Quasi-3D Modeling and Efficient Simulation of Laminar Flows in Microfluidic Devices
Abstract
:1. Introduction
2. Theoretical Background
- The boundary condition at a channel wall is assumed to be the “no-slip” condition, (wall) = 0. This condition is well established even at the submicron level [30];
- The flow is quasi-static. Therefore, the time derivative in Equation (2) can be neglected. By this, it is assumed that the fluid velocities are sufficiently low so that any change in the boundary conditions results in an instantaneous rearrangement of the velocity and pressure fields to conform to the new conditions;
- Low-Reynolds-number flow is dominated by viscous forces. So the term can be ignored for low Reynolds number; i.e., [31]. For aqueous flows in microchannels with scale lengths on the order of 50 μm, velocities only need to be much less than 1 m·s−1 for this to be valid. For the majority of the cases of interest, their velocities are significantly less than 1 m·s−1. Equation (2) then reduces to the much simpler equation:
- Finally, there are no free-moving bodies (particles) in the flow that can locally disrupt the velocity profile, a condition commonly assumed in microfluidic simulations containing cells or particles in low concentrations, so that the particle-induced hydro-dynamic disturbances in the flow field can be neglected [32].
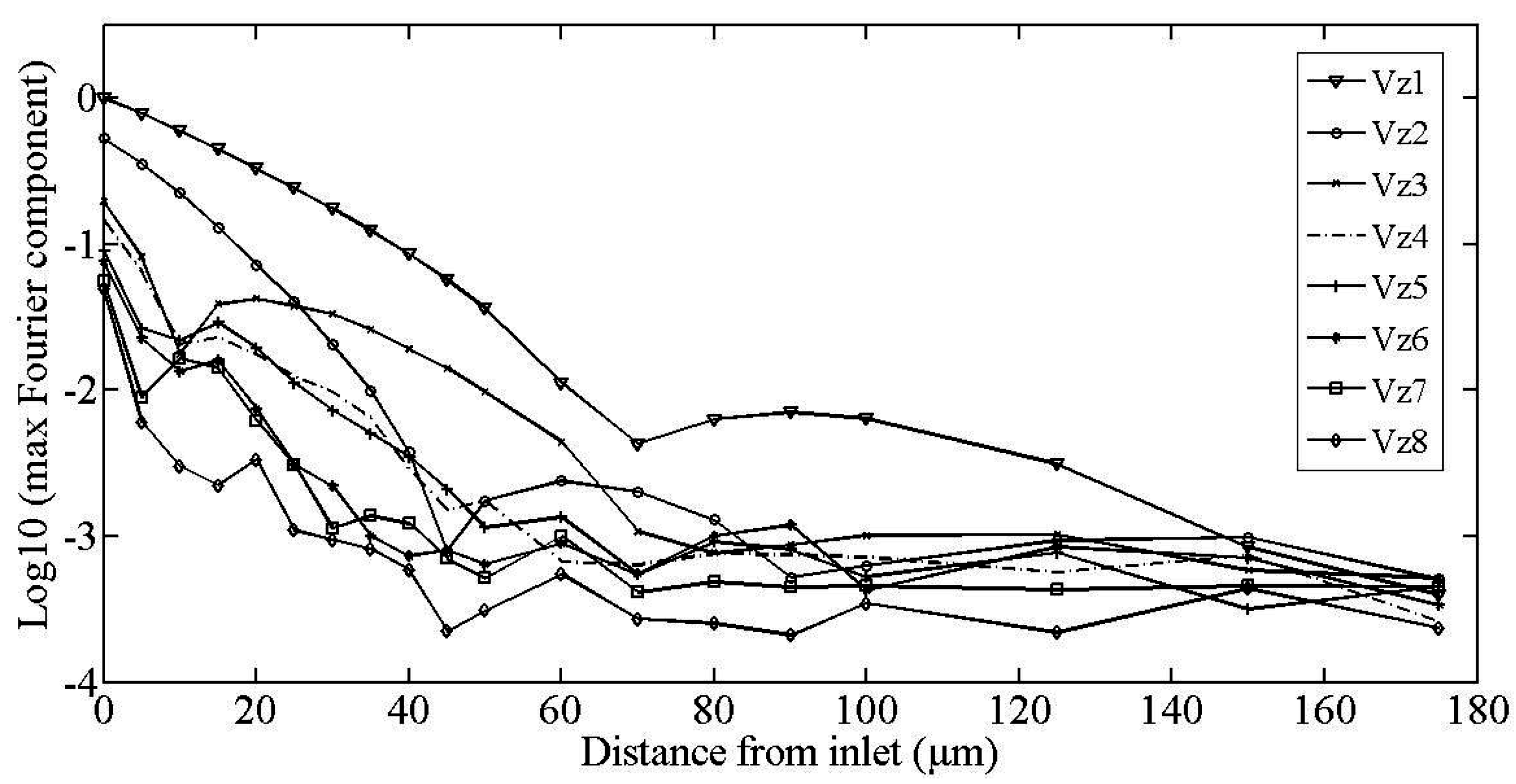
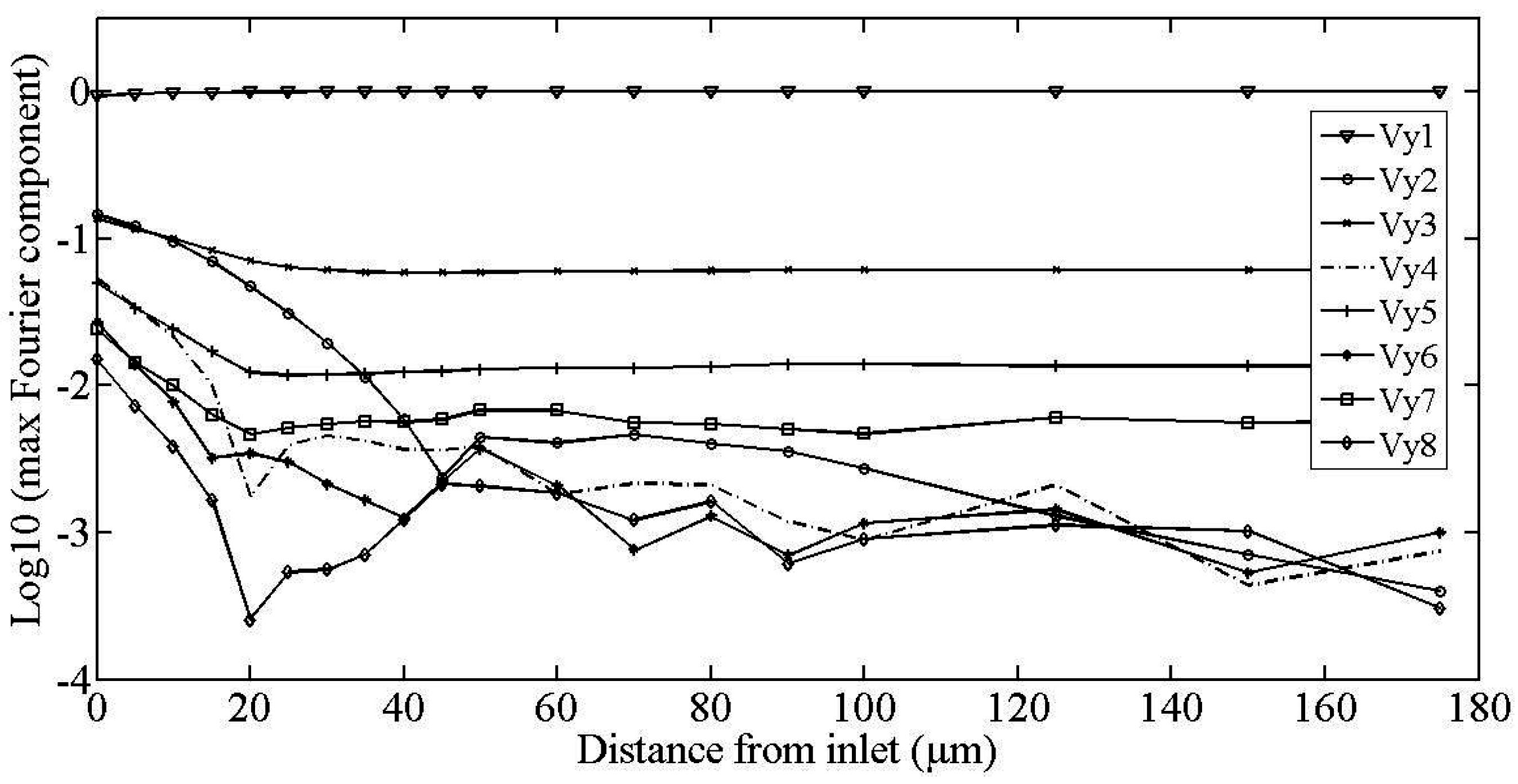
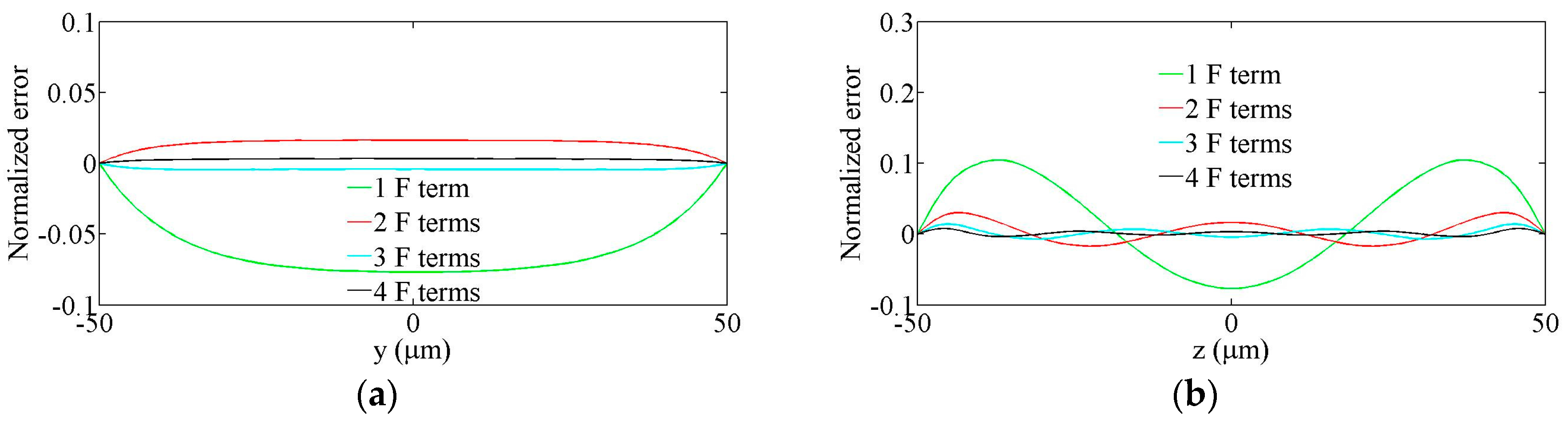
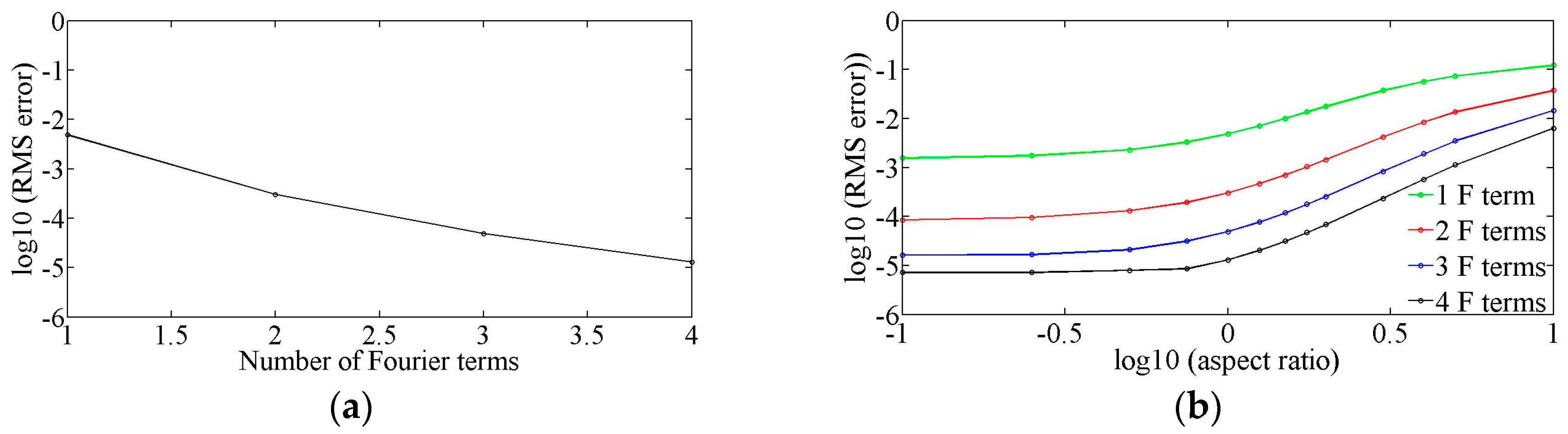
2.1. Fourier Expansion of the Flow Field
3. Testing of the Model
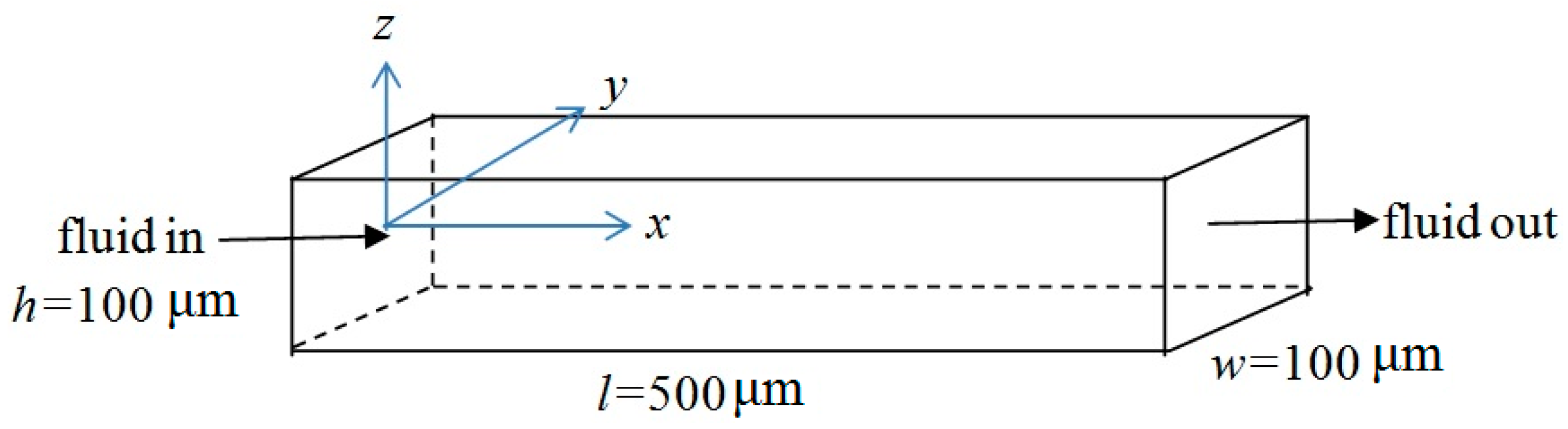
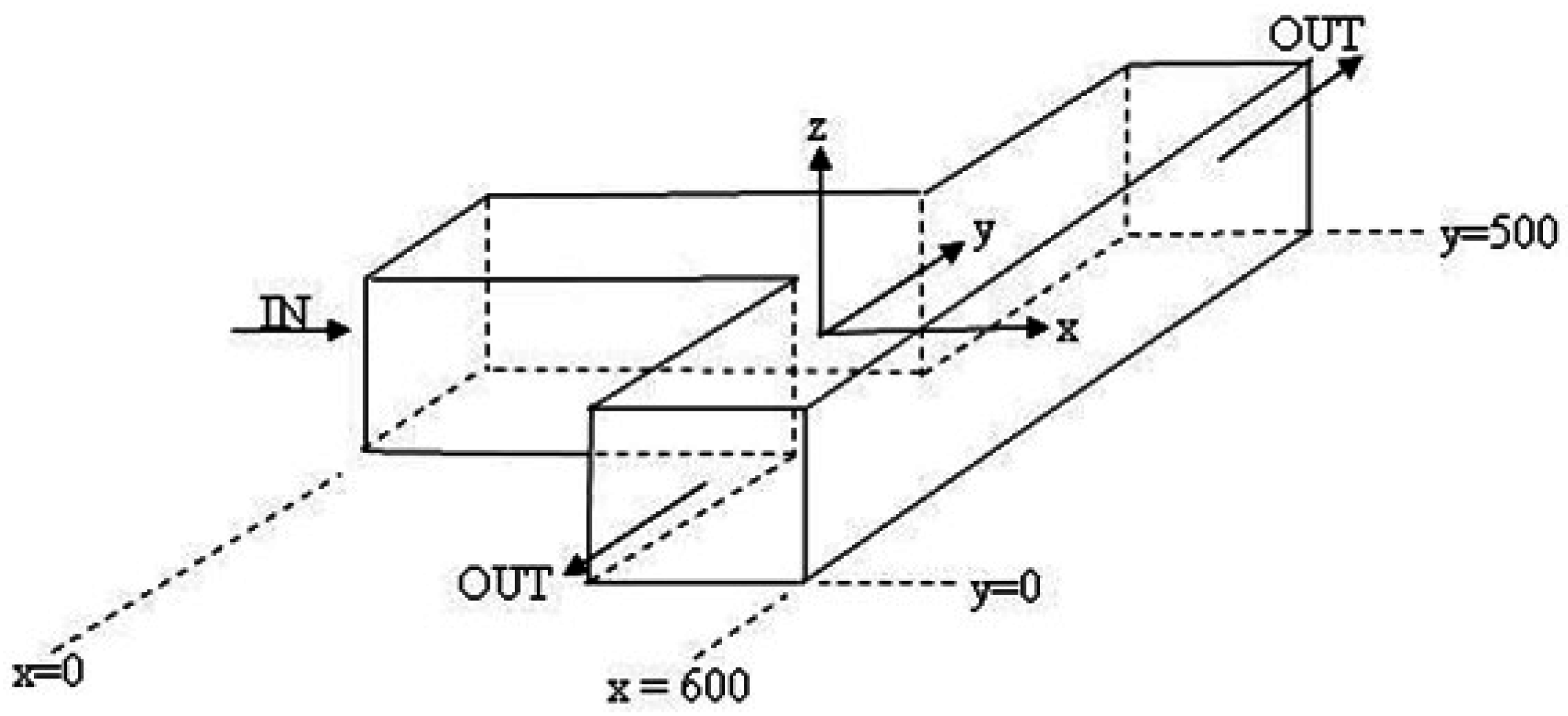
3.1. Straight Rectangular Channel
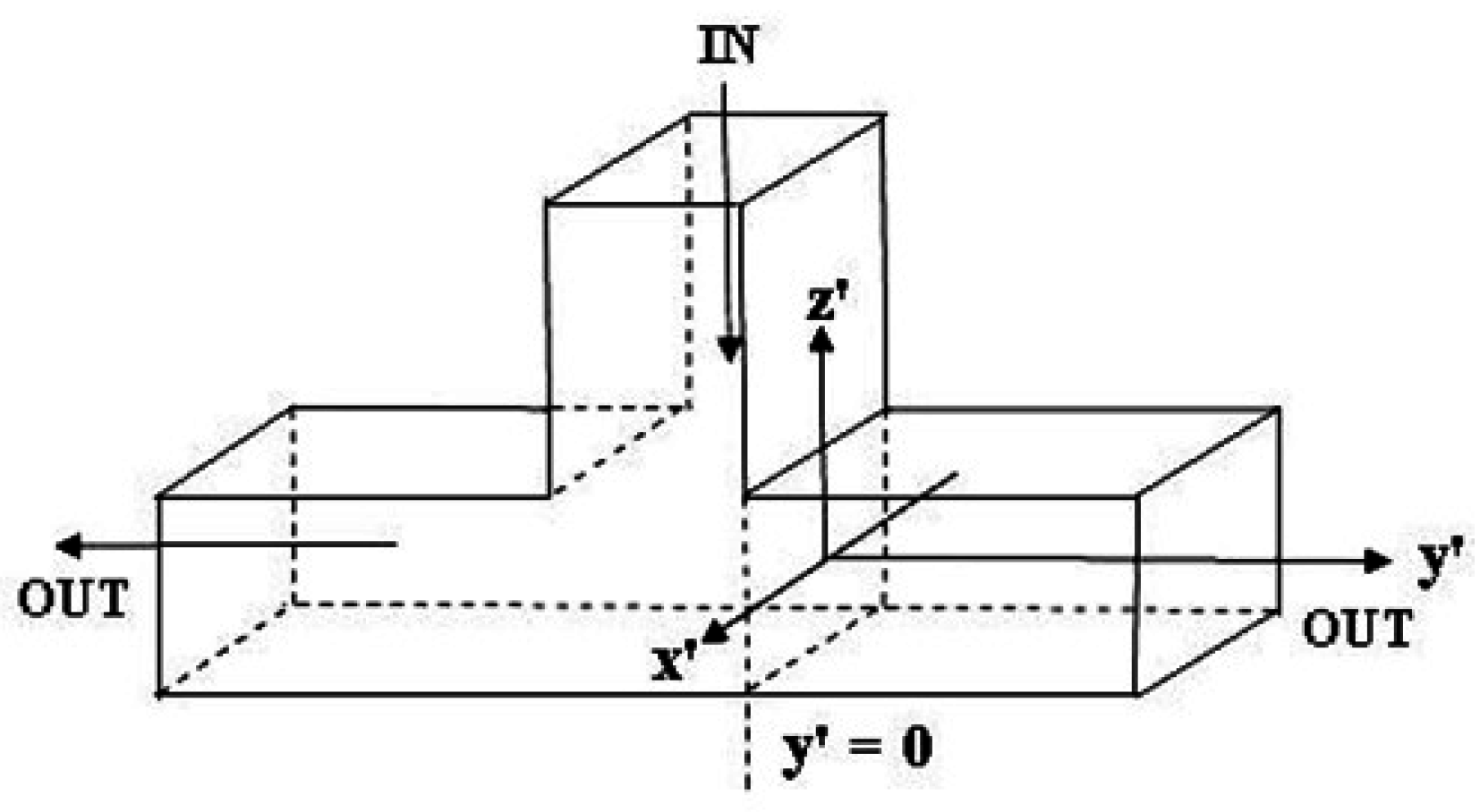
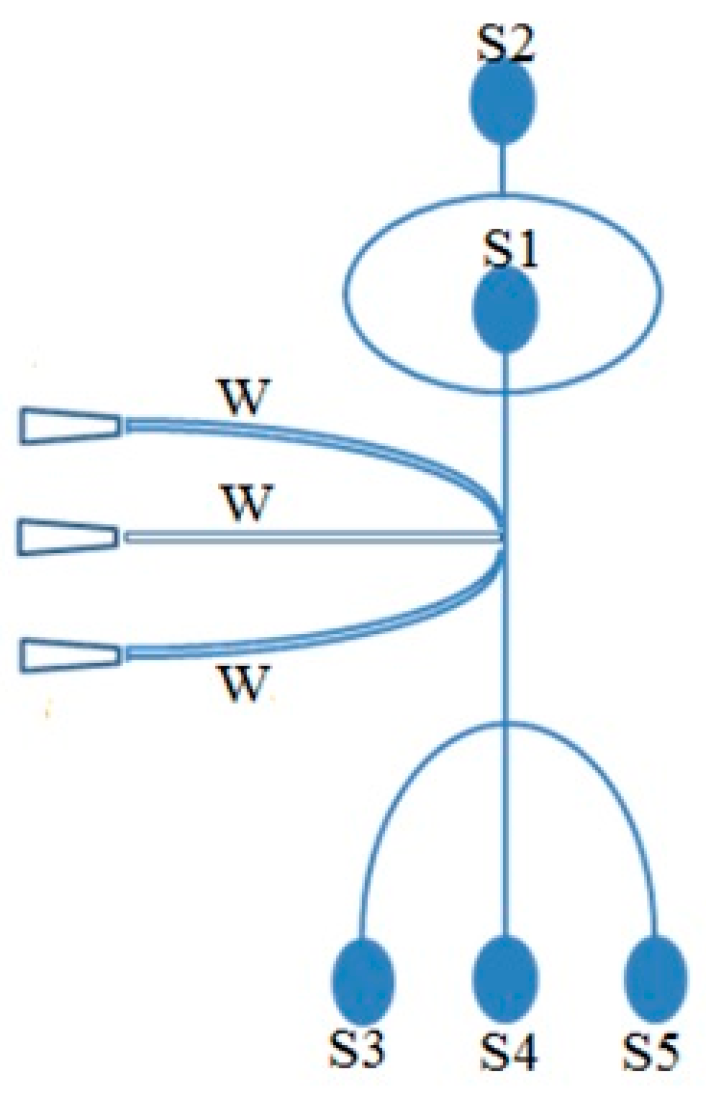
3.2. Flow in a T-cell
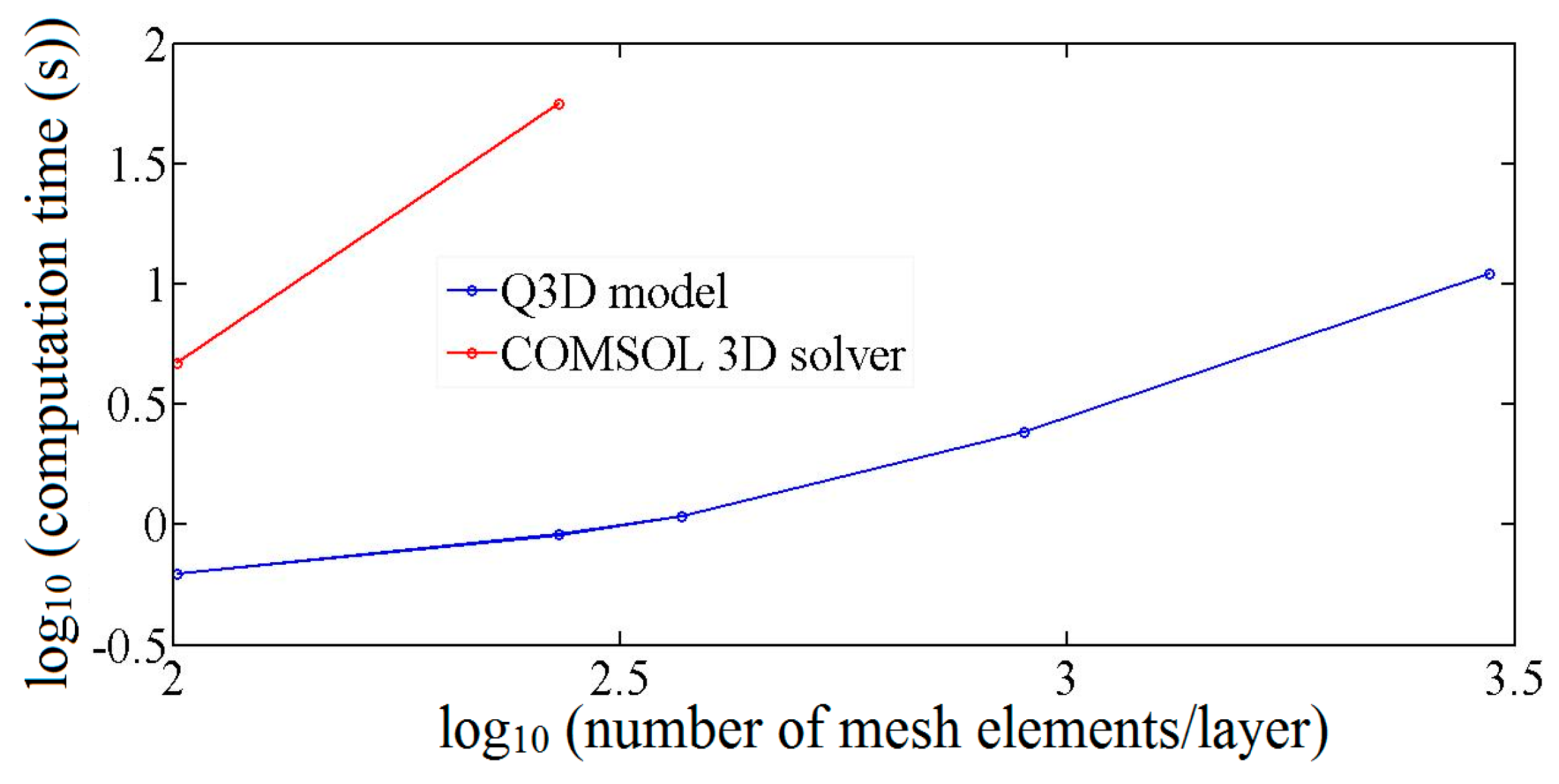
3.3. Computation Resources
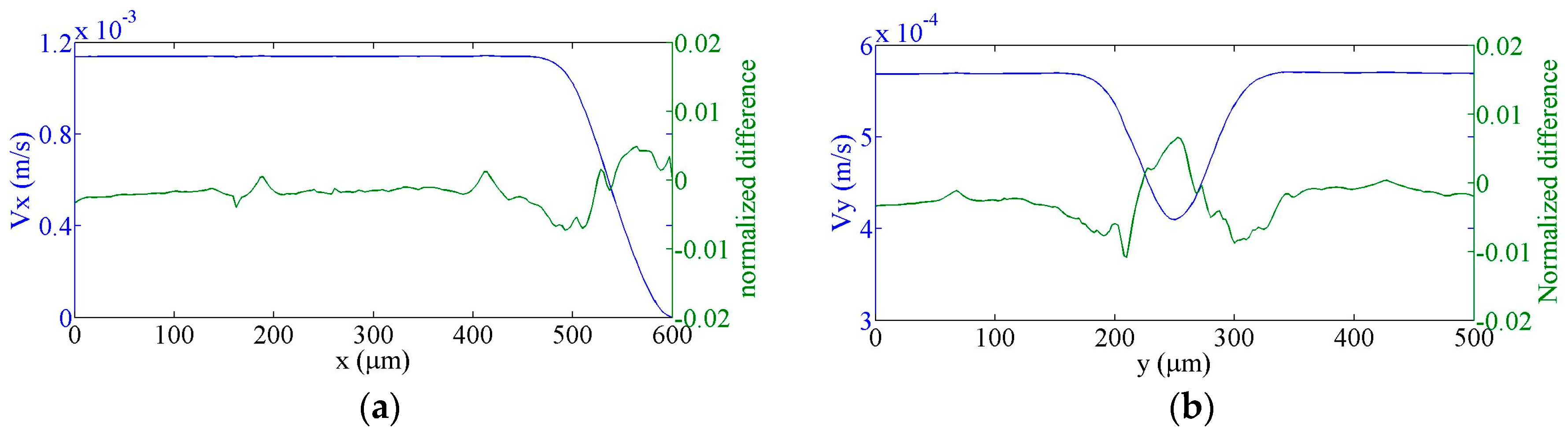
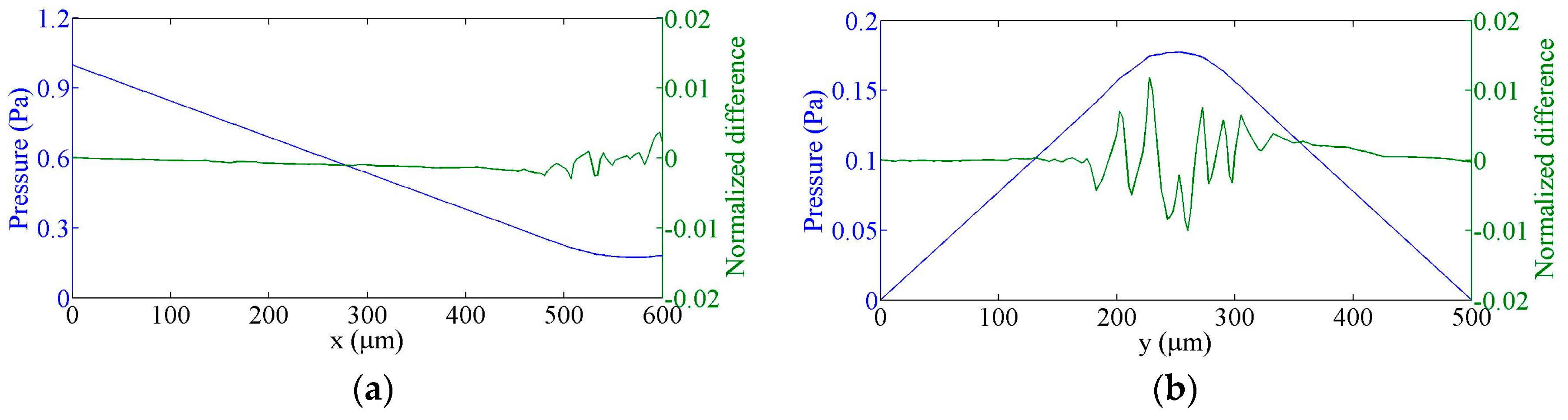
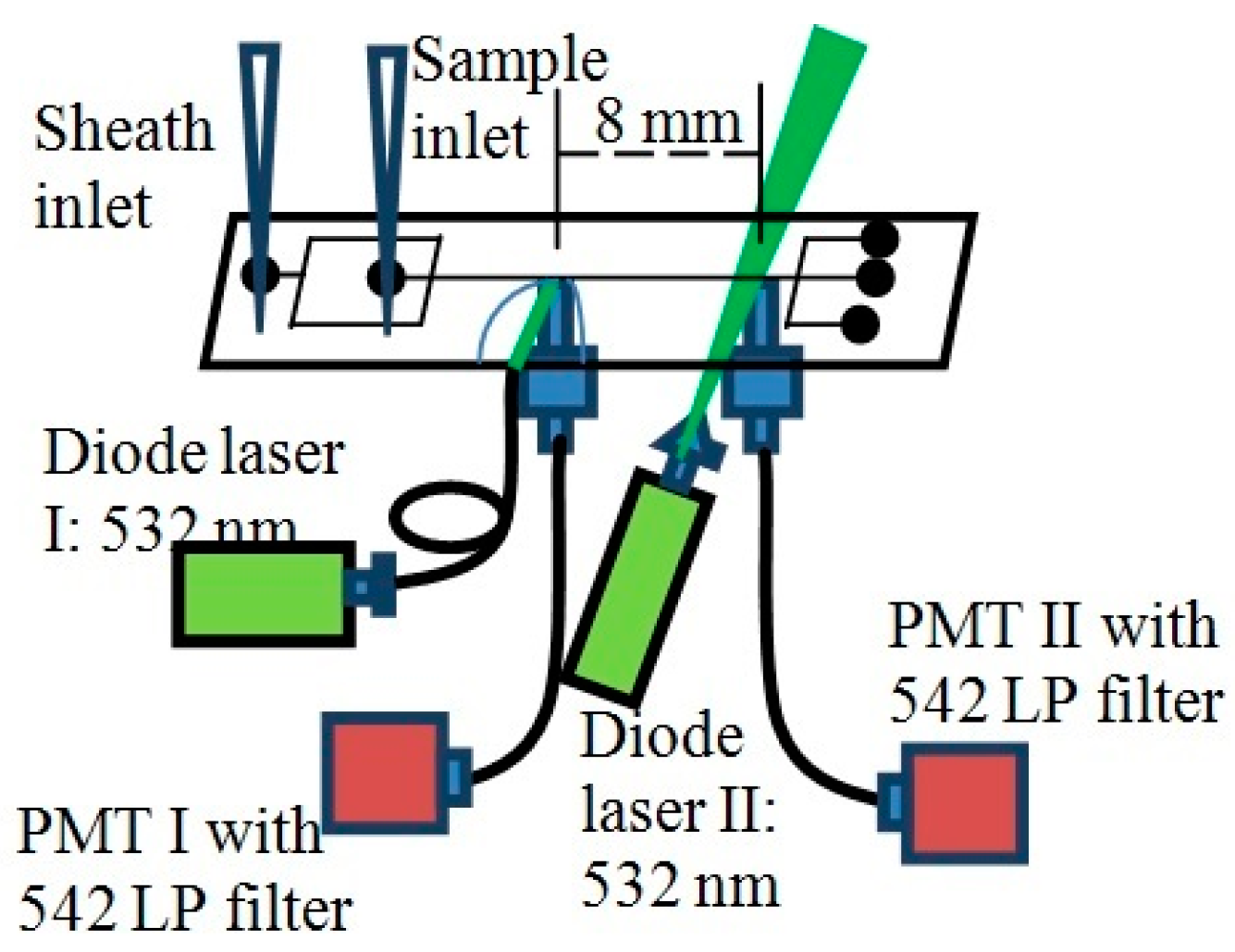
4. Comparison of Experimental and Simulation Results
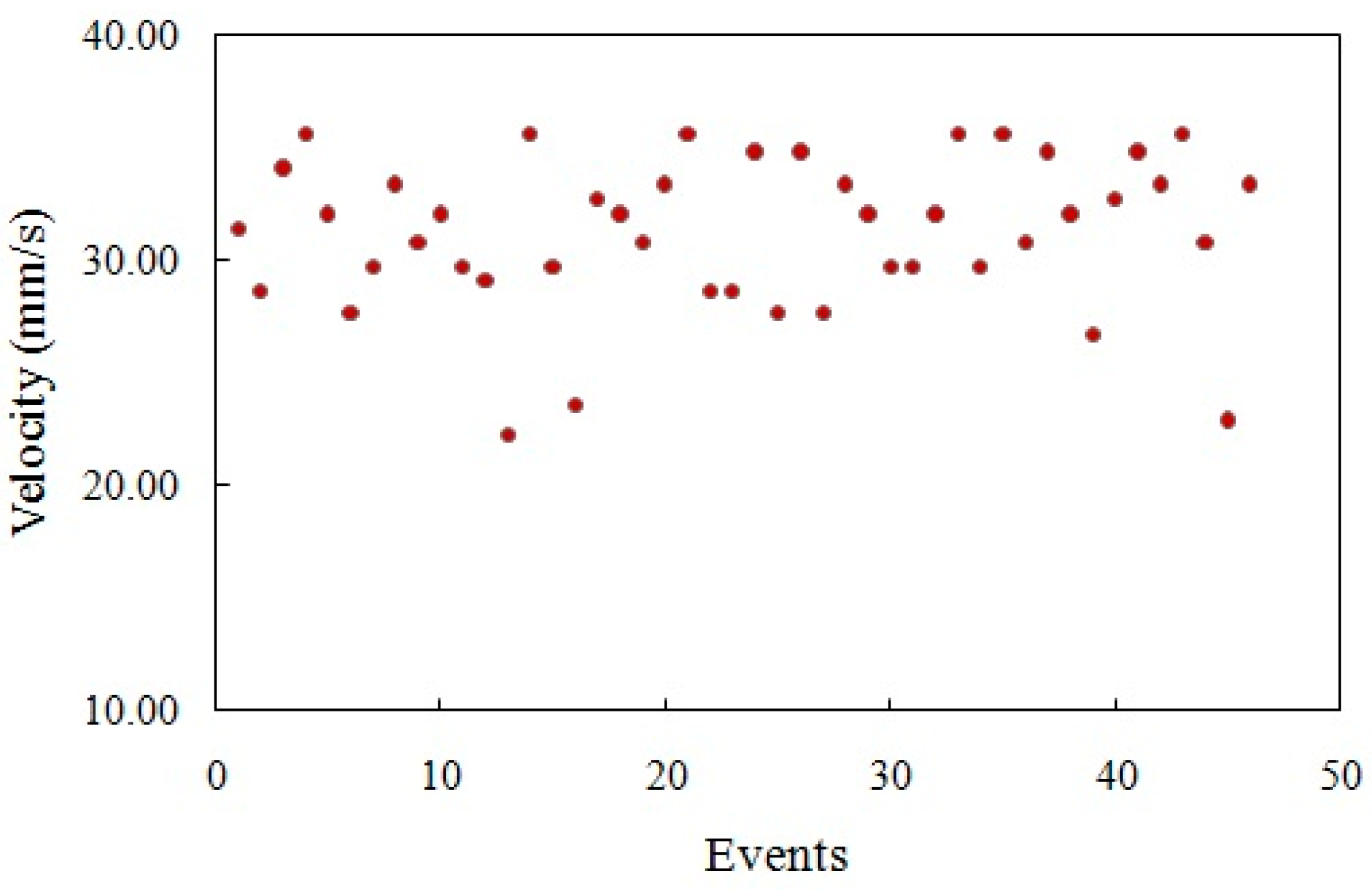
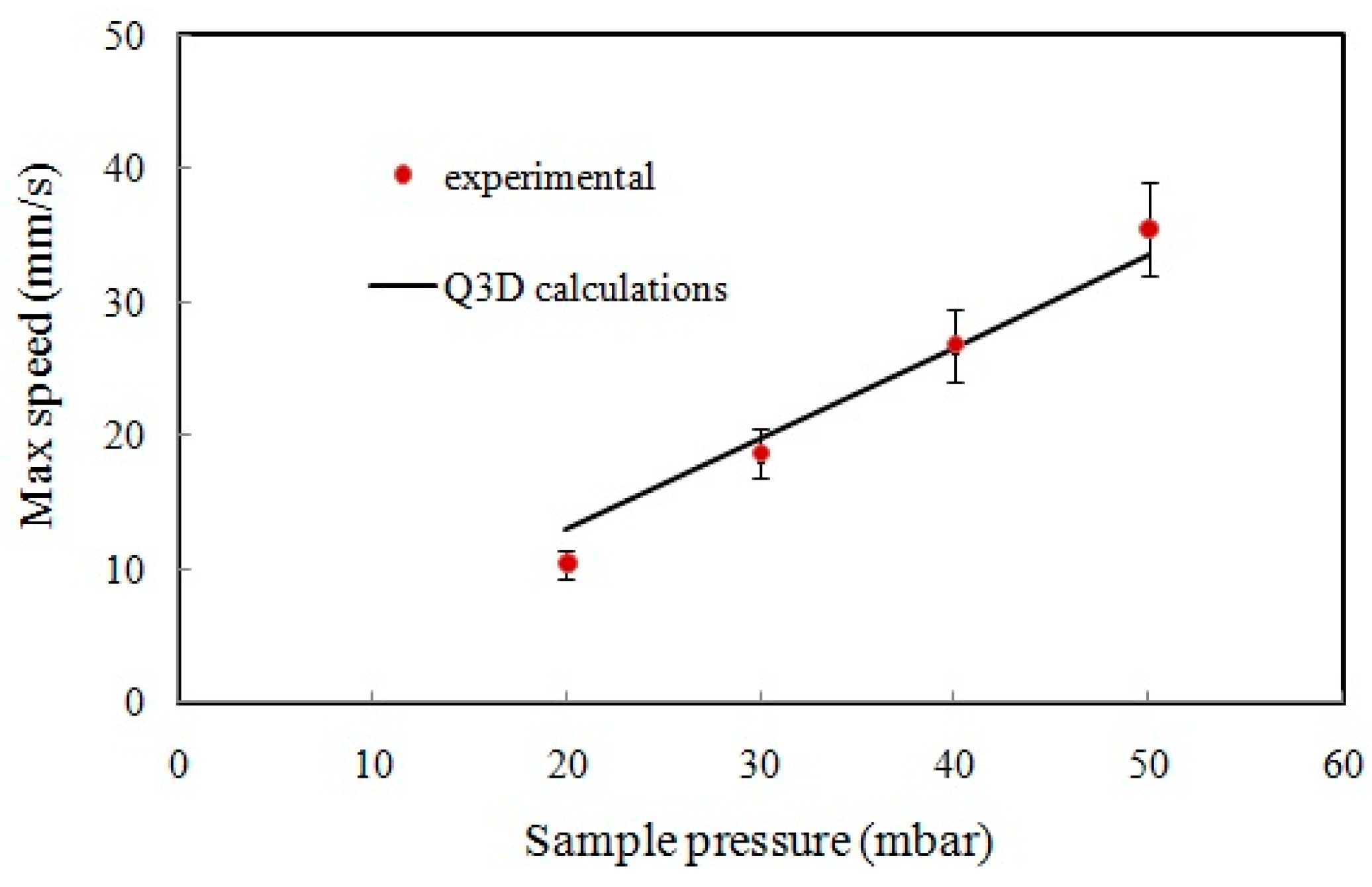
4.1. Flow Speed Characterization in the Main Microchannel
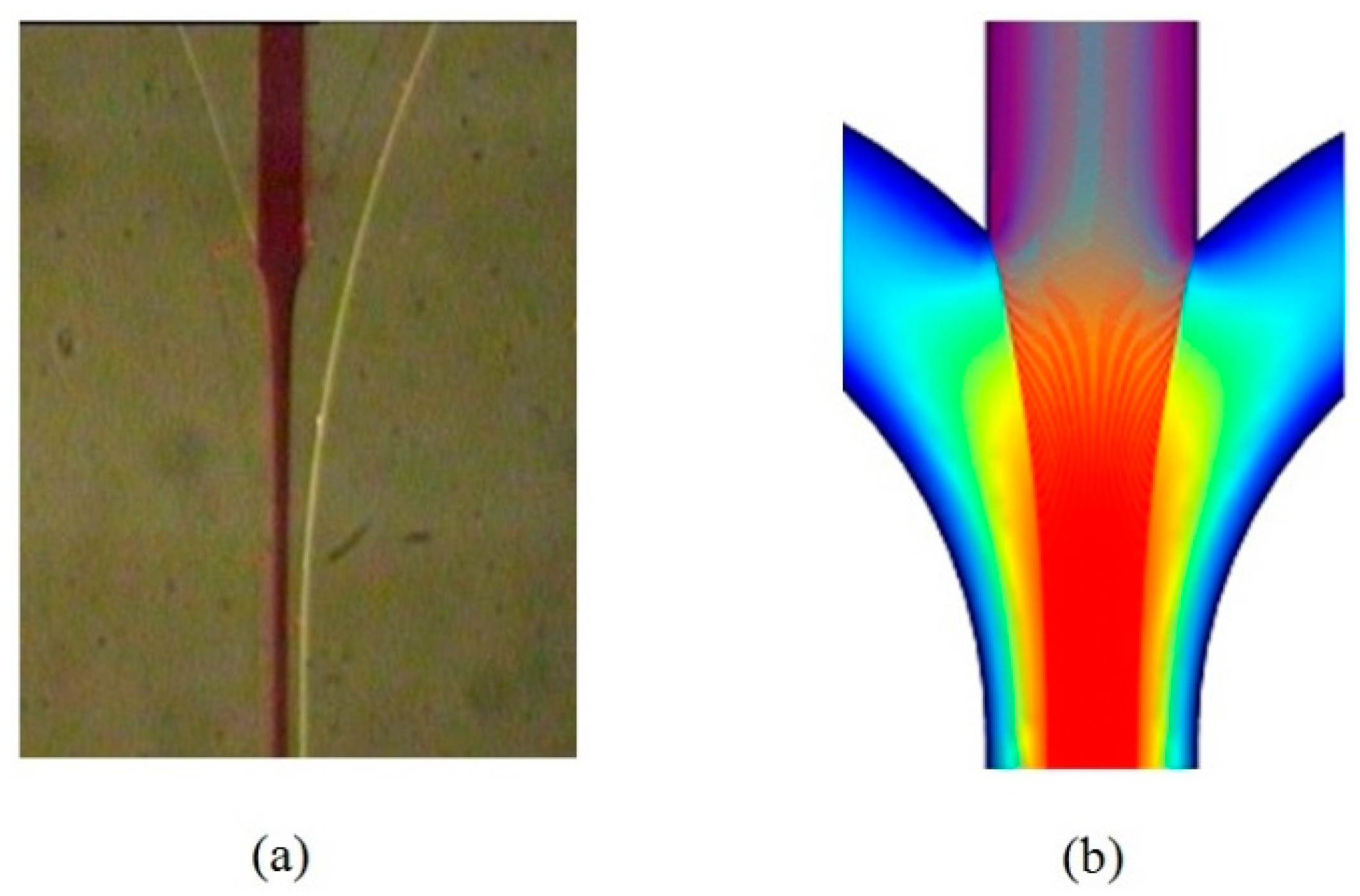
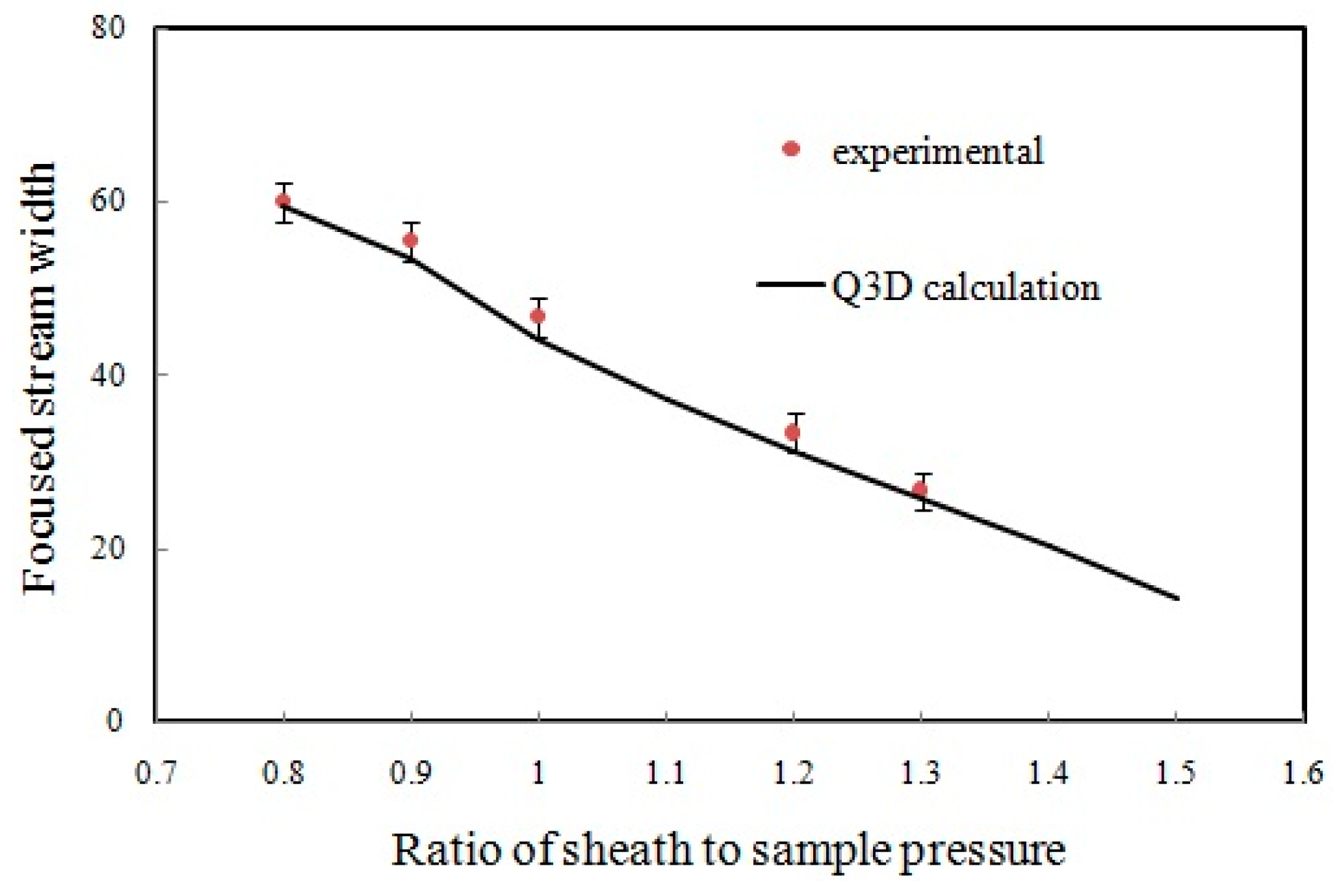
4.2. Flow Focusing Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Approximation of Symmetric Flow
References
- Gravesen, P.; Branebjerg, J.; Jensen, O.S. Microfluidics: A review. J. Micromech. Microeng. 1993, 3, 168182. [Google Scholar] [CrossRef]
