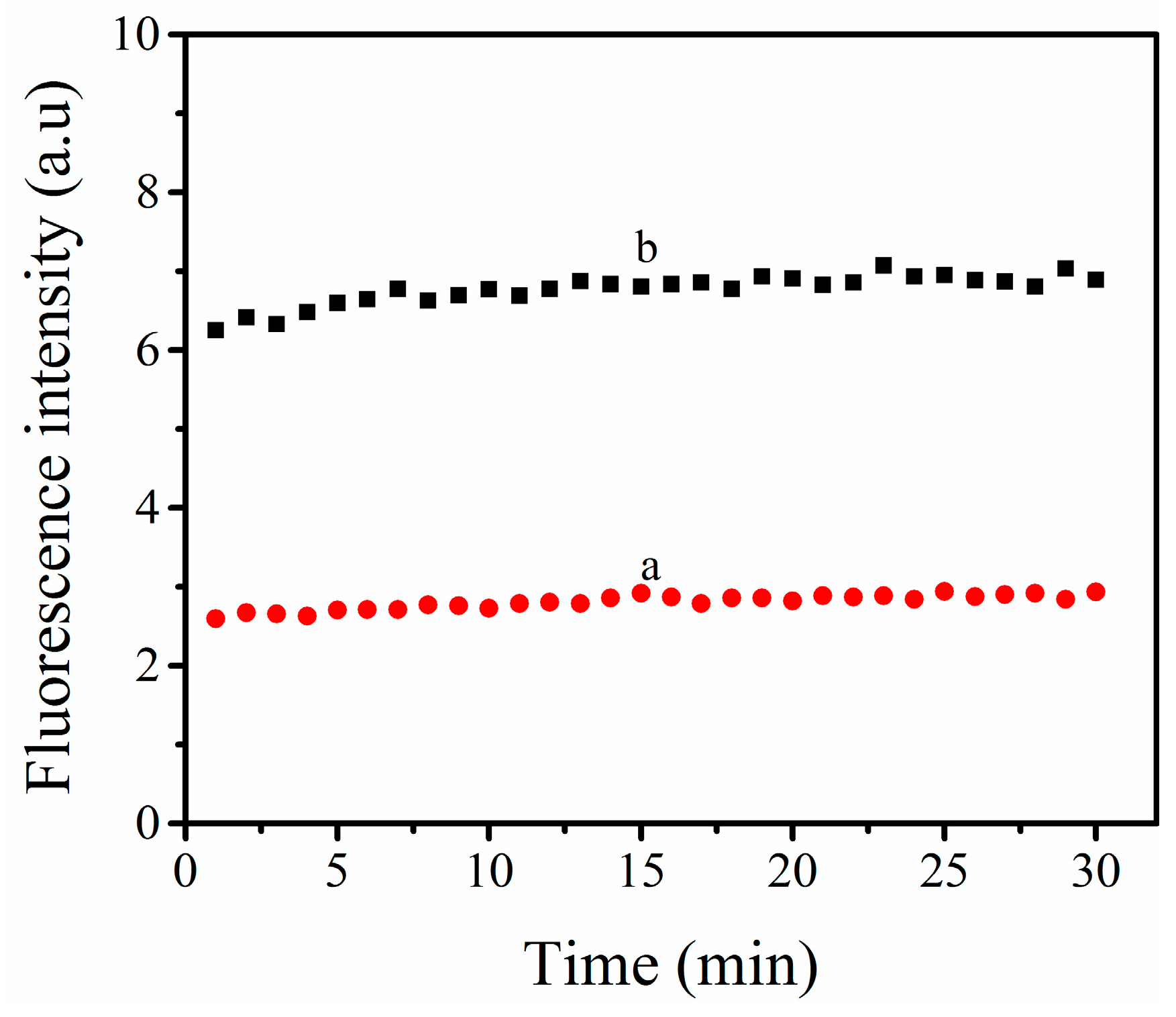
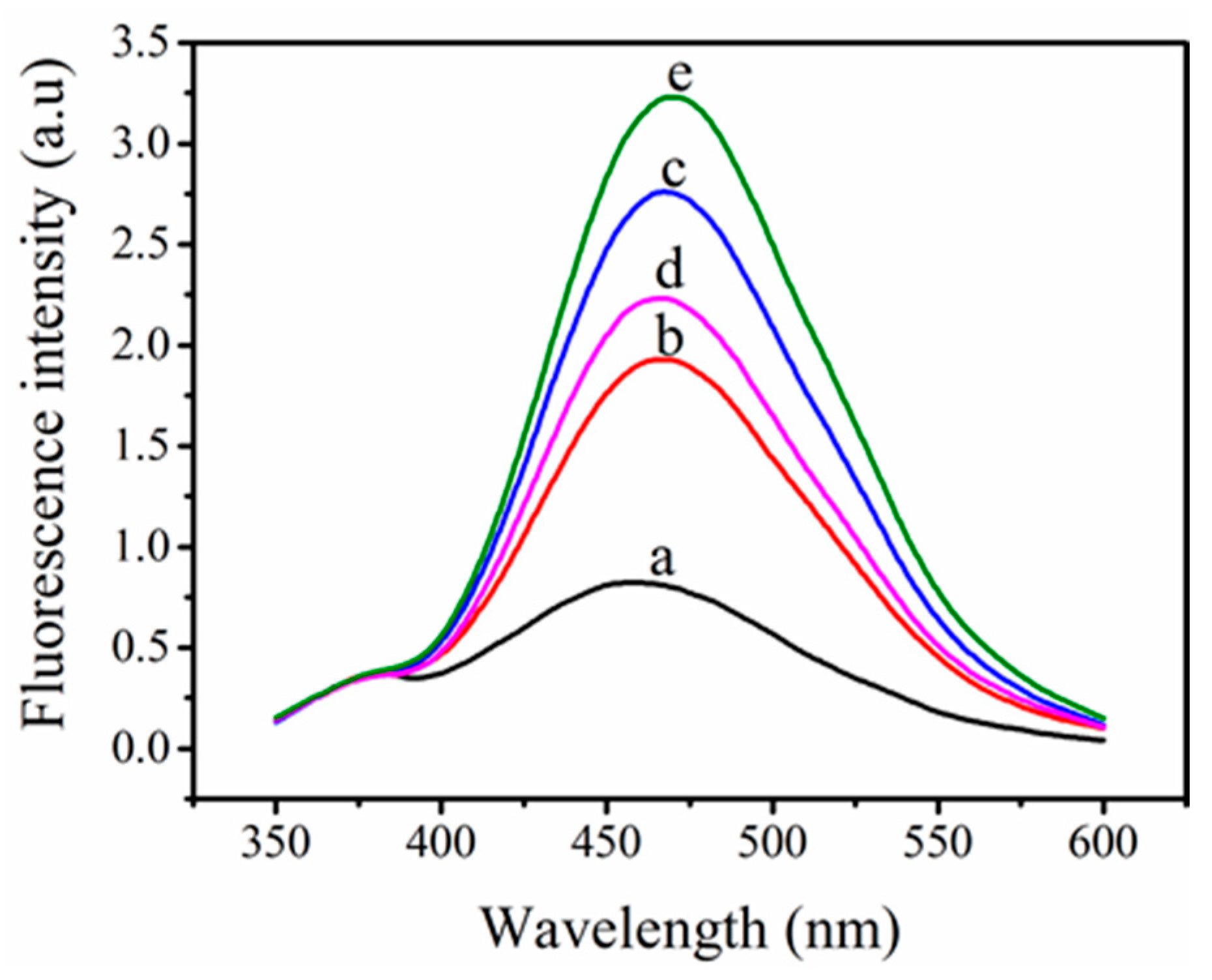
3.3. The Viability of (TPE-2N+) + ABA Probe
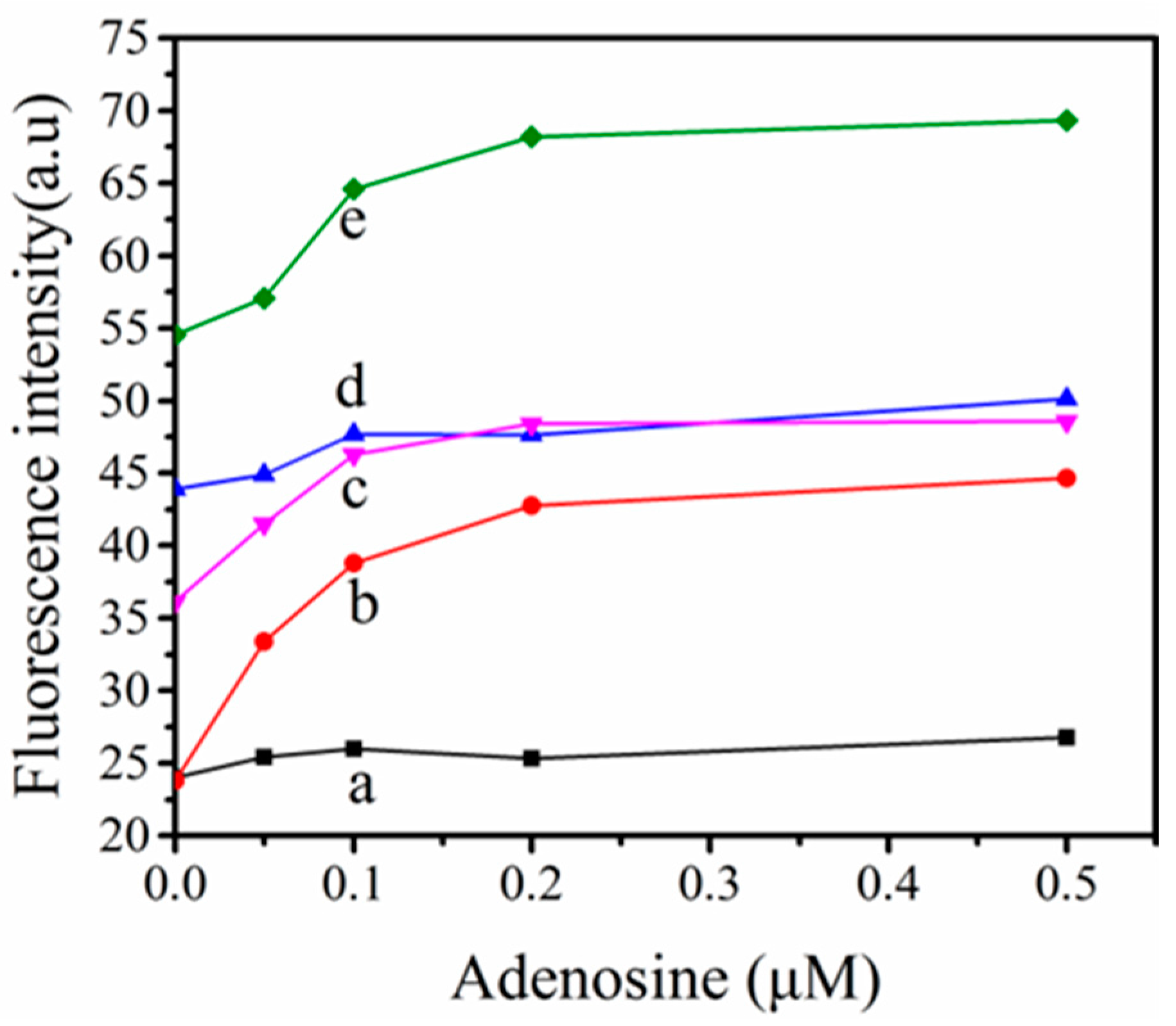
3.4. Optimization of the Optimal Ratio of TPE-2N+ and ABA Concentration
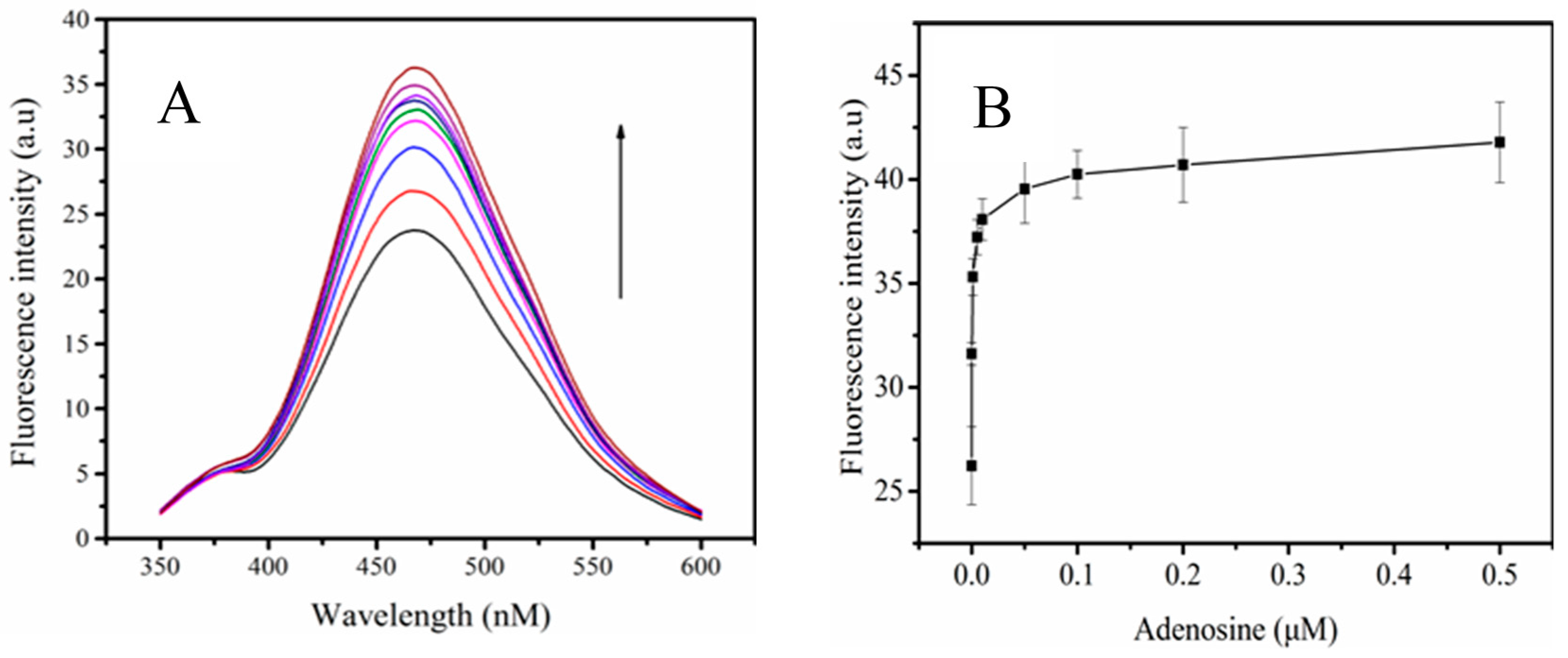
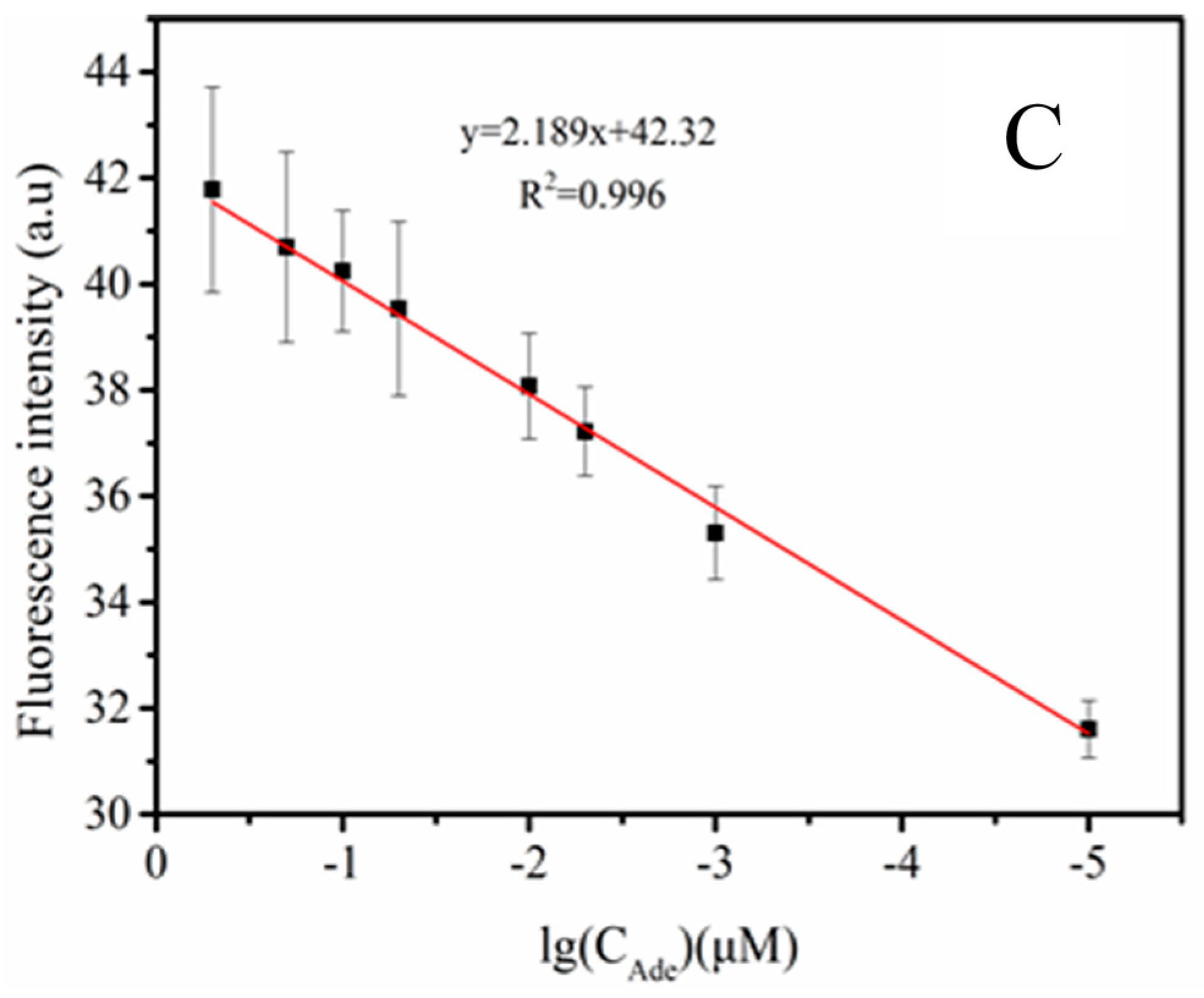
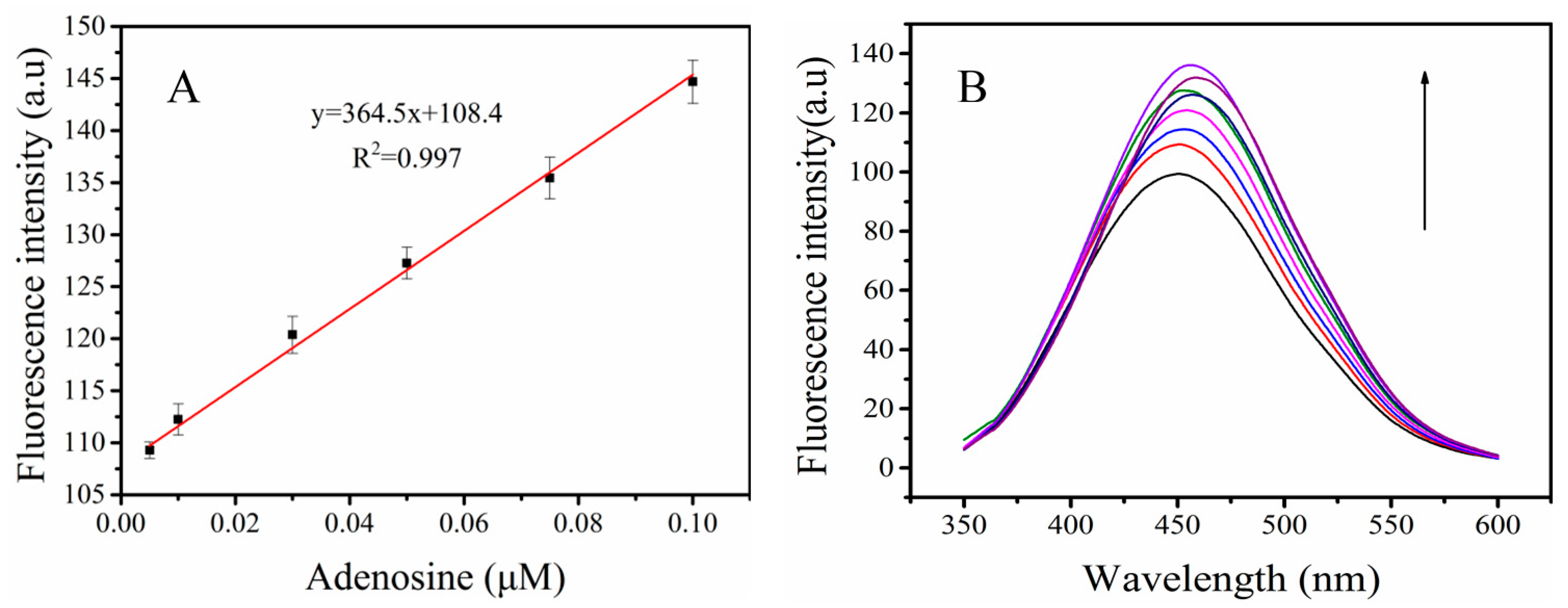
3.5. The Sensitivity of the Probe
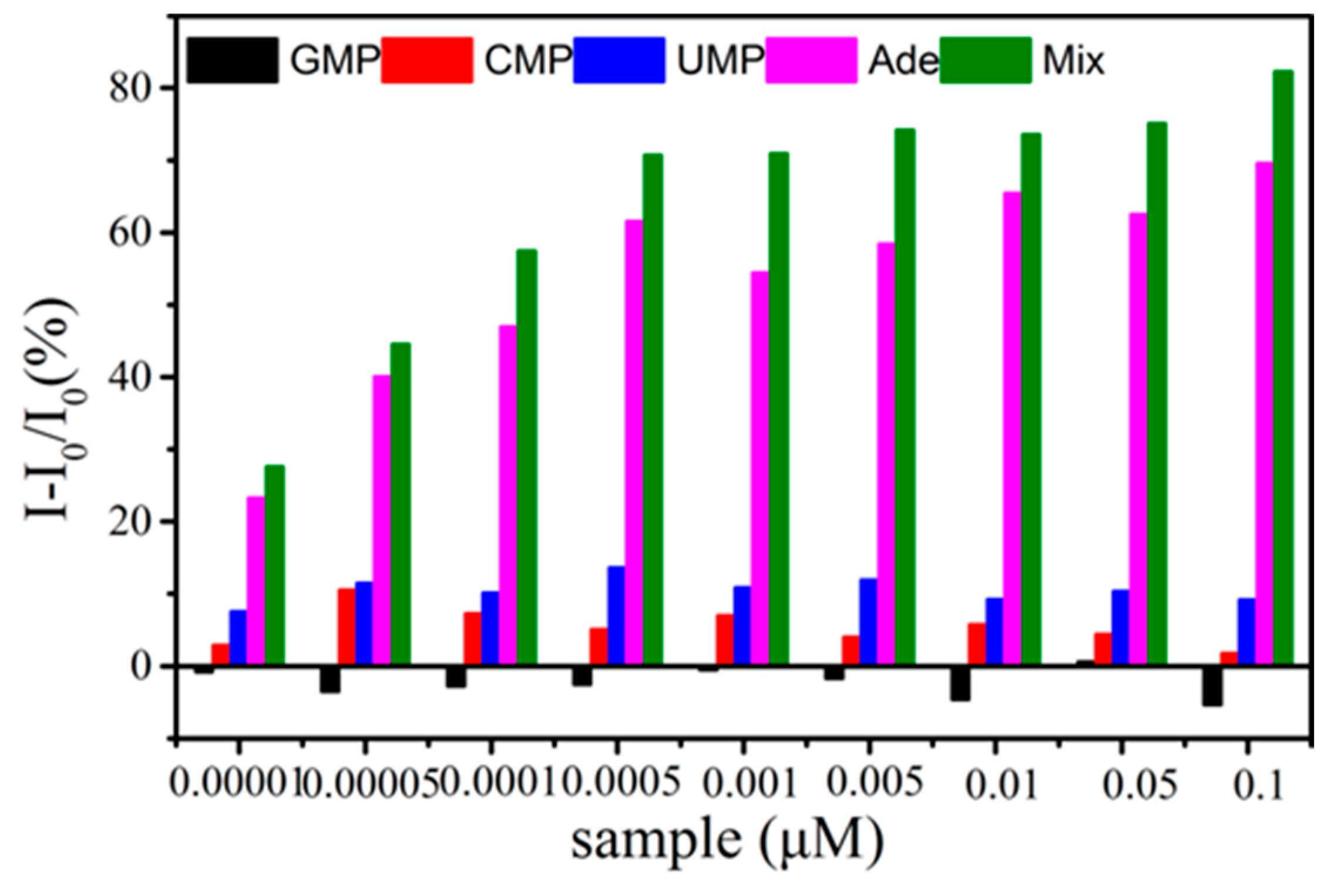
3.6. Specificity of TPE-ABA Detection System
3.7. Analytical Application to Urine Samples
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, H.; Hong, Y.; Tang, B.Z. Fabrication of fluorescent nanoparticles based on AIE luminogens (AIE dots) and their applications in bioimaging. Mater. Horiz. 