Nano-Engineered Biomimetic Optical Sensors for Glucose Monitoring in Diabetes
Abstract
:1. Introduction
2. Enzymatic Glucose Sensors: Advantages and Disadvantages
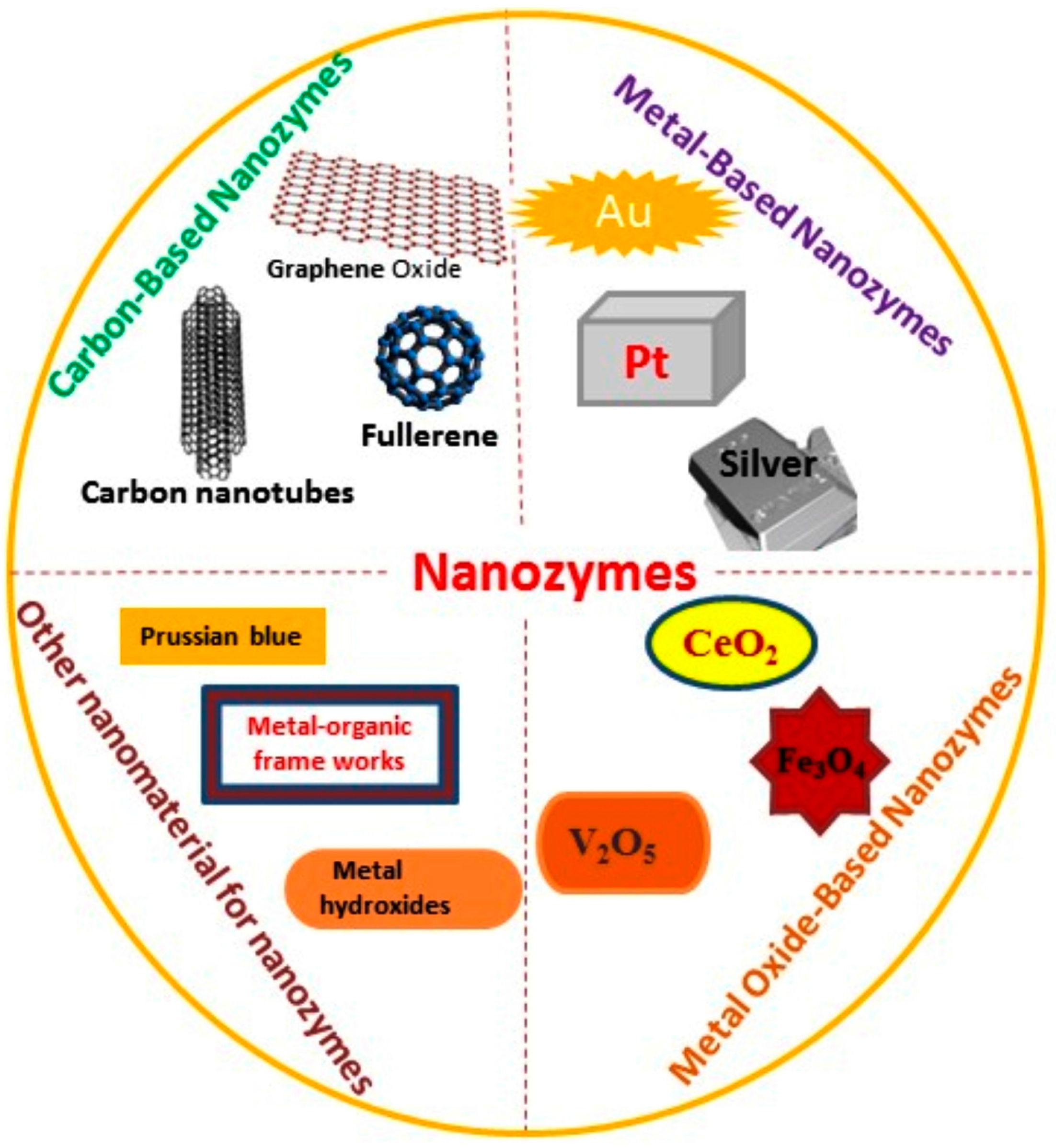
3. Types of Nanomaterials
3.1. Carbon-Based Nanozymes
3.2. Metal-Based Nanozymes
3.3. Metal Oxide-Based Nanozymes
3.4. Other Nanomaterial for Nanozymes
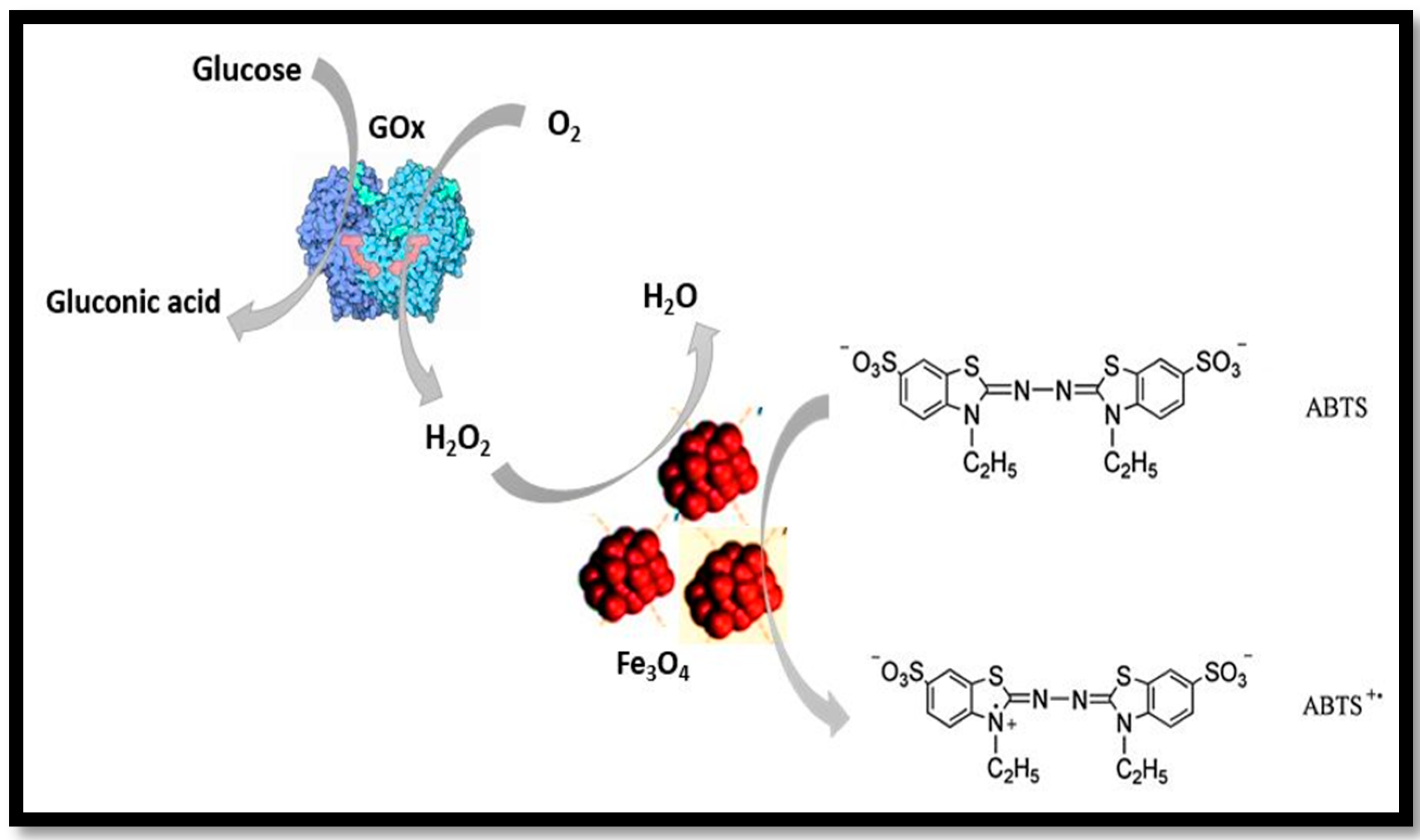
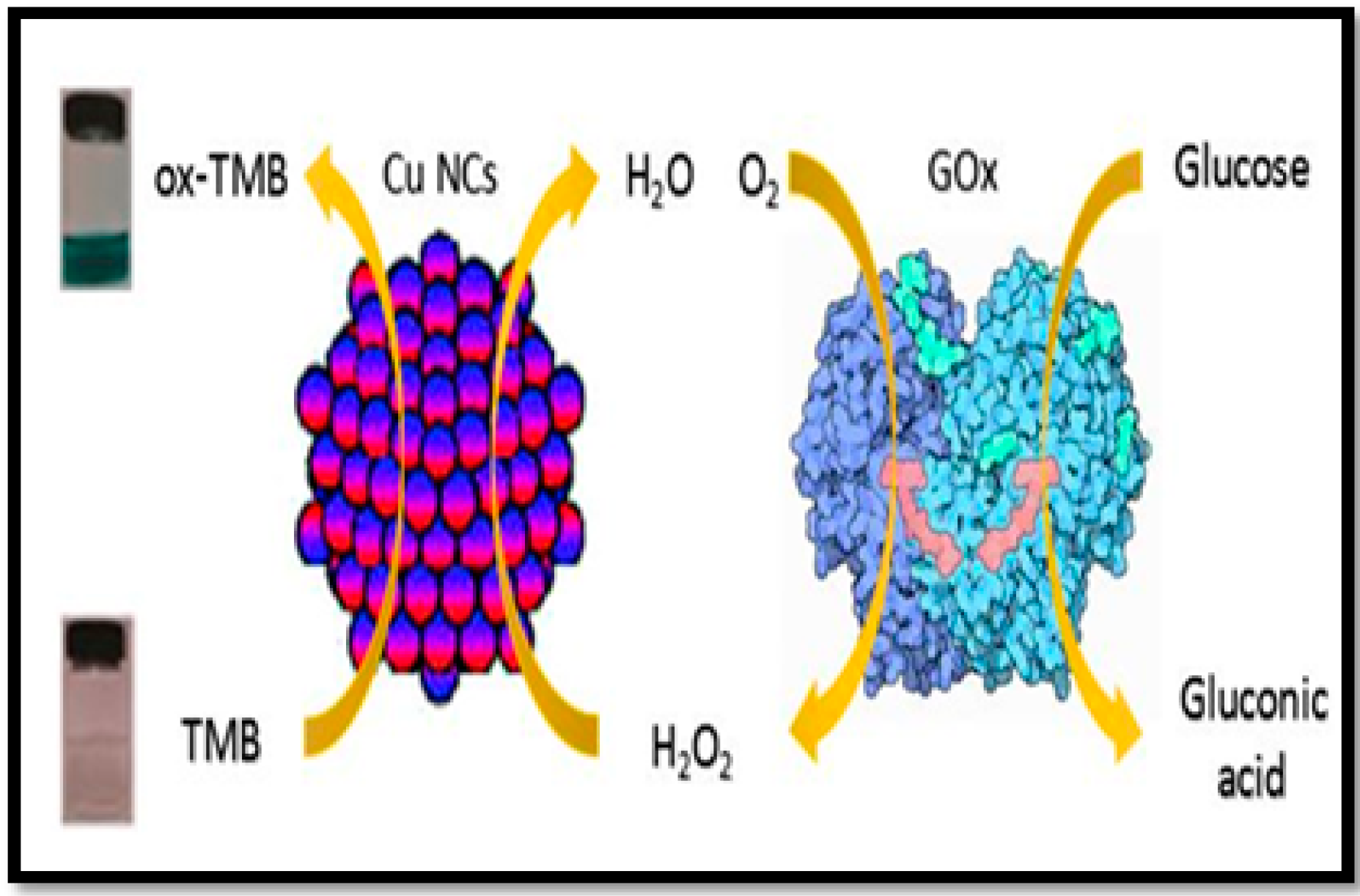
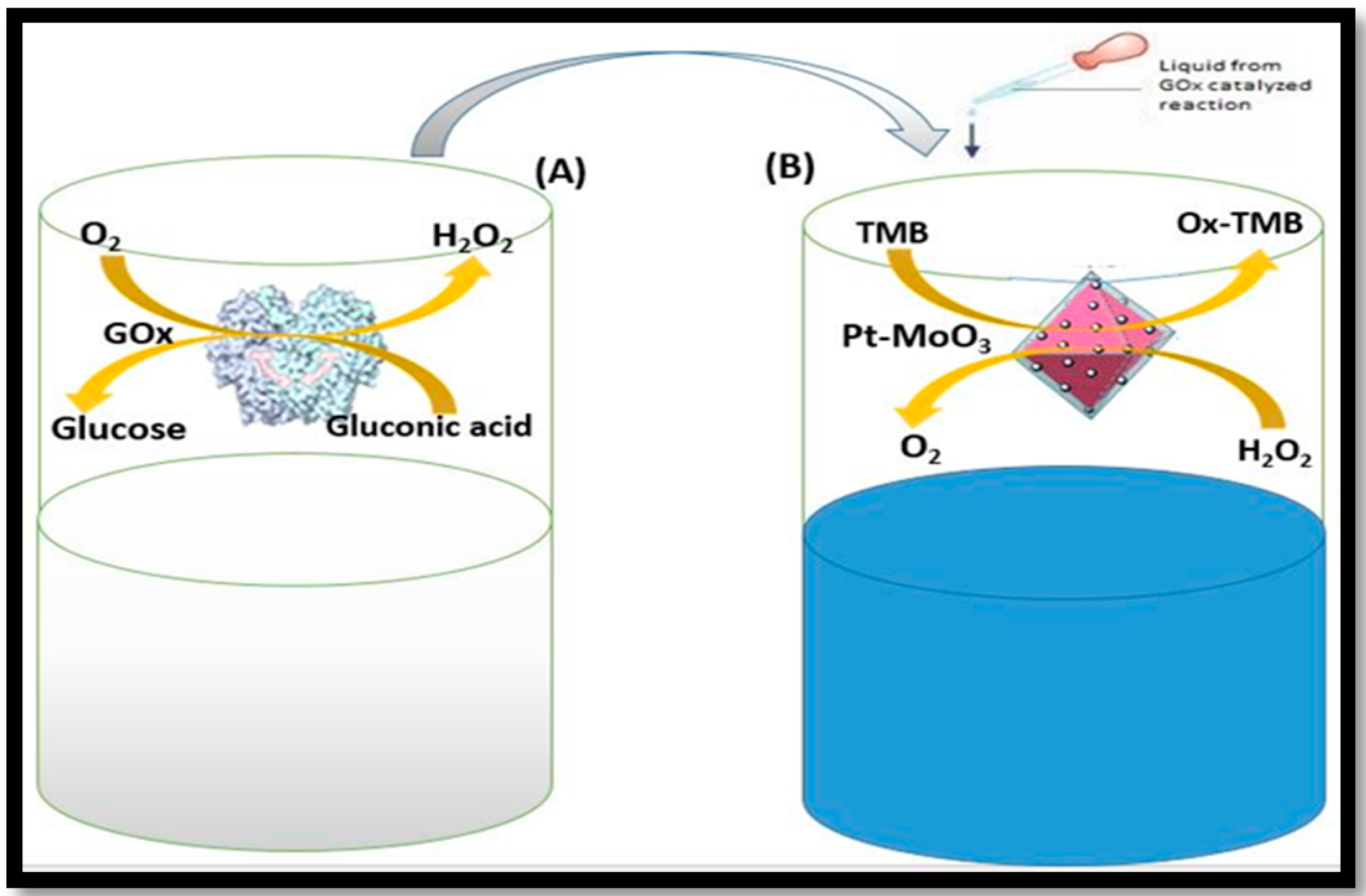
4. Application of Nanozymes in Colorimetric Sensing of Glucose
4.1. One Component System
4.2. Multi- Component System
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Petit, J.-M.; Bour, J.-B.; Galland-Jos, C.; Minello, A.; Verges, B.; Guiguet, M. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J. Hepatol. 2001, 35, 279–283. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Amos, A.F.; McCarty, D.; Zimmet, P. The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diabet. Med. 1997, 14, S7–S85. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.A.; Balkau, B.; Vauzelle-Kervröedan, F.; Thibult, N.; Eschwege, E. Revision of diagnostic criteria for diabetes. Lancet 1996, 348, 1657–1658. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Haider, W.; Raza, Y.; Marty, J.L. Colorimetric cholesterol sensor based on peroxidase like activity of zinc oxide nanoparticles incorporated carbon nanotubes. Talanta 2015, 143, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Hou, S.; Wen, T.; Liu, W.; Zhang, K. Research progress of nanoparticles as enzyme mimetics. Sci. China Phys. Mech. Astron. 2011, 54, 1749–1756. [Google Scholar] [CrossRef]
- Hayat, A.; Cunningham, J.; Bulbul, G.; Andreescu, S. Evaluation of the oxidase like activity of nanoceria and its application in colorimetric assays. Anal. Chim. Acta. 2015, 885, 140–147. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wamer, W.; Xia, Q.; Yin, J.-J.; Fu, P.P. Enzyme-like activity of nanomaterials. J. Environ. Sci. Health Part C 2014, 32, 186–211. [Google Scholar] [CrossRef] [PubMed]
- McLamore, E.S.; Taguchi, M.; Ptitsyn, A.; Claussen, J.C. Nanomaterial-mediated biosensors for monitoring glucose. J. Diabetes Sci. Technol. 2014, 8, 403–411. [Google Scholar]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dong, S. Graphene nanosheet: synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Interface engineering catalytic graphene for smart colorimetric biosensing. Acs Nano 2012, 6, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-Free Colorimetric Detection of Single Nucleotide Polymorphism by Using Single-Walled Carbon Nanotube Intrinsic Peroxidase-Like Activity. Chem. Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Han, Z.; Zhu, J.J. Helical carbon nanotubes: intrinsic peroxidase catalytic activity and its application for biocatalysis and biosensing. Chem. Eur. J. 2011, 17, 9377–9384. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, H.; Yamago, S.; Nakamura, E.; Shiraki, T.; Sugiura, Y. Photoinduced biochemical activity of fullerene carboxylic acid. J. Am. Chem. Soc. 1993, 115, 7918–7919. [Google Scholar] [CrossRef]