- Hou, H.H.; Wang, Y.N.; Chang, C.L.; Yang, R.J.; Fu, L.M. Rapid glucose concentration detection utilizing disposable integrated microfluidic chip. J. Microfluid. Nanofluid. 2011, 11, 479–487. [Google Scholar] [CrossRef]
- Weng, X.; Jiang, H.; Li, D. Microfluidic DNA hybridization assays. J. Microfluid. Nanofluid. 2012, 11, 367–383. [Google Scholar] [CrossRef]
- Weng, X.; Chon, C.H.; Jiang, H.; Li, D. Rapid detection of formaldehyde concentration in food on a polydimethylsiloxane (PDMS) microfluidic chip. Food Chem. 2009, 114, 1079–1082. [Google Scholar] [CrossRef]
- Jiang, H.; Weng, X.; Li, D. Microfluidic whole-blood immunoassays. J. Microfluid. Nanofluid. 2011, 10, 941–964. [Google Scholar] [CrossRef]
- Wu, H.W.; Hsu, R.C.; Lin, C.C.; Hwang, S.M.; Lee, G.B. An integrated microfluidic system for isolation, counting, and sorting of hematopoietic stem cells. Biomicrofluidics 2010, 4, 024112. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahon, A.R.; Gordon, J.; Nowak, C.; Sengupta, S.; Powell, T.H.Q.; Feder, J.; Lodge, D.M.; Chang, H. Rapid on-chip genetic detection microfluidic platform for real world applications. Biomicrofluidics 2009, 3, 022407. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.C.; Emory, J.M.; Henry, C.S. Advances in microfluidics for environmental analysis. Analyst 2012, 137, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kang, Q. Electrochemomechanical energy conversion efficiency in silica nanochannels. J. Microfluid. Nanofluid. 