Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation
Abstract
:1. Introduction
2. Materials and Methods
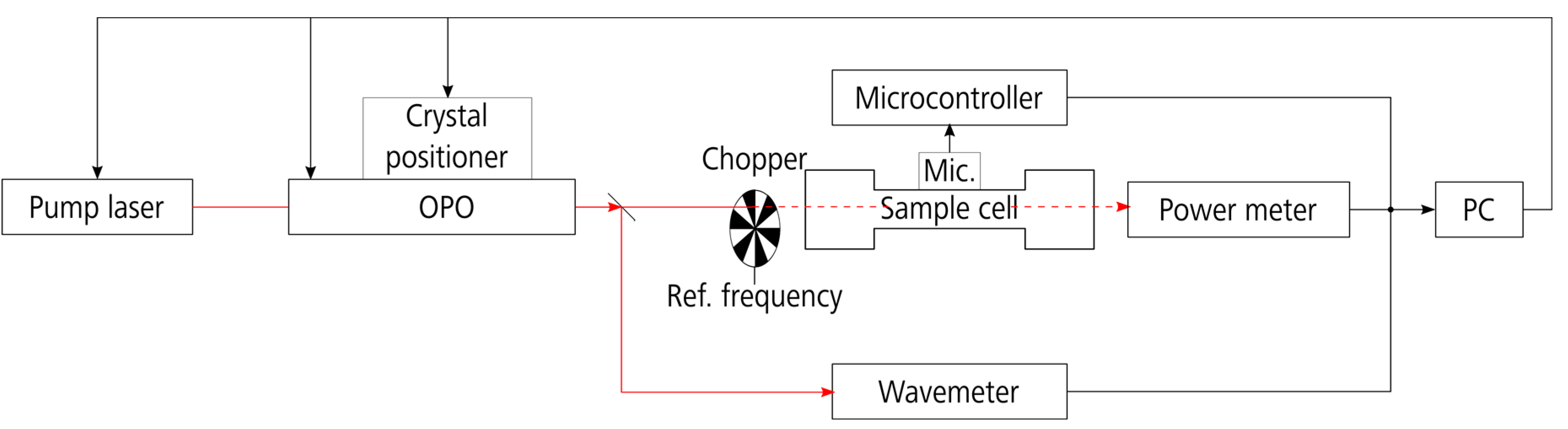
2.1. Experimental Setup
2.2. Lung Cancer Biomarkers
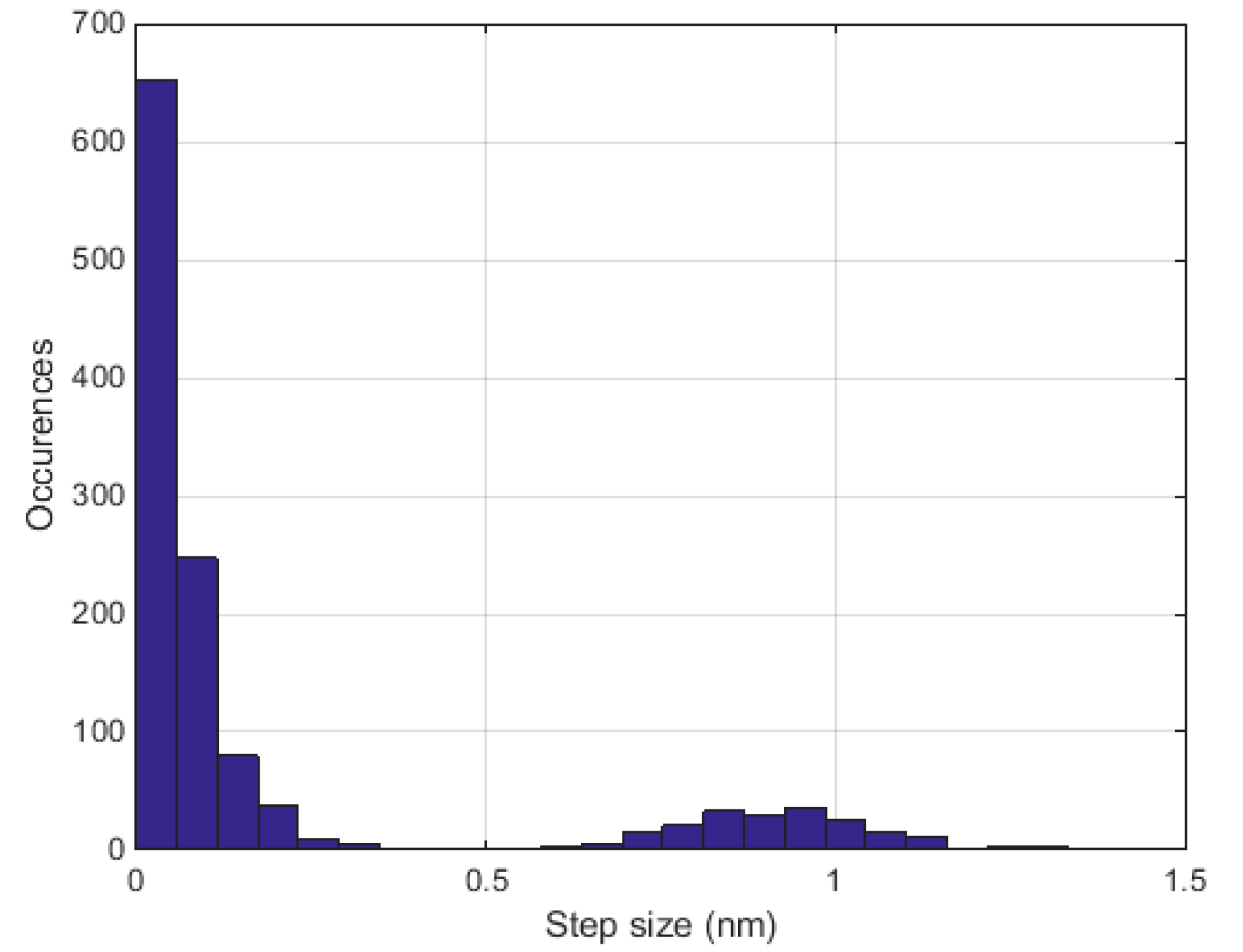
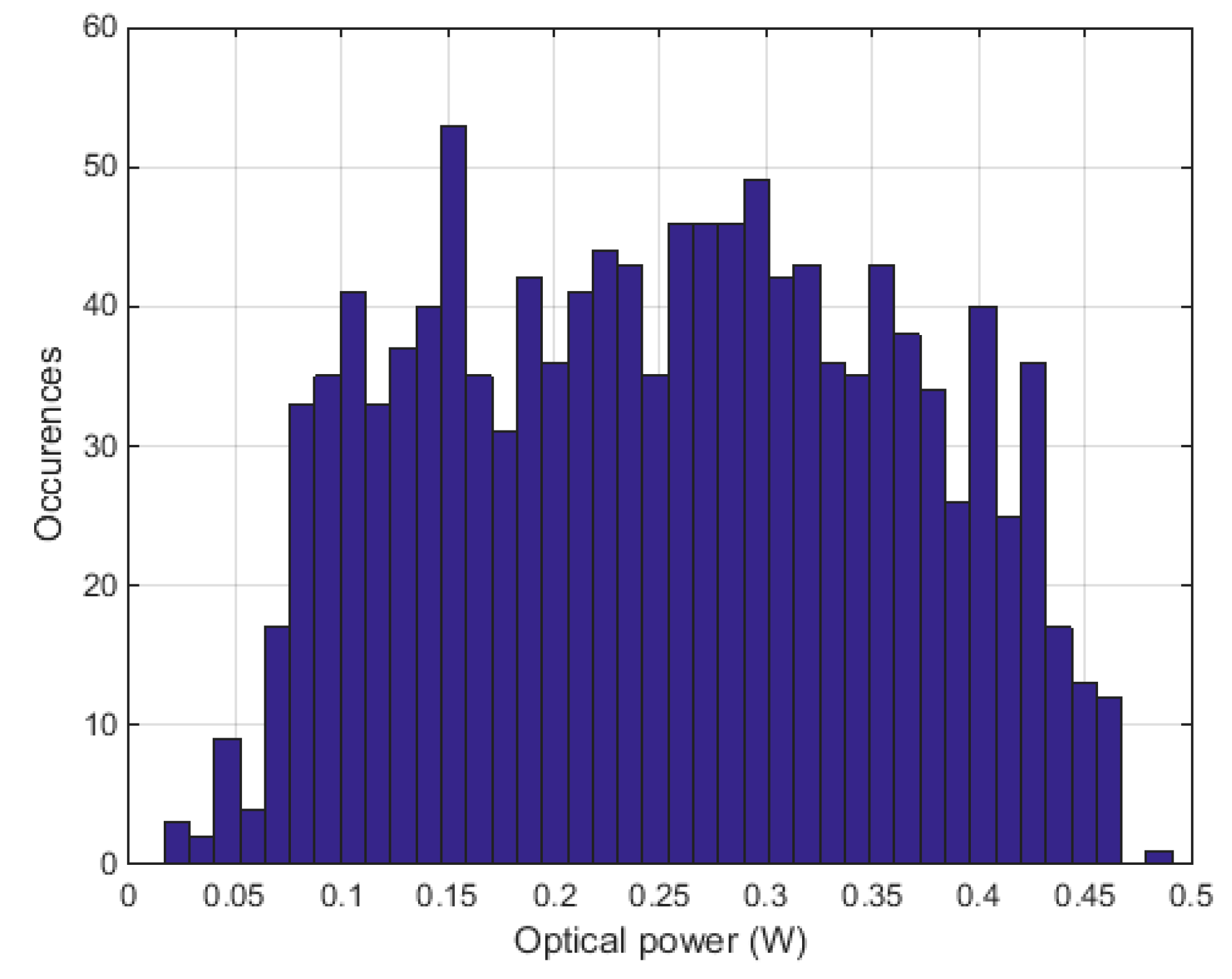
2.3. Measurements
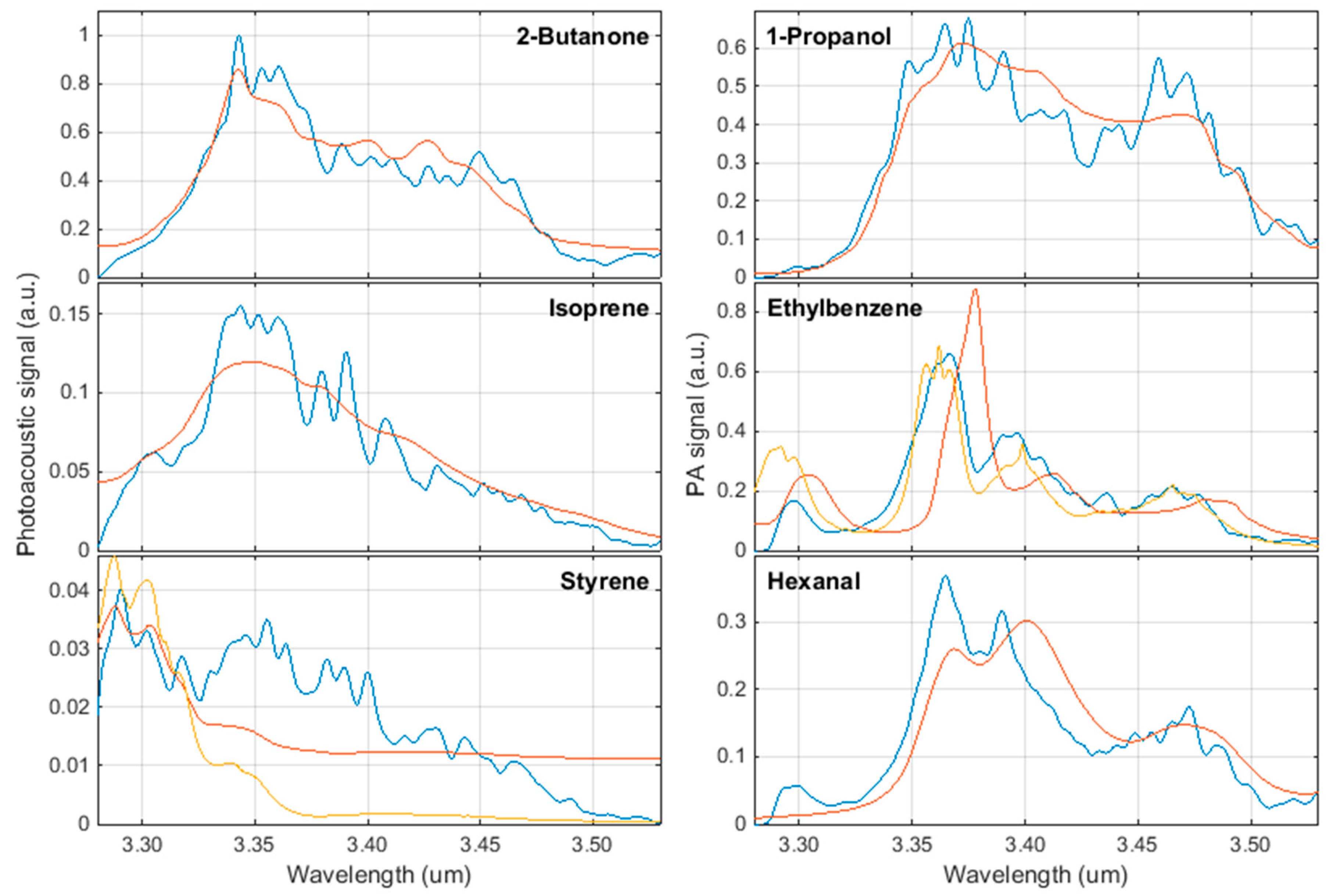
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; International Agency for Research on Cancer/World Health Organization: Lyon, France, 2014. [Google Scholar]
