1. Introduction
The synovial fluid is produced by the synovium membrane inside the articular cartilage. It can be considered a dialysate of plasma with a high content of hyaluronic acid [
1,
2,
3,
4]. The amount of the synovial fluid in a joint is no more than 3.5 mL (knee) [
4,
5] where the main functions of the synovial fluid are the lubrication of the joints and the absorption of the mechanical load produced when walking or running (knees) or holding up weight (cubit and shoulder) [
1,
2,
3,
4,
5]. The viscosity of the synovial fluid depends on the concentration of the hyaluronic acid. There are studies that relate the low viscosity of the synovial fluid (low concentration of hyaluronic acid) with arthrocentesis, osteoarthritis and inflammatory arthropathies [
1,
2,
3,
4,
6,
7]. Due to the concentration of hyaluronic acid in the synovial fluid being ≈ 3.5 mg/mL [
6], the sample may be considered a non-Newtonian fluid with viscoelastic properties [
5,
8]. Due to this lubricant function, the analysis of synovial fluid is important for the detection and diagnosis of arthritic diseases.
At present, the evaluation of the viscosity of synovial fluid samples is done through macroscopic analysis. This analysis evaluates the color, transparency and viscosity of the sample [
1,
2,
3,
9,
10]. The results of a macroscopic analysis make the classification of the samples possible in healthy, inflammatory or septic, according to
Table 1 [
5,
9,
11].
The viscosity test involves extending the sample on a surface and measuring the length before the thread is cut. If the resulting length is greater than 3 cm, the sample is healthy; if not, the sample displays a problem and more tests are necessary [
5].
Although the viscosity of the sample is a fundamental parameter in the macroscopic analysis [
3], the clinical method to obtain its value is primarily qualitative. The sample of synovial fluid is obtained through a puncture in the joint and it is extended on a surface with the same needle used to extract it. If the final length is greater than 3 cm, the sample is considered healthy; but if it is lower than 3 cm, the sample is considered abnormal [
11].
The macroscopic method provides a subjective measure of viscosity that depends, for example, on the skills and strength of the laboratory researcher, the stretching and the surface. Sometimes the viscosity is not even taken into account, only the appearance [
1].
Due to the importance of the hyaluronic acid concentration in the synovial fluid, the small amount of sample in the joints and the current subjective method to obtain a measure of the viscosity, it has been proposed to use a piezoelectric biosensor based on a quartz crystal resonator (QCR) to obtain the measure of viscosity of the sample.
There are several studies linking the use of the Quartz Crystal Microbalance (QCM) to the detection of diseases characterizing biological fluids as blood [
12,
13,
14,
15], urine [
16,
17], saliva [
17,
18,
19] and synovial fluid [
20,
21]. The methods proposed in these studies have proven to be successful according to their results. However, they are also more complex because they involve the functionalization of the electrode in the crystal in order to detect a specific protein related to the disease. There are also studies that relate similar piezoelectric techniques to sense the viscosity of biological samples, such as: the use of cantilevers [
22,
23,
24]. Sensors based on cantilevers are also valid to obtain the viscosity values in biological samples; the physics principle is the same. However, the cantilever transducer is more fragile because it only has one fixed contact point. In consequence, the manufacture and use of these biosensors have a higher level of difficulty.
In contrast to the standard method applied in clinical and others more complex piezoelectric techniques, the method proposed enables us to know the viscosity value for synovial fluid samples in a simple way because the proposed technique does not require the functionalization of the electrode and the manufacture of the biosensor is easy.
In preliminary tests, aqueous dilutions of hyaluronic acid at different concentrations have been used to emulate the synovial fluid (both healthy and abnormal). As reference equipment, the Fungilab viscometer has been used to obtain viscosity measures of each sample in order to compare them with experimental measures.