- Nakamura, E.; Isobe, H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhen, M.; Guan, M.; Chen, D.; Zhang, G.; Ge, J. A novel glucose colorimetric sensor based on intrinsic peroxidase-like activity of C60-carboxyfullerenes. Biosens. Bioelectron. 2013, 47, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Comotti, M.; Della, P.C.; Matarrese, R.; Rossi, M. The catalytic activity of “naked” gold particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Hu, X.; Wu, X.; Zheng, Z. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials 2013, 34, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, W.; Gao, X.; Lu, Z.; Wu, X.; Gao, X. Mechanisms of oxidase and superoxide dismutation-like activities of gold, silver, platinum, and palladium, and their alloys: A general way to the activation of molecular oxygen. J. Am. Chem. Soc. 2015, 137, 15882–15891. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, W.; Liu, A.L.; Hong, L.; Deng, H.H.; Lin, X.H. Comparison of the Peroxidase-Like Activity of Unmodified, Amino-Modified, and Citrate-Capped Gold Nanoparticles. ChemPhysChem 2012, 13, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, W.; Wu, X.; Gao, X. Mechanism of pH-switchable peroxidase and catalase-like activities of gold, silver, platinum and palladium. Biomaterials 2015, 48, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Wu, Q.; Shan, Z.; Huang, Q.-M. BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosens. Bioelectron. 2011, 26, 3614–3619. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yin, J.-J.; Ning, B.; Wu, X.; Hu, Y.; Ferrari, M. Direct evidence for catalase and peroxidase activities of ferritin–platinum nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, Y.; Gu, N. Peroxidase-like catalytic activity of cubic Pt nanocrystals. Colloids Surf. A 2011, 373, 6–10. [Google Scholar] [CrossRef]
- Lien, C.-W.; Huang, C.-C.; Chang, H.-T. Peroxidase-mimic bismuth–gold nanoparticles for determining the activity of thrombin and drug screening. Chem. Commun. 2012, 48, 7952–7954. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Y.; Yuan, J.; Yin, J.-J.; Wu, X.; Hu, X. Au@ Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials 2011, 32, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, X.; Liu, J.; Hu, X.; Zhang, K.; Hou, S. Design of AgM bimetallic alloy nanostructures (M = Au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem. Mater. 2010, 22, 2988–2994. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, Y. The effective peroxidase-like activity of chitosan-functionalized CoFe2O4 nanoparticles for chemiluminescence sensing of hydrogen peroxide and glucose. Analyst 2012, 137, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, H.; Huang, Y. Luminol–silver nitrate chemiluminescence enhancement induced by cobalt ferrite nanoparticles. Luminescence 2011, 26, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Baksi, A.; Banerjee, I.; Ananthakrishnan, R.; Maiti, T.K.; Pramanik, P. Development of phosphonate modified Fe(1−x)MnxFe2O4mixed ferrite nanoparticles: novel peroxidase mimetics in enzyme linked immunosorbent assay. Talanta 2011, 86, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, Y.