2010, 9, 181–190. [Google Scholar] [CrossRef]
- Su, X.; Kirkwood, S.E.; Gupta, M.; Curtis, L.M.; Qiu, Y.; Wieczorek, A.J.; Rozmus, W.; Tsui, Y.Y. Microscope-based label-free microfluidic cytometry. Opt. Express 2011, 19, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Moniz, A.; Triantis, I.; Michelakis, K.; Trzebinski, J.; Azarbadegan, A.; Field, B.; Toumazou, C.; Eames, I.; Cass, A. An integrated silicon sensor with microfuidic chip for monitoring potassium and pH. J. Microfluid. Nanofluid. 2011, 10, 1119–1125. [Google Scholar] [CrossRef]
- Lin, C.H.; Wang, J.H.; Fu, L.M. Improving the separation efficiency of DNA biosamples in capillary electrophoresis microchips using high-voltage pulsed dc electric fields. J. Microfluid. Nanofluid. 2008, 5, 403–410. [Google Scholar] [CrossRef]
- Kjeang, E.; Djilali, N.; Sinton, D. Microfluidic fuel cells: A review. J. Power Sources 2009, 186, 353–369. [Google Scholar] [CrossRef]
- Mairhofer, J.; Roppert, K.; Ertl, P. Microfluidic systems for pathogen sensing: A review. Sensors 2009, 9, 4804–4823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ozdemir, P. Microfluidic DNA