Development of a Room Temperature SAW Methane Gas Sensor Incorporating a Supramolecular Cryptophane A Coating
Abstract
:1. Introduction

2. Technique Realization
2.1. Two-Port SAW Resonator
| Parameters | Values | Parameters | Values |
|---|---|---|---|
| Operation frequency (MHz) | 300 | Wavelength (λ: μm) | 10.5 |
| IDT length (λ) | 41 | Gap between the reflectors and IDT (λ) | 0.75/0.5 |
| Reflector length (λ) | 300 | Length of the coating area (λ) | 150 |
| aperture (λ) | 200 | Gap between the IDT and coating area (λ) | 5 |
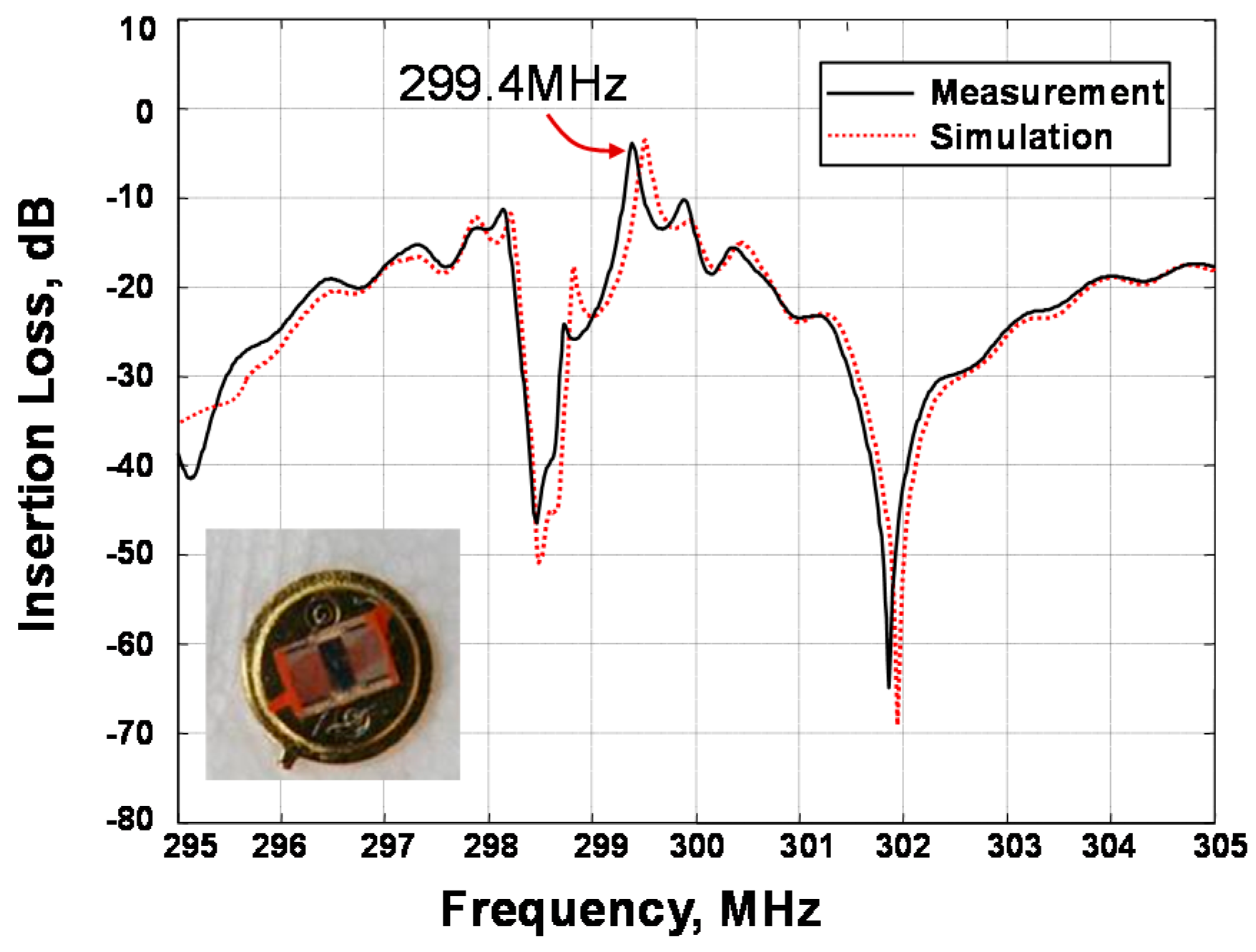