-S.; Yuan, J.; Zhu, L.; Liu, Z.; Tang, H. Ultrasensitive fluorometric determination of hydrogen peroxide and glucose by using multiferroic BiFeO3 nanoparticles as a catalyst. Talanta 2010, 81, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Chen, Z.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Sol–Gel Synthesis and Photo-Fenton-Like Catalytic Activity of EuFeO3 Nanoparticles. J. Am. Ceram. Soc. 2011, 94, 3418–3424. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; He, S.; Huang, Y. CoFe2O4 magnetic nanoparticles as a peroxidase mimic mediated chemiluminescence for hydrogen peroxide and glucose. Chem. Commun. 2011, 47, 10785–10787. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Feng, J.; Zhou, X.; Ren, C.; Li, H.; Chen, X. Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal. Chem. 2012, 84, 5753–5758. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Espi, V.; Alvarez-Paneque, A.; Torrens, M.; Otero-González, A.; Reguera, E. Conjugation of manganese ferrite nanoparticles to an anti Sticholysin monoclonal antibody and conjugate applications. Colloids Surf. A 2011, 387, 118–124. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J. Highly Dispersed CeO2 on TiO2 Nanotube: A Synergistic Nanocomposite with Superior Peroxidase-Like Activity. ACS Appl. Mater. Interfaces 2015, 7, 6451–6461. [Google Scholar] [CrossRef] [PubMed]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P. Electron localization determines defect formation on ceria substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, J.; Zhang, Z.; Gao, W.; Zhou, X.; Qu, Y. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials 2015, 59, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Artiglia, L.; Agnoli, S.; Paganini, M.C.; Cattelan, M.; Granozzi, G. TiO2@ CeO x Core–Shell Nanoparticles as Artificial Enzymes with Peroxidase-Like Activity. ACS Appl. Mater. Interfaces 2014, 6, 20130–20136. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, Y.; Yu, S.; Zhu, W.; Zheng, F.; Liu, W. Dual role of hydrogen peroxide on the oxidase-like activity of nanoceria and its application for colorimetric hydrogen peroxide and glucose sensing. RSC Adv. 2016, 6, 59939–59945. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Feng, Y.-B.; Hong, L.; Chen, Q.-Y.; Wu, L.-F. Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 2012, 137, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Liu, A.L.; Wang, L.M.; Li, G.W.; Lin, X.H. Peroxidase-like activity of cupric oxide nanoparticle. ChemCatChem 2011, 3, 1151–1154. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Hu, Y.; Wu, J.; Wei, H. Nanozymes: Next Wave of Artificial Enzymes; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hu, A.-L.; Liu, Y.-H.; Deng, H.-H.; Hong, G.-L.; Liu, A.-L.; Lin, X.-H. Fluorescent hydrogen peroxide sensor based on cupric oxide nanoparticles and its application for glucose and l-lactate detection. Biosens. Bioelectron. 