amplication—A review. Anal. Chim. Acta 2009, 638, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Chorlton, F. Textbook of Fluid Dynamics; Van Nostrand Company: London, UK, 1967. [Google Scholar]
- Sobek, D.; Young, A.M.; Gray, M.L.; Senturia, S.D. A microfabricated flow chamber for optical measurements in fluids. In Micro Electro Mechanical Systems, MEMS’s 93, Proceedings of the Investigation of Micro Structures, Sensors, Actuators, Machines and Systems, Fort Lauderdale, FL, USA, 7–10 February 1993; IEEE: New York, NY, USA, 1993. [Google Scholar]
- Gass, V.; van der Schoot, B.H.; de Rooij, N.F. Nanofluid handling by micro-flow sensor based on drag force measurements. In Micro Electro Mechanical Systems, MEMS’s 93, Proceedings of Investigation of Micro Structures, Sensors, Actuators, Machines and Systems, Fort Lauderdale, FL, USA, 7–10 February 1993; IEEE: New York, NY, USA, 1993. [Google Scholar]
- Manz, A.; Miyahara, Y.; Miura, J.; Watanabe, Y.; Miyagi, H.; Sato, K. Design of an open-tubular column liquid chromatograph using silicon chip technology. Sens. Actuators B Chem. 1990, 1, 249–255. [Google Scholar] [CrossRef]
- Tiren, J.; Tenerz, L.; Hok, B. A batch-fabricated non-reverse valve with cantilever beam manufactured by micromachining of silicon. Sens. Actuators 1989, 18, 389–396. [Google Scholar] [CrossRef]