3. Materials and Methods
3.1. Samples
The concentration of the hyaluronic acid in the healthy synovial fluid is around 3.5 mg/mL [
6,
7,
45]. In traumatic arthritis and osteoarthritis the hyaluronic acid concentration decreases to around 1.3 mg/mL, and for rheumatoid arthritis the concentration is lower, only 0.84 mg/mL [
6]. In this study, the hyaluronic acid dilutions were done at the same concentrations in order to emulate the healthy and abnormal synovial fluid. One additional sample at 7 mg/mL was made with the purpose of obtaining an extra point in the analysis of results. The hyaluronic acid (Mw = 1.5 MDa) was supplied by Sigma Aldrich (St. Louis, MO, USA).
Dilutions were prepared by weighing the hyaluronic acid salt (HA) using the analytic balance and mixing the HA with cold water, (water temperature was 4 °C). The amount of hyaluronic acid and the water volume were calculated according to the desired concentration. After mixing HA and cold water, the solution was stirred gently and continuously during 5 min. Finally, the samples were stored in the refrigerator for 8 h. This process is needed so that the mixture does not contain air bubbles (homogeneous solution). With the aim of evaluating the performance of the QCR biosensor and its detection limit, additional glycerol solutions were created at different concentrations in milli-q water (40%, 30%, 20%, 10%, 5%, 2.5%, 1.25%, 0.63%, 0.31%).
3.2. Crystals and Holder Cell
The 10 MHz quartz crystals were purchased from International Crystal Manufacturing (Oklahoma City, OK, USA). The static cell was obtained from Gamry Instruments Inc. (Warminster, PA, USA).
3.3. Viscometer of Reference