2014, 61, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Li, H.; Zhu, R.; Shao, Q.; Yang, S. NiO nanoparticles modified with 5, 10, 15, 20-tetrakis (4-carboxyl pheyl)-porphyrin: Promising peroxidase mimetics for H2O2 and glucose detection. Biosens. Bioelectron. 2015, 64, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tarlani, A.; Fallah, M.; Lotfi, B.; Khazraei, A.; Golsanamlou, S.; Muzart, J. New ZnO nanostructures as non-enzymatic glucose biosensors. Biosens. Bioelectron. 2015, 67, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Qi, P.; Zhang, D.; Wu, J.; Wang, Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2012, 33, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Teplensky, M.H.; Moghadam, P.Z.; Fairen-Jimenez, D. Metal-organic frameworks as biosensors for luminescence-based detection and imaging. Interface Focus 2016, 6, 20160027. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Li, L.; Zhang, C.; Fu, J.; Jiang, J. MIL-53 (Fe): A Metal–Organic Framework with Intrinsic Peroxidase-Like Catalytic Activity for Colorimetric Biosensing. Chem. A Eur. J. 2013, 19, 15105–15108. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.-X.; Jia, S.-Y.; Wang, F.-F.; Wu, S.-H.; Song, J.; Liu, Y. Hemin@metal–organic framework with peroxidase-like activity and its application to glucose detection. Catal. Sci. Technol. 2013, 3, 2761–2768. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, X.J.; Yang, X.X.; Li, Y.F. A nanosized metal–organic framework of Fe-MIL-88NH2 as a novel peroxidase mimic used for colorimetric detection of glucose. Analyst 2013, 138, 4526–4531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Gong, S.-W.; Zhang, Y.; Yang, T.; Wang, C.-Y.; Gu, N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J. Mater. Chem. 2010, 20, 5110–5116. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, S.; Yin, J.-J.; He, W.; Lu, W.; Ma, M. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Ni, F.; Gao, F.; Li, M. Peroxidase-Like Layered Double Hydroxide Nanoflakes for Electrocatalytic Reduction of H2O2. Electroanalysis 2009, 21, 2125–2132. [Google Scholar] [CrossRef]
- Cui, L.; Yin, H.; Dong, J.; Fan, H.; Liu, T.; Ju, P. A mimic peroxidase biosensor based on calcined layered double hydroxide for detection of H2O2. Biosens. Bioelectron. 2011, 26, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, J.; Liu, S.; Wang, L.; Qin, X.; Lu, W. Novel application of CoFe layered double hydroxide nanoplates for colorimetric detection of H2O2 and glucose. Analyst 2012, 37, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yang, D.; Peng, S.; Chen, X.; Huang, Y.; Liu, Y. Single nanoparticle to 3D supercage: framing for an artificial enzyme system. J. Am. Chem. Soc. 2015, 137, 13957–13963. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, Z.; Cao, H.; Huang, Y. Peroxidase-like activity of chitosan stabilized silver nanoparticles for visual and colorimetric detection of glucose. Analyst 2012, 137, 5560–5564. [Google Scholar] [CrossRef] [PubMed]