- Bruss, H. Theoretical Microfluidics, 1st ed.; Oxford University Press: London, UK, 2008. [Google Scholar]
- Bourouina, T.; Grandchamp, J.P. Modeling micropumps with electrical equivalent networks. J. Micromech. Microeng. 1996, 6, 398–404. [Google Scholar] [CrossRef]
- Auerbach, F.J.; Meiendres, G.; Mller, R.; Schelle, G.J.E. Simulation of the thermal behaviour of thermal flow sensors by equivalent electrical circuits. Sens. Actuators A Phys. 1994, 41, 275–278. [Google Scholar] [CrossRef]
- Swart, N.R.; Nathan, A. Flow-rate microsensor modeling and optimization using SPICE. Sens. Actuators A Phys. 1992, 34, 109–122. [Google Scholar] [CrossRef]
- Erickson, D. Towards numerical prototyping of labs-on-chip: Modeling for integrated microfluidic devices. J. Microfluid. Nanofluid. 2005, 1, 301–318. [Google Scholar] [CrossRef]
- Ulrich, J.; Zengerle, R. Static and dynamic flow simulation of a KOH-etched microvalve using the finite-element method. Sens. Actuators A Phys. 1996, 53, 379–385. [Google Scholar] [CrossRef]
- Koch, M.; Evans, A.G.R.; Brunnschweiler, A. Coupled fem simulation for the characterization of the fluid flow within a micromachined cantilever valve. J. Micromech. Microeng. 1996, 6, 112–114. [Google Scholar] [CrossRef]