The rotational viscometer Alpha series manufactured by Fungilab has been used as reference equipment for the viscosity measures. Its resolution is 0.1 mPa·s according to manufacturer. This equipment is in the Center for Biomedical Technology (CTB).
3.4. Biosensor
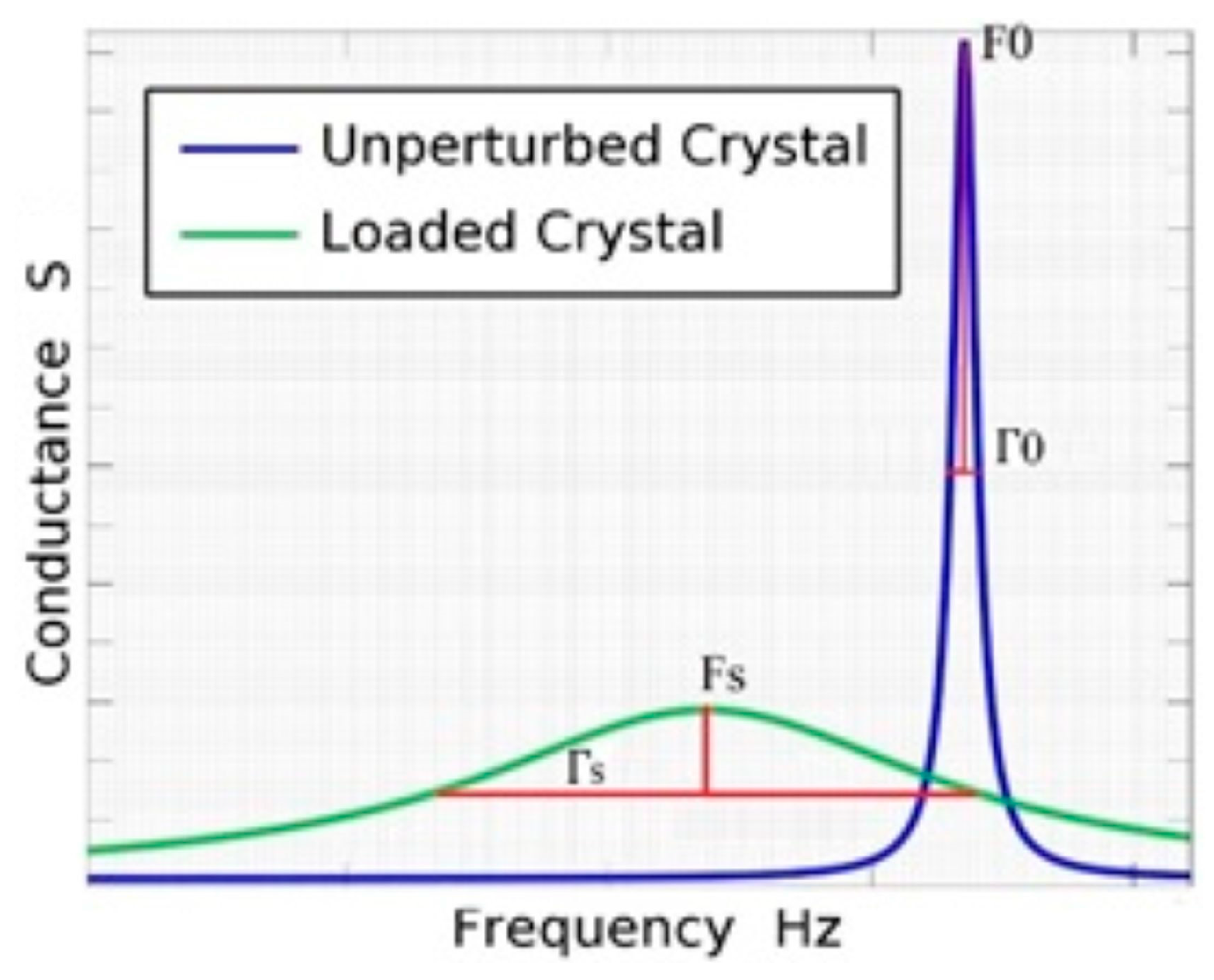
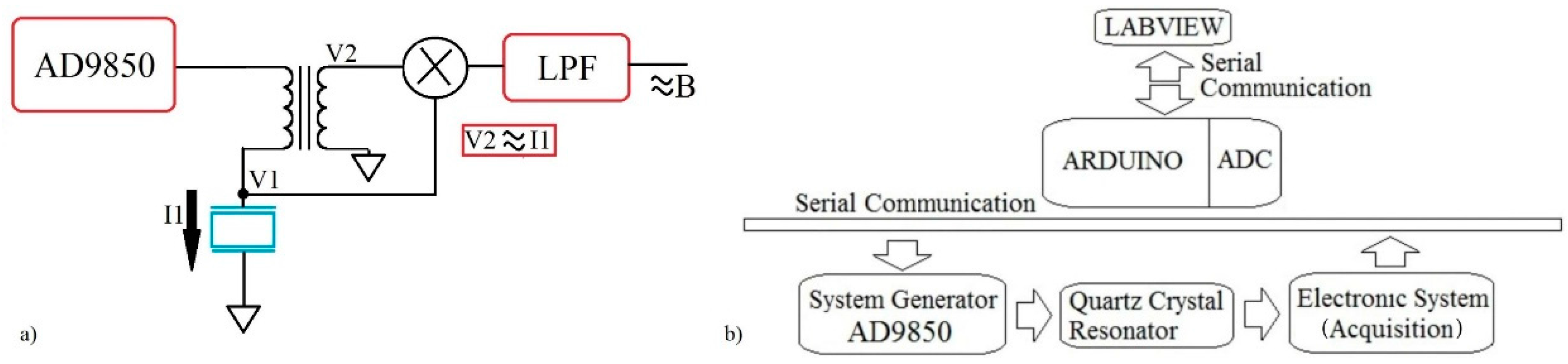
The quartz crystal has two electrodes, one on each side. The active electrode receives the sample on its surface and is connected to the electric ground to give more stability to the measures. The proposed circuit gets the voltage and current signal for several frequencies in a configurable bandwidth close to the crystal series resonance frequency (Fs). The circuit is a variation of the Nakamoto and Kobayashi proposal [
46]. The electronic system includes a DAC (AD9850 module, Analog Devices, Norwood, MA, USA) to get a high resolution in the measures. The AD9850 module is capable of creating a sinusoidal signal of between 1 and 40 MHz with a frequency step of 1 Hz. The amplitude of the input signal in the crystal is 1 Vp. The sweep frequency is configured close to the resonance frequency of the quartz crystal to identify the expected behavior. The quartz crystal is placed in an array with a toroidal-core transformer used as a current sensor [
30,
46]. The secondary coil of the transformer gives a voltage proportional to the current in the primary which is the crystal current. The voltage is shifted 90° with respect to the original current signal. The voltage signal (V2) related to the current through the crystal (I1) and the voltage used to stimulate the crystal (V1) are multiplied by using an AD835 mixer (manufacturer) according to
Figure 4a.