- Jv, Y.; Li, B.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-M.; Stoeva, S.I.; Mirkin, C.A. Bio-bar-code-based DNA detection with PCR-like sensitivity. J. Am. Chem. Soc. 2004, 126, 5932–5933. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, Z.-F.; Shi, M.-J.; Xu, Y.; Wu, Y.-L. Light emission of gold nanoparticles induced by the reaction of bis (2, 4, 6-trichlorophenyl) oxalate and hydrogen peroxide. Anal. Chem. 2005, 77, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Ornatska, M.; Sharpe, E.; Andreescu, D.; Andreescu, S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal. Chem. 2011, 83, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Hayes, S.A.; O’Keefe, T.J.; O’Keefe, M.J.; Stoffer, J.O. The Phase Stability of Cerium Species in Aqueous Systems II. The Systems. Equilibrium Considerations and Pourbaix Diagram Calculations. J. Electrochem. Soc. 2006, 153, C74–C79. [Google Scholar] [CrossRef]
- Jiao, X.; Song, H.; Zhao, H.; Bai, W.; Zhang, L.; Lv, Y. Well-redispersed ceria nanoparticles: Promising peroxidase mimetics for H2O2 and glucose detection. Anal. Methods 2012, 4, 3261–3267. [Google Scholar] [CrossRef]
- Wang, T.; Sun, D.-C. Preparation and characterization of nanometer-scale powders ceria by electrochemical deposition method. Mater. Res. Bull. 2008, 43, 1754–1760. [Google Scholar] [CrossRef]
- Orge, C.A.; Órfão, J.J.; Pereira, M.F.; de Farias, A.M.D.; Neto, R.C.R.; Fraga, M.A. Ozonation of model organic compounds catalysed by nanostructured cerium oxides. Appl. Catal. B 2011, 103, 190–199. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Hola, K.; Bourlinos, A.B.; Kozak, O.; Berka, K.; Siskova, K.M.; Havrdova, M. Photoluminescence effects of graphitic core size and surface functional groups in carbon dots: COO− induced red-shift emission. Carbon 2014, 70, 279–286. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon nanoparticles: Synthesis, characterization, and bioimaging application. J. Phys. Chem. 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, J. A review of organic nanomaterials in photothermal cancer therapy. Cancer Res. Front. 2016, 2, 67–84. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Tang, J.Y.; Wang, G.L.; Zhang, M.; Hu, X.Y. Facile synthesis of submicron Cu2O and CuO crystallites from a solid metallorganic molecular precursor. J. Cryst. Growth 2006, 294, 278–282. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Irudayaraj, J. Fluorescent Ag clusters via a protein-directed approach as a Hg (II) ion sensor. Anal. Chem. 2011, 83, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yuan, J.; Dong, Q.; Wang, E. Highly sequence-dependent formation of fluorescent silver nanoclusters in hybridized DNA duplexes for single nucleotide mutation identification. J. Am. Chem. Soc. 2009, 132, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.-Y.; Huang, C.-C.; Chang, H.-T. Silver nanoclusters as fluorescent probes for selective and sensitive detection of copper ions. Chem. Commun. 2010, 46, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Nie, S. Etching colloidal gold nanocrystals with hyperbranched and multivalent polymers: A new route to fluorescent and water-soluble atomic clusters. J. Am. Chem. Soc. 2007, 129, 2412–2413. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Yeh, H.