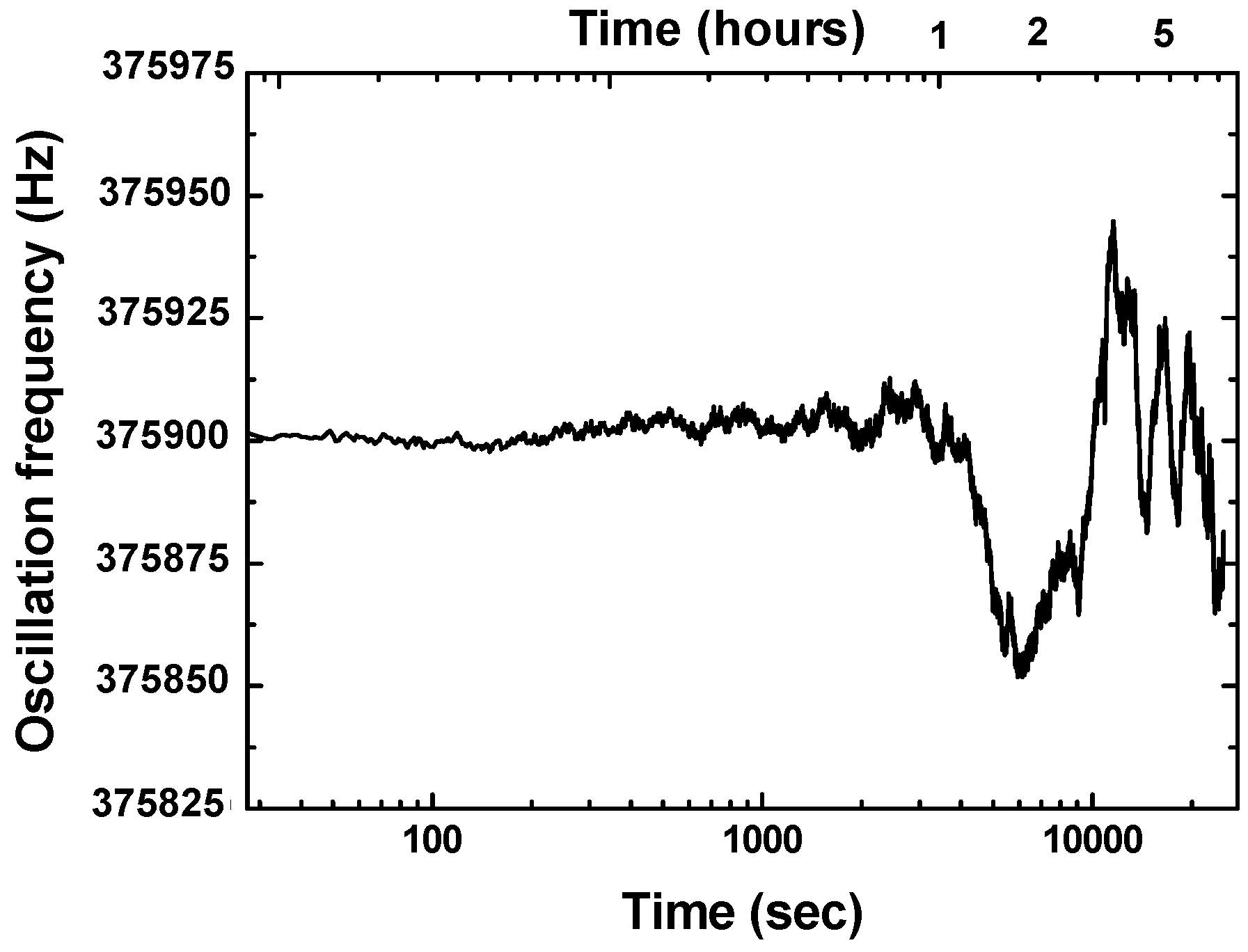
2.2. Differential Resoantor-Oscillator

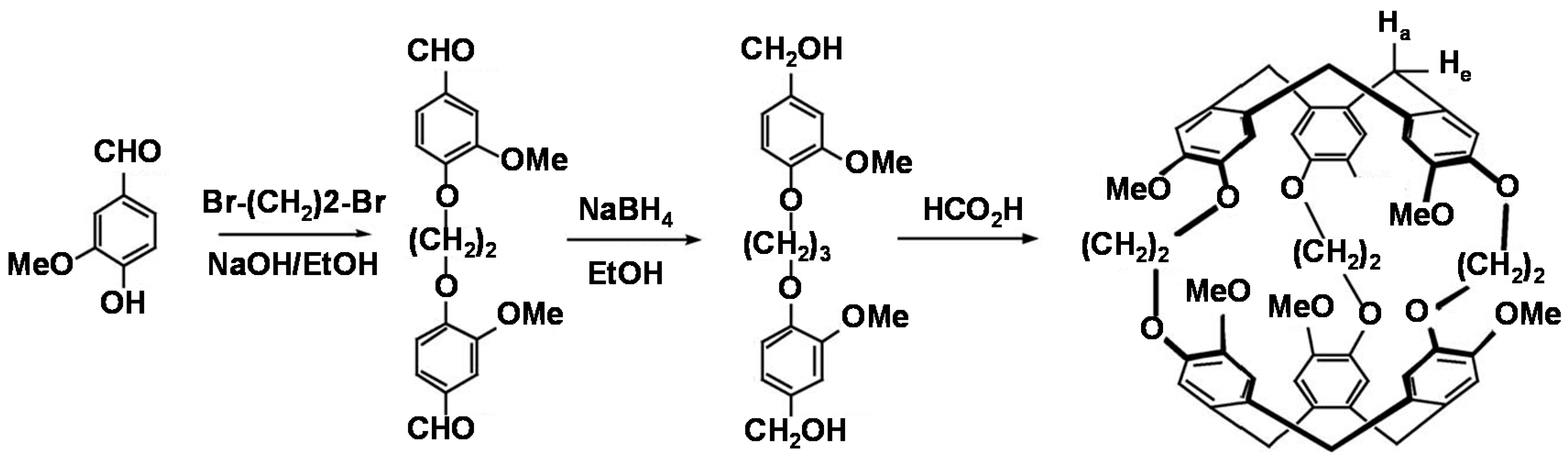
2.3. Synthesis and Characterization of CrypA