The output signal in the mixer is filtered using an active filter with an LT1057 amplifier (Linear Technology, Milpitas, CA, USA) to obtain the DC component. According to [
30], the DC component is related to the susceptance of the quartz crystal.
The acquisition system uses the ADC port of the Arduino DUE card (
https://www.arduino.cc/) to obtain the output signals (voltage, current, susceptance). The digital resolution of the acquisition is 12 bits. With this system, the acquisition time per sweep cycle takes a few seconds.
Figure 4b shows the blocks diagram of the prototype.
A Labview code has been developed to control the Arduino card. Through this program the user can choose the initial parameters (range of frequency sweep and amplitude of signal) and obtain the voltage and current signals on the quartz crystal for every frequency from it. The conductance and susceptance graphs are shown in the Labview interface, ΔF, ΔΓ and ΔR are calculated with the same code.
Figure 5 displays the QCR prototype system.
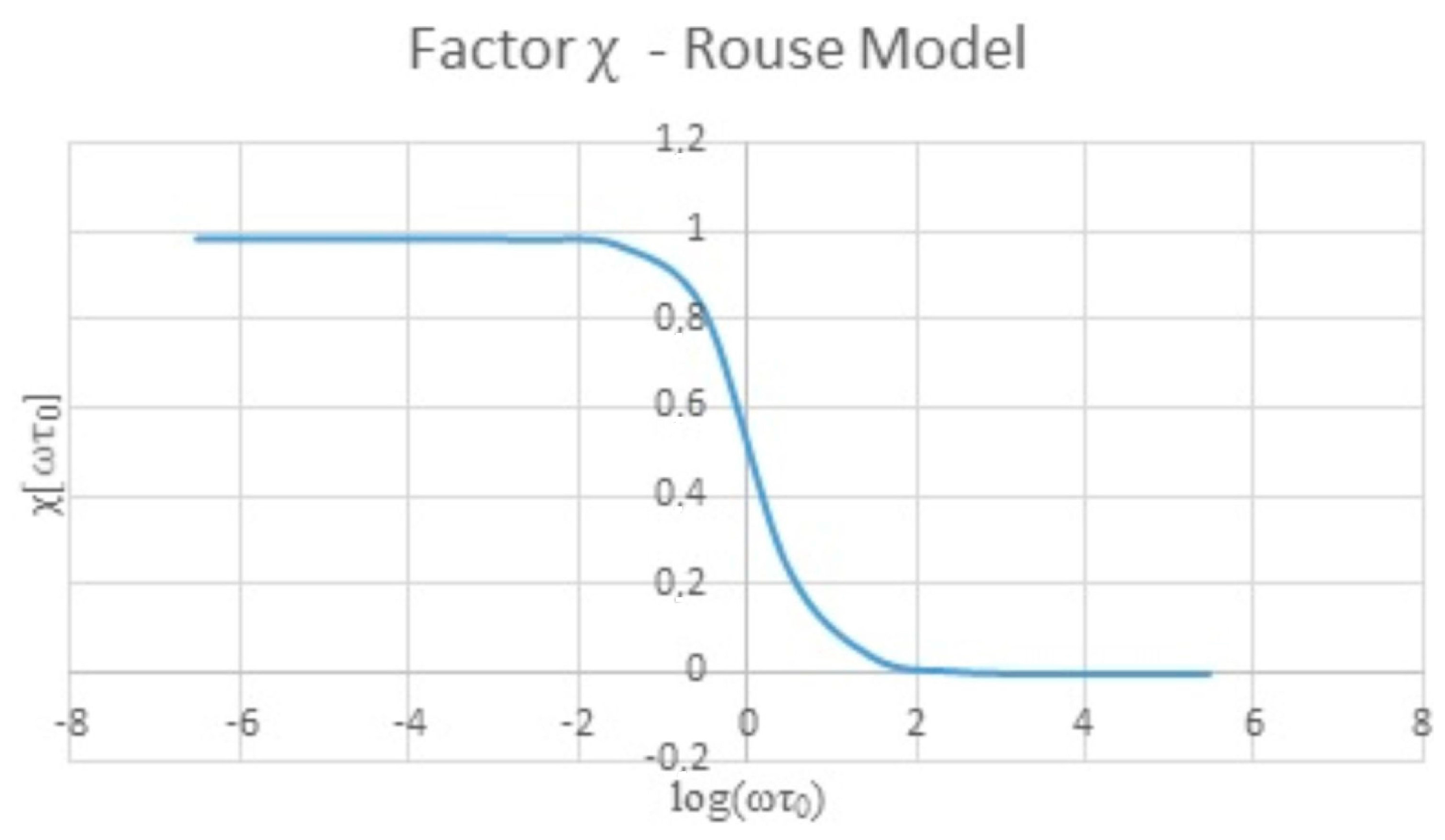
Additionally, the Labview software has access to a database created with the external variables needed to obtain the correction factor χ: zero shear viscosity, molecular weight, the gas constant, the density of the fluid, temperature and the Riemann Zeta Function value for the Rouse model. The software uses the database in order to calculate the factor χ. The user can edit the zero shear viscosity, the density or the temperature if the value is known.
In this study, the concentration of the samples is always known. However, for an unknown sample, the biosensor uses the change in the motional resistance (ΔR) in order to select the correct factor χ. In the results section is shown the ΔR values for each sample. These resistance values are also saved in the database for reference.