- McMullin, J.N. Propagating Beam Method Simulation of Planar Multimode Optical Waveguides. In Proceedings of the SPIE 1389, Microelectronic Interconnects and Packages: Optical and Electrical Technologies, Boston, MA, USA, 1 April 1991; pp. 621–629.
- Smits, A.J. A Physical Introduction to Fluid Mechanics, 1st ed.; John Wiley and Sons Inc.: San Francisco, CA, USA, 2000. [Google Scholar]
- Bocquet, L.; Barrat, J.L. Hydrodynamic boundary conditions and correlation functions of confined fluids. Phys. Rev. Lett. 1993, 70, 2726–2729. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.P.; Yager, P.; Goldstein, R.E.; Austin, R.H. Biotechnology at low Reynolds numbers. BioPhys. J. 1996, 71, 3430–3441. [Google Scholar] [CrossRef]
- Amini, H.; Sollier, E.; Weaver, W.M.; Carlo, D.D. Intrinsic particle-induced lateral transport in microchannels. Proc. Natl. Acad. Sci. USA 2012, 109, 11593–11598. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; McMullin, J.N.; Tsui, Y.Y. Rapid and cheap prototyping of a micro uidic cell sorter. Cytometry A 2011, 79A, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z. Development of an Efficient Quasi-3D Microfluidic Flow Model and Fabrication and Characterization of an All-PDMS Opto-Microfluidic Flow Cytometer. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2012. [Google Scholar]