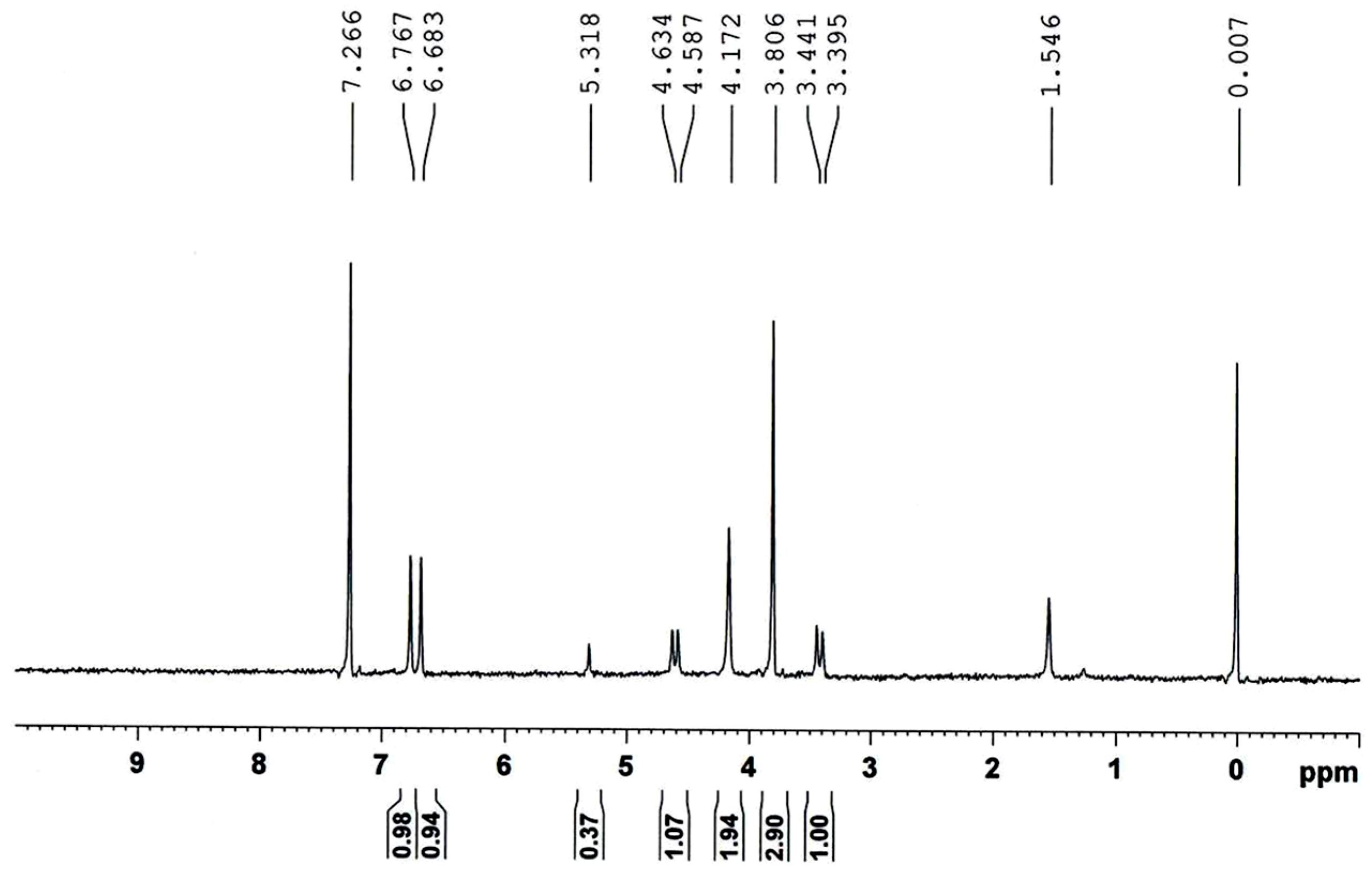
2.4. CrypA Deposition
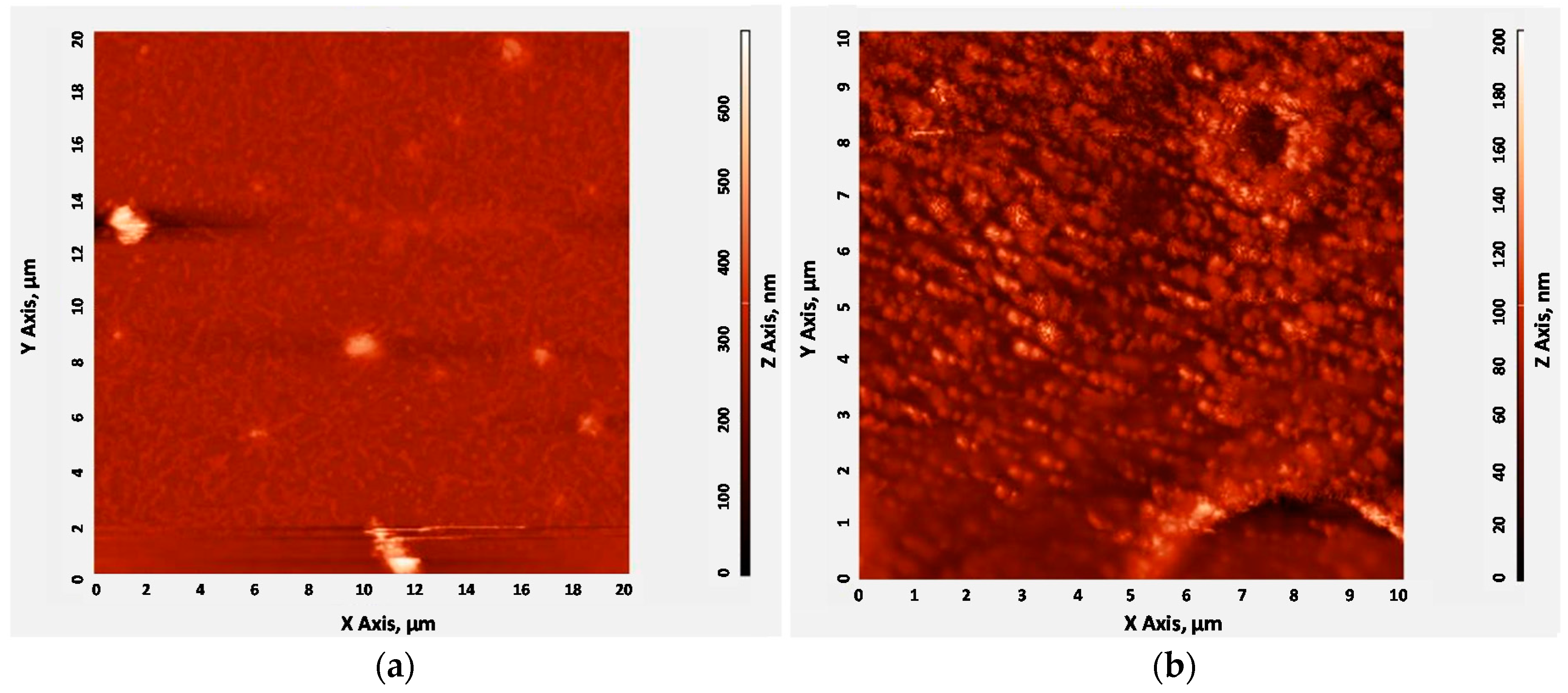
3. Sensor Experiments