3.5. Measurement Protocol
(1) Select a frequency range to make the sweep and to obtain the conductance curve in the frequency domain for the bare crystal (without sample). Calculate the serial resonance frequency (F0), the half band half width (Γ0) and the motional resistance (R0) using the developed code in Labview for each cycle. These measures are taken 10 times and the final values are the average of the measures; (2) Have the sample analyzed in the electrode surface of the quartz crystal (50 μL of volume); (3) Adjust the frequency range in Labview software to obtain the conductance curve of the quartz crystal in contact with liquid sample; (4) The measurements with samples (Fs, Γs and Rs) are taken 100 times (approx. 3 min) and the final values are the average among the measures; (5) Obtain ΔF, ΔΓ and ΔR.
The density values were obtained using an analytical balance. The mass of each solution was determined from 1 mL of its volume.
3.6. Protocol to Clean the Crystal
(1) Add abundant water to wash the residues on the crystal; (2) Add alcohol (isopropanol) to clean the crystal surface; (3) Add acetone to complete the electrode cleaning (remove solvents); (4) Dry the electrode surface with a liquid nitrogen pistol.
5. Discussion
The proposed QCR prototype has a good performance. The results illustrate the capability of the QCR sensor to measure small changes in the composition of glycerol aqueous solutions.
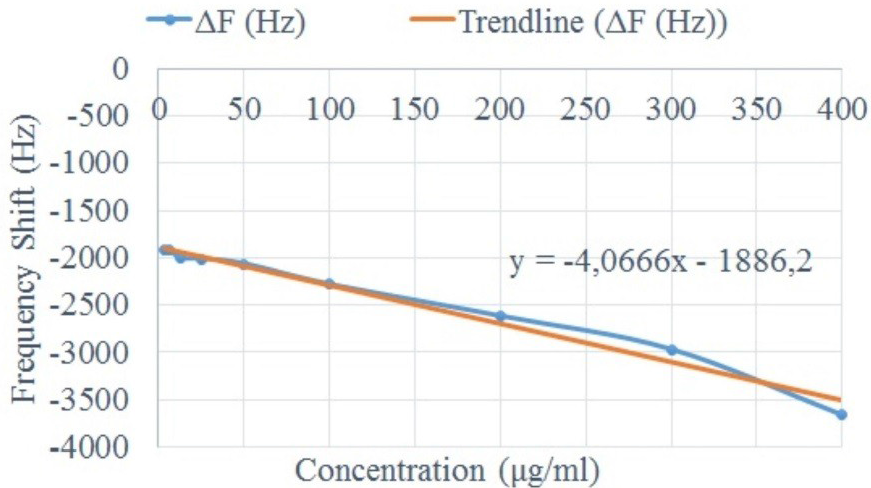
Figure 7 shows the relationship between the concentration of the sample and the sensor response ΔF. According to
Figure 7, the sensitivity of the sensor is ≈ 4.06 Hz per 1 μg/mL concentration sample. The smallest incremental change of concentration that could be detected in the biosensor (resolution) was 6.25 μg/mL.