-C.; Yoo, H.; Werner, J.H.; Martinez, J.S. Silver nanocluster aptamers: In situ generation of intrinsically fluorescent recognition ligands for protein detection. Chem. Commun. 2011, 47, 2294–2296. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Han, S.; Parveen, S.; Yuan, Y.; Zhang, L.; Xu, G. Highly sensitive fluorescent detection of trypsin based on BSA-stabilized gold nanoclusters. Biosens. Bioelectron. 2012, 32, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Giri, A.; Bootharaju, M.; Xavier, P.L.; Pradeep, T.; Pal, S.K. Copper quantum clusters in protein matrix: potential sensor of Pb2+ ion. Anal. Chem. 2011, 83, 9676–9680. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yuan, Y.; Zhang, L.; Zhao, J.; Majeed, S.; Xu, G. Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal. Chim. Acta 2013, 762, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ye, J.; Tan, K.; Wang, J.; Yang, G. Colorimetric visualization of glucose at the submicromole level in serum by a homogenous silver nanoprism–glucose oxidase system. Anal.Chem. 2013, 85, 6241–6247. [Google Scholar] [CrossRef] [PubMed]
- Radhakumary, C.; Sreenivasan, K. Naked eye detection of glucose in urine using glucose oxidase immobilized gold nanoparticles. Anal. Chem. 2011, 83, 2829–2833. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, X.; Wu, H.; Jin, Y. Enzymatic plasmonic engineering of Ag/Au bimetallic nanoshells and their use for sensitive optical glucose sensing. Adv. Mater. 2012, 24, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, X.; Wu, H.; Zhai, Y.; Jin, Y. In situ nanoplasmonic probing of enzymatic activity of monolayer-confined glucose oxidase on colloidal nanoparticles. Anal. Chem. 2013, 85, 4546–4553. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, Y.; Rong, P.; Yang, J.; Wang, W.; Liu, D. A high-throughput colorimetric assay for glucose detection based on glucose oxidase-catalyzed enlargement of gold nanoparticles. Nanoscale 2015, 7, 15584–15588. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Zhang, X.; Wang, J. Nanostructured mimic enzymes for biocatalysis and biosensing. In NanoBiosensing; Springer: New York, NY, USA, 2011; pp. 85–109. [Google Scholar]
- Karakoti, A.; Singh, S.; Dowding, J.M.; Seal, S.; Self, W.T. Redox-active radical scavenging nanomaterials. Chem. Soc. Rev. 2010, 39, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, X.; Wang, H.; Zheng, H.; Huang, Y. Analytical and environmental applications of nanoparticles as enzyme mimetics. TrAC Trends Anal. Chem. 2012, 39, 114–129. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Haider, W.; Hayat, A.; Raza, Y.; Anwar, C.A.; Rehman, I.-U.; Marty, J.L. Gold nanoparticle decorated single walled carbon nanotube nanocomposite with synergistic peroxidase like activity for d-alanine detection. RSC Adv. 2015, 5, 24853–24858. [Google Scholar] [CrossRef]
- Premkumar, T.; Mezzenga, R.; Geckeler, K.E. Carbon nanotubes in the liquid phase: addressing the issue of dispersion. Small 2012, 8, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Weng, D.; Lee, H.; Levon, K.; Mao, J.; Scrivens, W.; Stephens, E. The influence of Buckminsterfullerenes and their derivatives on polymer properties. Eur. Polym. J. 1999, 35, 867–878. [Google Scholar] [CrossRef]