- Smith, R.A.; Manassaram-Baptiste, D.; Brooks, D.; Cokkinides, V.; Doroshenk, M.; Saslow, D.; Wender, R.C.; Brawley, O.W. Cancer screening in the United States, 2014: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2014, 64, 30–51. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Crowley, J.J.; Goldstraw, P. The revised TNM staging system for lung cancer. Ann. Thorac. Cardiovasc. Surg. 2009, 15, 4–9. [Google Scholar] [PubMed]
- Taivans, I.; Bukovskis, M.; Strazda, G.; Jurka, N. Breath testing as a method for detecting lung cancer. Expert Rev. Anticancer Ther. 2014, 14, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Van’t Westeinde, S.C.; van Klaveren, R.J. Screening and early detection of lung cancer. Cancer J. 2011, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ligor, T.; Pater, Ł.; Buszewski, B. Application of an artificial neural network model for selection of potential lung cancer biomarkers. J. Breath Res. 2015, 9, 027106. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chrom. B. 2010, 878, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Cummin, A.R.C.; Gagliardi, A.J.; Gleeson, K.; Greenberg, J.; Maxfield, R.A.; Rom, W.N. Detection of lung cancer with volatile markers in the breath. Chest 2003, 123, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- WHO International Programme on Chemical Safety Biomarkers in Risk Assessment: Validity and Validation. Available online: http://www.inchem.org/documents/ehc/ehc/ehc222.htm (accessed on 18 October 2016).