2016, 3, 283–293. [Google Scholar] [CrossRef]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by Luminogens with Aggregation-induced Emission Characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes based on AIE fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Rananaware, A.; Bhosale, R.S.; Patil, H.; Al Kobaisi, M.; Abraham, A.; Shukla, R.; Bhosale, S.V.; Bhosale, S.V. Precise aggregation-induced emission enhancement via H+ sensing and its use in ratiometric detection of intracellular pH values. RSC Adv. 2014, 4, 59078–59082. [Google Scholar] [CrossRef]
- Rananaware, A.; Bhosale, R.S.; Ohkubo, K.; Patil, H.; Jones, L.A.; Jackson, S.L.; Fukuzumi, S.; Bhosale, S.V.; Bhosale, S.V. Tetraphenylethene-based star shaped porphyrins: Synthesis, self-assembly, and optical and photophysical study. J. Org. Chem. 2015, 80, 3832–3840. [Google Scholar] [CrossRef] [PubMed]
- Rananaware, A.; Abraham, A.N.; La, D.D.; Mistry, V.; Shukla, R.; Bhosale, S.V. Synthesis of a tetraphenylethene-substituted tetrapyridinium salt with multifunctionality: Mechanochromism, cancer cell imaging, and DNA marking. Aust. J. Chem. 2017, 70, 652–659. [Google Scholar] [CrossRef]
- McGown, L.B.; Joseph, M.J.; Pitner, J.B.; vonk, G.P.; Linn, C.P. The nucleic acid ligand. A new tool for molecular recognition. Anal. Chem. 1995, 67, 663A–668A. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Li, J.Y.; Chen, L.Q.; Huang, C.Z.; Li, Y.F. Adenosine-aptamer recognition-induced assembly of gold nanorods and a highly sensitive plasmon resonance coupling assay of adenosine in the brain of model sd rat. Analyst 2010, 135, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, Z.; Khayamian, T.; Saraji, M.; Shirani, M.P. Aptasensor based on fluorescence resonance energy transfer for the analysis of adenosine in urine samples of lung cancer patients. Biosens. Bioelectron. 2016, 79, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Gupta, M. Adenosine and its receptors as therapeutic targets: An overview. Saudi Pharm. J. 2013, 21, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, B.M.; Stirdivant, S.M.; Mitchell, M.W.; Wulff, J.E.; McDunn, J.E.; Li, Z.; Dennis-Barrie, A.; Neri, B.P.; Milburn, M.V.; Lotan, Y.; Wolfert, R.L. Bladder cancer biomarker discovery using global metabolomic profiling of urine. PLoS ONE 2014, 9, e115870. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Häußler, M.; Lam, J.W.Y.; Li, Z.; Sin, K.K.; Dong, Y.; Tong, H.; Liu, J.; Qin, A.; Renneberg, R.; et al. Label-free fluorescent probing of g-quadruplex formation and real-time monitoring of DNA folding by a quaternized tetraphenylethene salt with aggregation-induced emission characteristics. Chem. A Eur. J. 2008, 14, 6428–6437. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cao, Z.; Lau, C.; Lu, J. DNA aptamer folding on magnetic beads for sequential detection of adenosine and cocaine by substrate-resolved chemiluminescence technology. Analyst 2010, 135, 2400–2407. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-W.; Niu, C.-G.; Zeng, G.-M.; Ruan, M. Time-resolved fluorescence biosensor for adenosine detection based on home-made europium complexes. Biosens. Bioelectron. 