- She, Z.W.; Liu, S.; Low, M.; Zhang, S.Y.; Liu, Z.; Mlayah, A. Janus Au-TiO2 Photocatalysts with Strong Localization of Plasmonic Near-Fields for Efficient Visible-Light Hydrogen Generation. Adv. Mater. 2012, 24, 2310–2314. [Google Scholar]
- Kokubo, K.; Shirakawa, S.; Kobayashi, N.; Aoshima, H.; Oshima, T. Facile and scalable synthesis of a highly hydroxylated water-soluble fullerenol as a single nanoparticle. Nano Res. 2011, 4, 204–215. [Google Scholar] [CrossRef]
- Chen, S.; Hai, X.; Chen, X.-W.; Wang, J.-H. In situ growth of silver nanoparticles on graphene quantum dots for ultrasensitive colorimetric detection of H2O2 and glucose. Anal. Chem. 2014, 86, 6689–6694. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-J.; Lin, C.-Y.; Liu, C.-H.; Cheng, T.-L.; Tseng, W.-L. Synthesis of poly (diallyldimethylammonium chloride)-coated Fe3O4 nanoparticles for colorimetric sensing of glucose and selective extraction of thiol. Biosens. Bioelectron. 2010, 26, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Maji, S.K.; Biswas, P.; Adhikary, B. New peroxidase-substrate 3,5-di-tert-butylcatechol for colorimetric determination of blood glucose in presence of Prussian Blue-modified iron oxide nanoparticles. Sens. Actuators B 2013, 177, 676–683. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Wu, S.; Wang, K.; He, X. Colorimetric detection of hydrogen peroxide and glucose using the magnetic mesoporous silica nanoparticles. Talanta 2015, 134, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hao, J.; Zhang, Z.; Lu, B.; Zhang, B.; Tang, J. CoxFe3−xO4 hierarchical nanocubes as peroxidase mimetics and their applications in H2O2 and glucose detection. Rsc Adv. 2014, 4, 35500–35504. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, C.; Guo, Y.; Nie, G.; Xu, L. Peroxidase-like activity of apoferritin paired gold clusters for glucose detection. Biosens. Bioelectron. 2015, 64, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Hou, X.; Xu, K. Highly sensitive colorimetric detection of glucose in a serum based on DNA-embeded Au@Ag core–shell nanoparticles. Nanotechnology 2015, 26, 405707. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Wang, Z.; Xu, K.; Ai, S. Double enzymatic cascade reactions within FeSe–Pt@SiO2 nanospheres: synthesis and application toward colorimetric biosensing of H2O2 and glucose. Analyst 2015, 140, 6684–6691. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, X.; Li, Y.; Yu, F.; Tang, L.; Hu, Y. Multifunctional janus hematite–silica nanoparticles: mimicking peroxidase-like activity and sensitive colorimetric detection of glucose. ACS Appl. Mater. Interfaces 2015, 7, 15395–15402. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zeng, L.; Wei, M.; Li, C.M.; Liu, A. AV2O3-ordered mesoporous carbon composite with novel peroxidase-like activity towards the glucose colorimetric assay. Nanoscale 2015, 7, 11678–11685. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Luo, Z.; Huang, X.; Tan, C.; Li, H. Liquid-phase growth of platinum nanoparticles on molybdenum trioxide nanosheets: An enhanced catalyst with intrinsic peroxidase-like catalytic activity. Nanoscale 2014, 6, 12340–12344. [Google Scholar] [CrossRef] [PubMed]