- Saalberg, Y.; Wolff, M. VOC breath biomarkers in lung cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Tittel, F.K.; Richter, D.; Fried, A. Mid-Infrared Laser Applications in Spectroscopy. In Solid-State Mid-Infrared Laser Sources; Sorokina, I.T., Vodopyanov, K.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 458–529. [Google Scholar] [CrossRef]
- Klingbeil, A.E. Mid-IR Laser Absorption Diagnostics for Hydrocarbon Vapor Sensing in Harsh Environments. Ph.D Thesis, Stanford University, Stanford, CA, USA, 2007. [Google Scholar]
- Bell, A.G. Upon the Production of Sound by Radiant Energy. Philos. Mag. 1881, 11, 150. [Google Scholar] [CrossRef]
- Michaelian, K.H. Photoacoustic IR Spectroscopy: Instrumentation, Applications and Data Analysis, 2nd ed.; Wiley: Somerset, NJ, USA, 2010. [Google Scholar]
- Sigrist, M.W. Laser photoacoustic spectrometry for trace gas monitoring. Analyst 1994, 119, 525–531. [Google Scholar] [CrossRef]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, H.; Saalberg, Y.; Wolff, M. Photoacoustic Hydrocarbon Spectroscopy Using a Mach-Zehnder Modulated cw OPO. Sens. Transducers 2015, 188, 40–47. [Google Scholar]
- Elia, A.; Lugarà, P.M.; Di Franco, C.; Spagnolo, V. Photoacoustic techniques for trace gas sensing based on semiconductor laser sources. Sensors 2009, 9, 9616–9628. [Google Scholar] [CrossRef] [PubMed]
- Pacific Northwest National Laboratory. Available online: https://secure2.pnl.gov/nsd/nsd.nsf/Welcome (accessed on 20 November 2016).
- Goertzel, G. An Algorithm for the Evaluation of Finite Trigonometric Series. Am. Math. Monthly 1958, 65, 34–35. [Google Scholar] [CrossRef]
- Oppenheim, A.V.; Schafer, R.W. Discrete-Time Signal Processing, 3rd ed.; Pearson: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Sysel, P.; Rajmic, P. Goertzel algorithm generalized to non-integer multiples of fundamental frequency. J. Adv. Signal Process. 2012, 2012, 56. [Google Scholar] [CrossRef]
- Bruhns, H.; Marianovich, A.; Wolff, M. Photoacoustic Spectroscopy Using a MEMS Microphone with Inter-IC Sound Digital Output. Int. J. Thermophys. 2014, 35, 2292–2301. [Google Scholar] [CrossRef]
- Bruhns, H.; Marianovich, A.; Rhein, S.; Wolff, M. Digital MEMS Microphone with Inter-IC Sound Interface for Photoacoustic Spectroscopy. In Proceedings of the 14th International Meeting on Chemical Sensors, IMCS 2012, Nuremberg, Germany, 20–23 May 2012; pp. 1217–1219.
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; Szidon, J.P.; Krotoszynski, B.K.; Gibbons, R.D.; O’Neill, H.J. Volatile organic compounds in exhaled air from patients with lung cancer. Clin. Chem. 1985, 31, 1278–1282. [Google Scholar] [PubMed]
- Ligor, M.; Ligor, T.; Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Denz, H.; Fiegl, M.; Hilbe, W.; Weiss, W.; et al. Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clin. Chem. Lab. Med. 2009, 47, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, A.; Kowalkowski, T.; Trawińska, E.; Buszewski, B. The application of statistical methods using VOCs to identify patients with lung cancer. J. Breath Res. 2011, 5, 046008. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, F.; Wang, Y.; Pan, Y.; Lu, D.; Wang, P.; Ying, K.; Chen, E.; Zhang, W. A study of the volatile organic compounds exhaled by lung cancer cells in vitro for breath diagnosis. Cancer 2007, 110, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: cross sectional and nested short-term follow-up study. Respir. Res. 2005, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Handa, H.; Usuba, A.; Maddula, S.; Baumbach, J.I.; Mineshita, M.; Miyazawa, T. Exhaled breath analysis for lung cancer detection using ion mobility spectrometry. PLoS One 2014, 9, e114555. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. J. Chromatogr. B 2011, 879, 3360–3366. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Szulejko, J.E.; McCulloch, M.; Jackson, J.; McKee, D.L.; Walker, J.C.; Solouki, T. Evidence for Cancer Biomarkers in Exhaled Breath. IEEE Sensors J. 2010, 10, 185–210. [Google Scholar] [CrossRef]