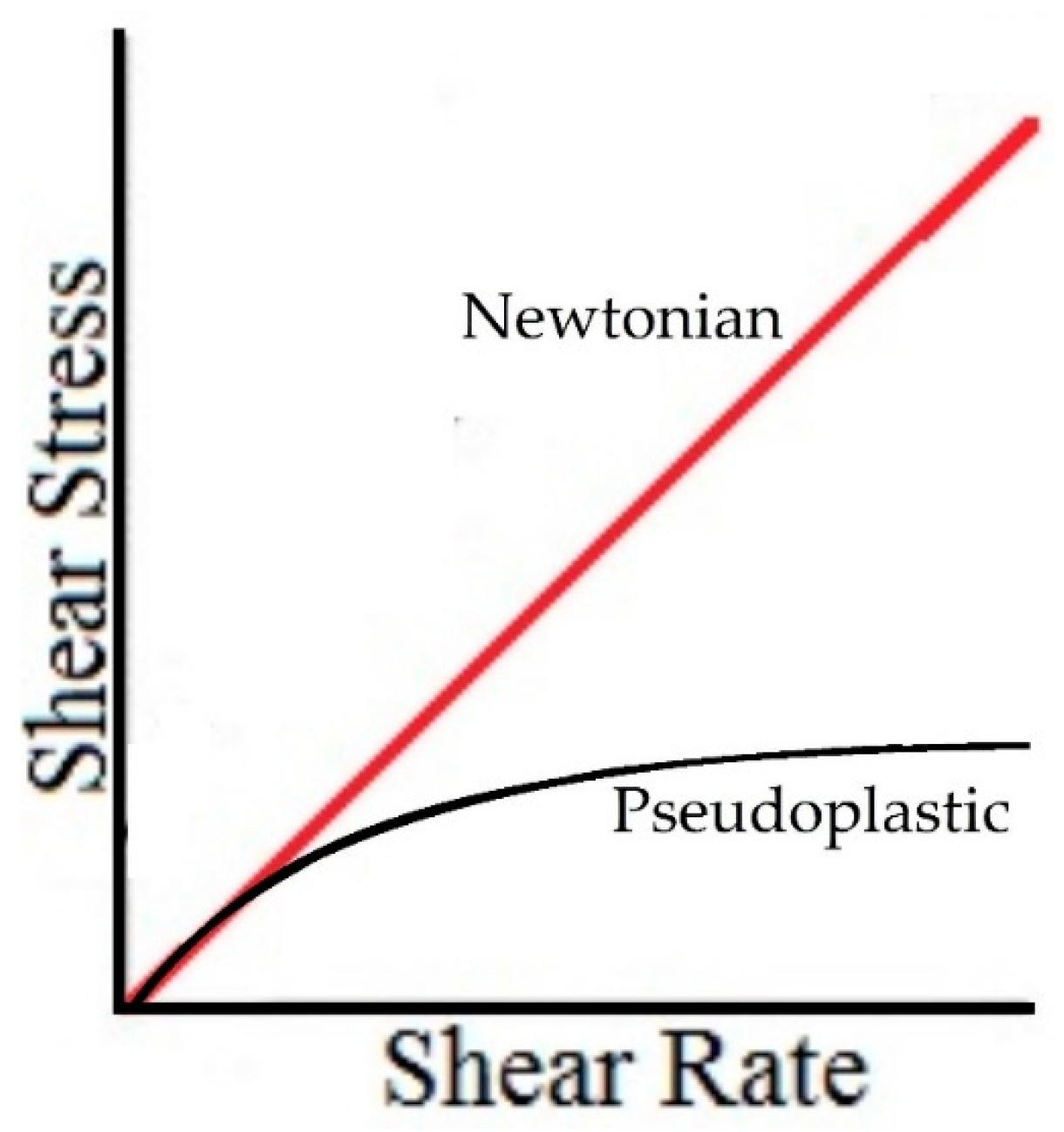
According to the results for hyaluronic acids solutions, the presence of hyaluronic acid in an aqueous solution results in a complex rheological behavior; this solution is catalogued into the pseudoplastic fluids. Due to this viscoelastic and pseudoplastic behavior, it is not possible to compare the QCR measures (high shear rate) and the viscometer measures (low shear rate) for the same sample.