| Nanozymes | Limit of Detection (LOD) | Linear Range | Real Sample Test | Ref |
|---|---|---|---|---|
| Fe3O4 MNPs | 30 µM | 50–1 × 103 µM | N/A | [34] |
| Positively-charged AuNPs | 4 µM | 18–1100 µM | N/A | [67] |
| Nanoceria | 500 µM | 5 × 102–1 × 105 µM | Human Serum | [70] |
| C-dots | 0.4 µM | 1–5 × 102 µM | Human Serum | [81] |
| Water soluble CuO NPs | N/A | 1 × 102–8 × 103 µM | N/A | [49] |
| Re-dispersed CeO2 NPs | 3 µM | 6.6–130 µM | Human Serum | [72] |
| Copper nanoclusters | 100 µM | 1 × 102–2 × 103 µM | N/A | [91] |
| Ag nanoplates | 0.2 µM | 0.2–1 × 102 µM | Human Serum | [92] |
| AuNPs | 49 µM | 1 × 102–1 × 103 µM | Human Serum | [96] |
| MPs | 3.74 µM | N/A | N/A | [32] |
| Nanozymes | Limit of Detection (LOD) | Linear Range | Real Sample Test | Ref |
|---|---|---|---|---|
| GO-COOH | 1 µM | 1–20 µM | Human Serum, juices | [16] |
| Ch-Ag NPs | 0.1 µM | 5–200 µM | Human Serum | [66] |
| PDDA-Fe2O3 | 30 µM | 30–1 × 103 µM | Human Serum | [108] |
| ZnFe2O4 MNPs | 0.3 µM | 1.25–18.75 µM | Urine sample | [41] |
| C60[C(COOH)2]2 | 0.5 µM | N/A | Human Serum | [21] |
| PB-Fe2O3 | 0.16 µM | 1–80 µM | Human Serum | [109] |
| Fe3O4@MSN | 4 µM | 10–500 µM | N/A | [110] |
| GQDs/AgNPs | 0.17 µM | 0.5–400 µM | N/A | [107] |
| CF nano-cubes | 2.47 µM | 8–90 µM | Human Serum | [111] |
| Apoferritin paired gold clusters (Au-Ft) | N/A | 2 × 103–1 × 104 µM | N/A | [112] |
| DNA-embedded core-shell Au@Ag NPs | 0.01 µM | 0–2 × 102 µM | Fetal bovine serum | [113] |
| FeSe-Pt@SiO2 nanospheres | 1.136 nM | 0.01136–227 µM | Human Serum | [114] |
| V2O3-OMC | 3.3 µM | 10–4 × 103 µM | Serum | [116] |
| Janus γ-Fe2O3/SiO2 NPs | 3.2 µM | 0–20 µM | Human Serum | [115] |
| H2TCPP-NiO nanocomposites | 20 µM | 50–5 × 102 µM | N/A | [53] |
| Nitrogen-doped graphene quantum dots | 16 µM | 25–375 µM | Serum | [117] |
| Pt-MoO3 hybrid nanomaterials | 0.1874 µM | 5–500 µM | Serum | [118] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauf, S.; Hayat Nawaz, M.A.; Badea, M.; Marty, J.L.; Hayat, A. Nano-Engineered Biomimetic Optical Sensors for Glucose Monitoring in Diabetes. Sensors 2016, 16, 1931. https://doi.org/10.3390/s16111931
Rauf S, Hayat Nawaz MA, Badea M, Marty JL, Hayat A. Nano-Engineered Biomimetic Optical Sensors for Glucose Monitoring in Diabetes. Sensors. 2016; 16(11):1931. https://doi.org/10.3390/s16111931
Chicago/Turabian StyleRauf, Sajid, Muhammad Azhar Hayat Nawaz, Mihaela Badea, Jean Louis Marty, and Akhtar Hayat. 2016. "Nano-Engineered Biomimetic Optical Sensors for Glucose Monitoring in Diabetes" Sensors 16, no. 11: 1931. https://doi.org/10.3390/s16111931
APA StyleRauf, S., Hayat Nawaz, M. A., Badea, M., Marty, J. L., & Hayat, A. (2016). Nano-Engineered Biomimetic Optical Sensors for Glucose Monitoring in Diabetes. Sensors, 16(11), 1931. https://doi.org/10.3390/s16111931