2011, 29, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Wang, Y.S.; Xue, J.H.; He, Y.; Yang, H.X.; Liang, J.; Shi, L.F.; Xiao, X.L. A gold nanoparticles-modified aptamer beacon for urinary adenosine detection based on structure-switching/fluorescence-“turning on” mechanism. J. Pharm. Biomed. Anal. 2012, 70, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, W.; Zhu, J.; Li, W.; Wang, L. Label-free fluorescence dual-amplified detection of adenosine based on exonuclease iii-assisted DNA cycling and hybridization chain reaction. Biosens. Bioelectron. 2015, 70, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Y.S.; Tang, X.; Zhou, B.; Xue, J.H.; Liu, H.; Liu, S.D.; Cao, J.X.; Li, M.H.; Chen, S.H. An enzyme-free strategy for ultrasensitive detection of adenosine using a multipurpose aptamer probe and malachite green. Anal. Chim. Acta 2015, 887, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Xiong, H.; Lam, J.W.Y.; Häußler, M.; Liu, J.; Yu, Y.; Zhong, Y.; Sung, H.H.Y.; Williams, I.D.; Wong, K.S.; et al. Fluorescent bioprobes: Structural matching in the docking processes of aggregation-induced emission fluorogens on DNA surfaces. Chem. A Eur. J. 2010, 16, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
| Samples | Adenosine Spiked (μM) | Adenosine Found (μM) | Recovery (%) | RSD (%, n = 6) |
|---|---|---|---|---|
| 1 | 0.01 | 0.0090 | 90.0 | 1.9 |
| 2 | 0.05 | 0.0434 | 86.8 | 1.2 |
| 3 | 0.1 | 0.0892 | 89.2 | 2.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Liu, J.; You, X.; Wang, C.; Li, Z.; Xie, W. A Light-Up Probe for Detection of Adenosine in Urine Samples by a Combination of an AIE Molecule and an Aptamer. Sensors 2017, 17, 2246. https://doi.org/10.3390/s17102246
Hu Y, Liu J, You X, Wang C, Li Z, Xie W. A Light-Up Probe for Detection of Adenosine in Urine Samples by a Combination of an AIE Molecule and an Aptamer. Sensors. 2017; 17(10):2246. https://doi.org/10.3390/s17102246
Chicago/Turabian StyleHu, Yingying, Jingjing Liu, Xiangyu You, Can Wang, Zhen Li, and Weihong Xie. 2017. "A Light-Up Probe for Detection of Adenosine in Urine Samples by a Combination of an AIE Molecule and an Aptamer" Sensors 17, no. 10: 2246. https://doi.org/10.3390/s17102246
APA StyleHu, Y., Liu, J., You, X., Wang, C., Li, Z., & Xie, W. (2017). A Light-Up Probe for Detection of Adenosine in Urine Samples by a Combination of an AIE Molecule and an Aptamer. Sensors, 17(10), 2246. https://doi.org/10.3390/s17102246