Table 4 shows these results.
The QCR measures require an additional factor to obtain results comparable with the viscometer results. The Rouse model is a good alternative to obtain the additional factor χ in order to correct the experimental measures.
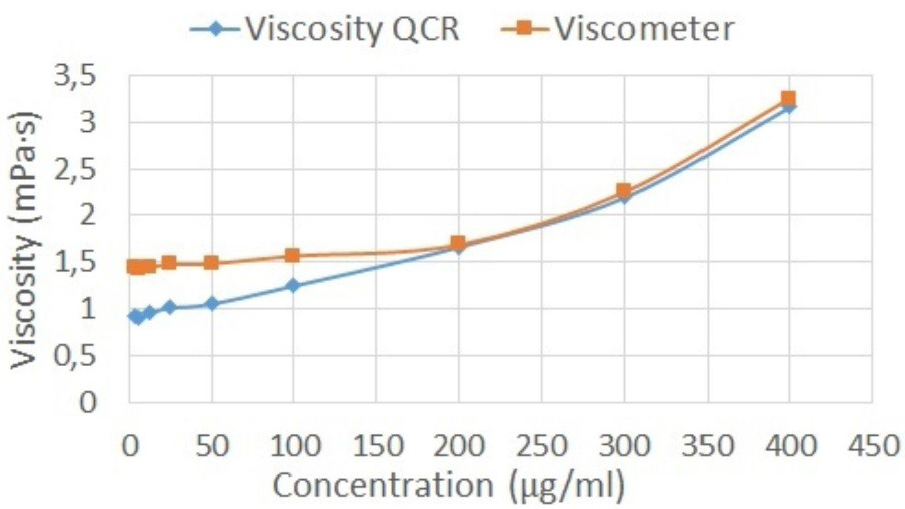
By applying the Rouse model to the experimental results, the new apparent viscosities were obtained for each sample. These apparent viscosities are only related to the viscous component of hyaluronic acid solutions. In consequence, the new apparent viscosities exhibit the same behavior as the viscometer measures (
Figure 9). In other words, the new frequency shifts obtained correspond to an equivalent fluid with the viscous component alone. The new apparent viscosities obtained were calculated using Equation (11).
Table 6 shows the results obtained after applying the Rouse model. The relative error increases as the concentration of the sample decreases. However, the viscosities obtained after applying the Rouse model are comparable with the reference viscosities obtained with the viscometer.
In this study, the concentration of the hyaluronic acid is already known for every sample. However, for future clinical samples, the concentration, the zero shear viscosity and the density are unknown variables. In such cases, it is necessary to use the motional resistance value in order to relate the response of the QCR with the correction factor χ. For unknown samples, the ΔR value obtained experimentally is compared with the ΔR values in the database and the system selects the parameters required to calculate the factor χ and adjust the viscosity value.
Table 3 shows the ΔR values obtained for each sample.
The obtained results confirm the relationship between hyaluronic acid concentration and the viscosity of the fluid. Considering the concentration samples used in this study are very similar to the ones reported for arthritic diseases [
6,
7] and the results shown, the QCR biosensor would be capable of identifying synovial fluid samples (healthy or with any medical affectation).