- Microfluidic Solutions and Systems, Pumps and Flow Controller: Fluigent. Available online: http://www.fluigent.com (accessed on 3 October 2016).
- Kim, Y.W.; Yoo, J.Y. Transport of solid particles in microfluidic channels. Opt. Laser. Eng. 2012, 50, 8798. [Google Scholar] [CrossRef]
- Park, J.S.; Song, S.H.; Jung, I.H. Continuous focusing of microparticles using inertial lift force and vorticity via multi-orifice microfluidic channels. Lab Chip 2009, 9, 939948. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.Z.; Tsui, Y.Y. Quasi-3D Modeling and Efficient Simulation of Laminar Flows in Microfluidic Devices. Sensors 2016, 16, 1639. https://doi.org/10.3390/s16101639
Islam MZ, Tsui YY. Quasi-3D Modeling and Efficient Simulation of Laminar Flows in Microfluidic Devices. Sensors. 2016; 16(10):1639. https://doi.org/10.3390/s16101639
Chicago/Turabian StyleIslam, Md. Zahurul, and Ying Yin Tsui. 2016. "Quasi-3D Modeling and Efficient Simulation of Laminar Flows in Microfluidic Devices" Sensors 16, no. 10: 1639. https://doi.org/10.3390/s16101639
APA StyleIslam, M. Z., & Tsui, Y. Y. (2016). Quasi-3D Modeling and Efficient Simulation of Laminar Flows in Microfluidic Devices. Sensors, 16(10), 1639. https://doi.org/10.3390/s16101639