- Krilaviciute, A.; Heiss, J.A.; Leja, M.; Kupcinskas, J.; Haick, H.; Brenner, H. Detection of cancer through exhaled breath: a systematic review. Oncotarget 2015, 6, 38643–38657. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; King, J.; Unterkofler, K.; Amann, A. Stability of selected volatile breath constituents in Tedlar, Kynar and Flexfilm sampling bags. Analyst 2013, 138, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the use of Tedlar® bags for breath-gas sampling and analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Steeghs, M.M.L.; Cristescu, S.M.; Harren, F.J.M. The suitability of Tedlar bags for breath sampling in medical diagnostic research. Physiol. Meas. 2007, 28, 73–84. [Google Scholar]
- Heymes, F.; Aprin, L.; Bony, A.; Forestier, S.; Cirocchi, S.; Dusserre, G. An experimental investigation of evaporation rates for different volatile organic compounds. Proc. Safety Prog. 2013, 32, 193–198. [Google Scholar] [CrossRef]
- Hirschmann, C.B.; Koivikko, N.S.; Raittila, J.; Tenhunen, J.; Ojala, S.; Rahkamaa-Tolonen, K.; Marbach, R.; Hirschmann, S.; Keiski, R.L. FT-IR-cPAS--new photoacoustic measurement technique for analysis of hot gases: a case study on VOCs. Sensors 2011, 11, 5270–5289. [Google Scholar] [CrossRef] [PubMed]
- Kessler, W. Multivariate Datenanalyse: Für die Pharma-, Bio- und Prozessanalytik; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Kohl, I.; Beauchamp, J.; Cakar-Beck, F.; Herbig, J.; Dunkl, J.; Tietje, O.; Tiefenthaler, M.; Boesmueller, C.; Wisthaler, A.; Breitenlechner, M.; et al. First observation of a potential non-invasive breath gas biomarker for kidney function. J. Breath Res. 2013, 7, 017110. [Google Scholar] [CrossRef] [PubMed]
| Priority | CAS | Names |
|---|---|---|
| 1 | 78-93-3 | 2-butanone, methyl ethyl ketone |
| 71-23-8 | 1-propanol, n-propanol | |
| 2 | 78-79-5 | isoprene, 2-methyl-1,3-butadiene |
| 100-41-4 | ethylbenzene | |
| 100-42-5 | styrene, ethenylbenzene | |
| 66-25-1 | hexanal | |
| 3 | 67-64-1 | acetone, propanone |
| 107-87-9 | 2-pentanone, methyl propyl ketone | |
| 67-63-0 | 2-propanol, isopropylalcohol, isopropanol | |
| 124-18-5 | decane | |
| 71-43-2 | benzene | |
| 111-71-7 | heptanal | |
| 106-97-8 | butane | |
| 123-38-6 | propanal | |
| 109-66-0 | n-pentane |
| Name | SNR (100 ppm) | LOD (ppb) |
|---|---|---|
| 2-butanone | 17,629 => 84.92 dB | 5.7 |
| 1-propanol | 11,969 => 81.56 dB | 8.4 |
| isoprene | 2,729 => 68.72 dB | 36.6 |
| ethylbenzene | 11,645 => 81.32 dB | 8.6 |
| styrene | 706 => 56.98 dB | 141.6 |
| hexanal | 6491 => 76.25 dB | 15.4 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saalberg, Y.; Bruhns, H.; Wolff, M. Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation. Sensors 2017, 17, 210. https://doi.org/10.3390/s17010210
Saalberg Y, Bruhns H, Wolff M. Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation. Sensors. 2017; 17(1):210. https://doi.org/10.3390/s17010210
Chicago/Turabian StyleSaalberg, Yannick, Henry Bruhns, and Marcus Wolff. 2017. "Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation" Sensors 17, no. 1: 210. https://doi.org/10.3390/s17010210
APA StyleSaalberg, Y., Bruhns, H., & Wolff, M. (2017). Photoacoustic Spectroscopy for the Determination of Lung Cancer Biomarkers—A Preliminary Investigation. Sensors, 17(1), 210. https://doi.org/10.3390/s17010210