3.1. Gas Sensor Experimental Setup
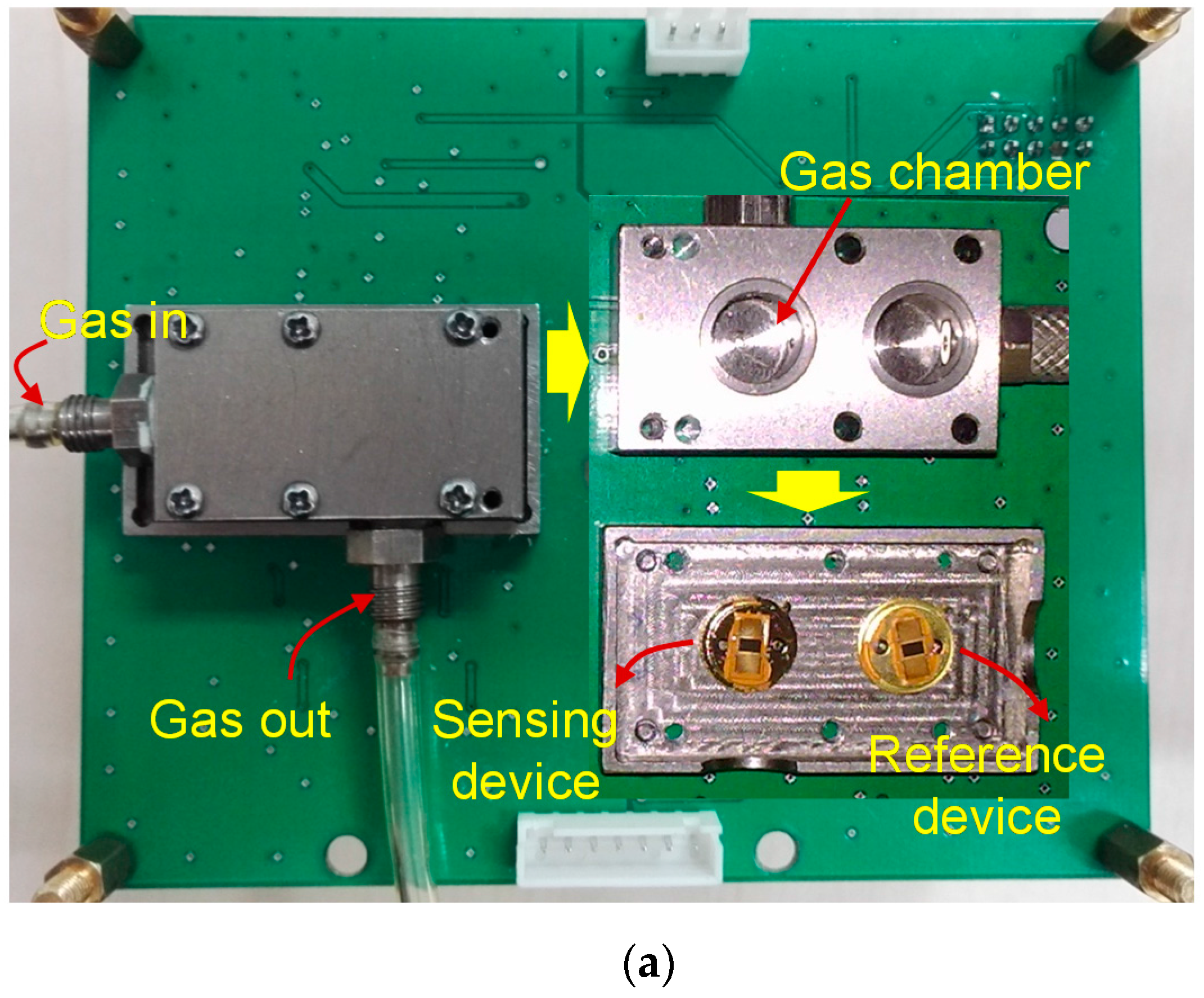
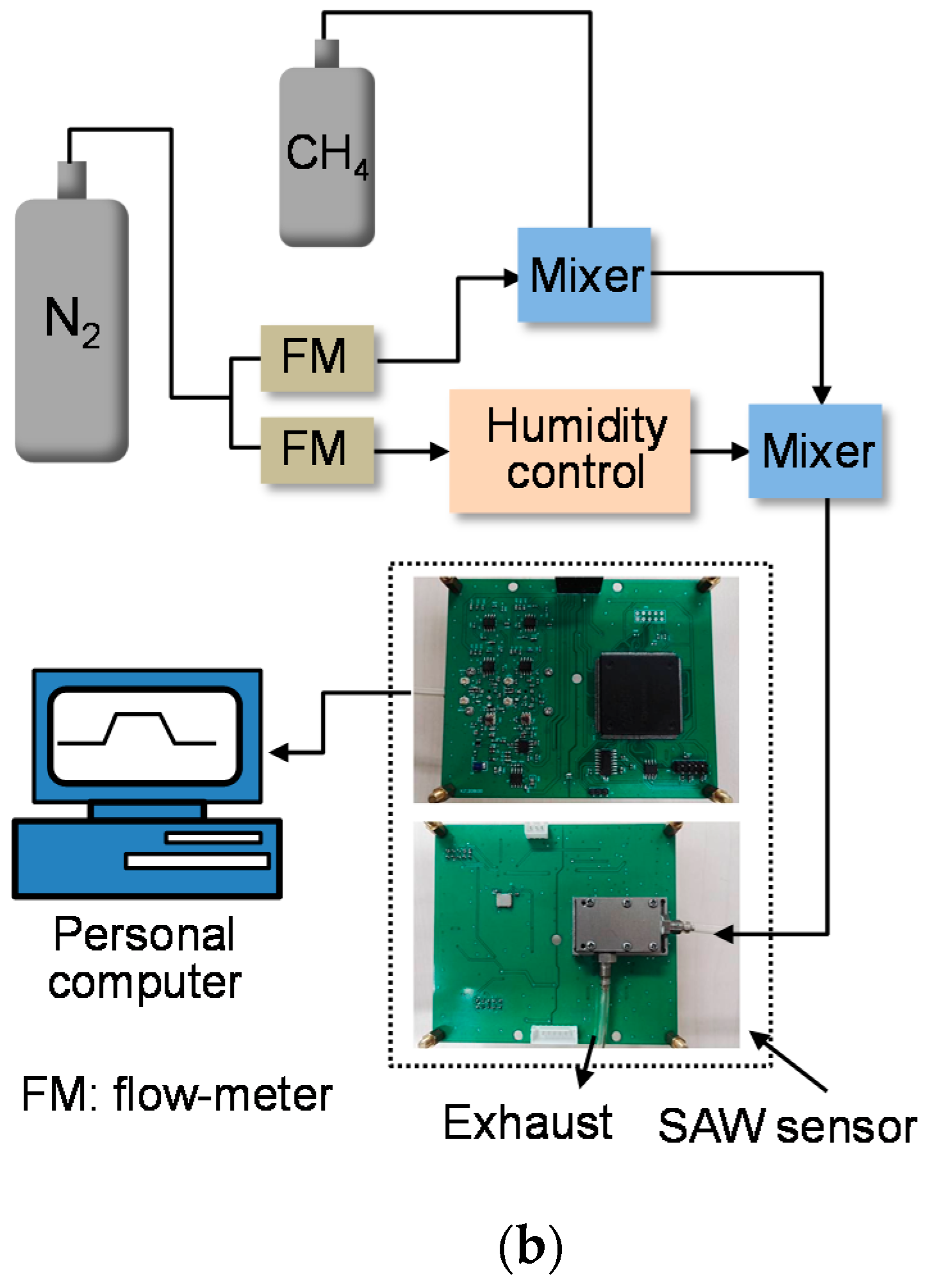
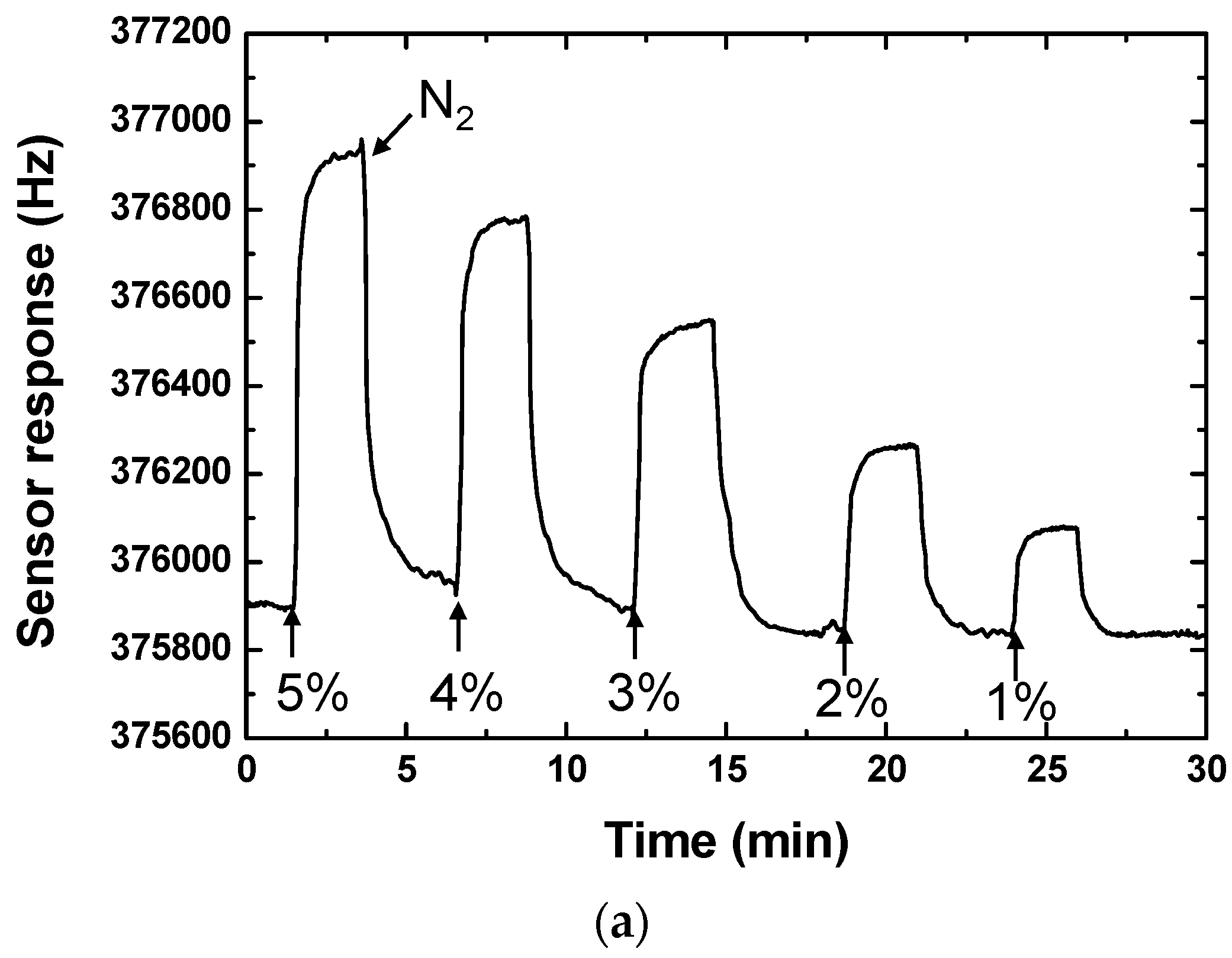
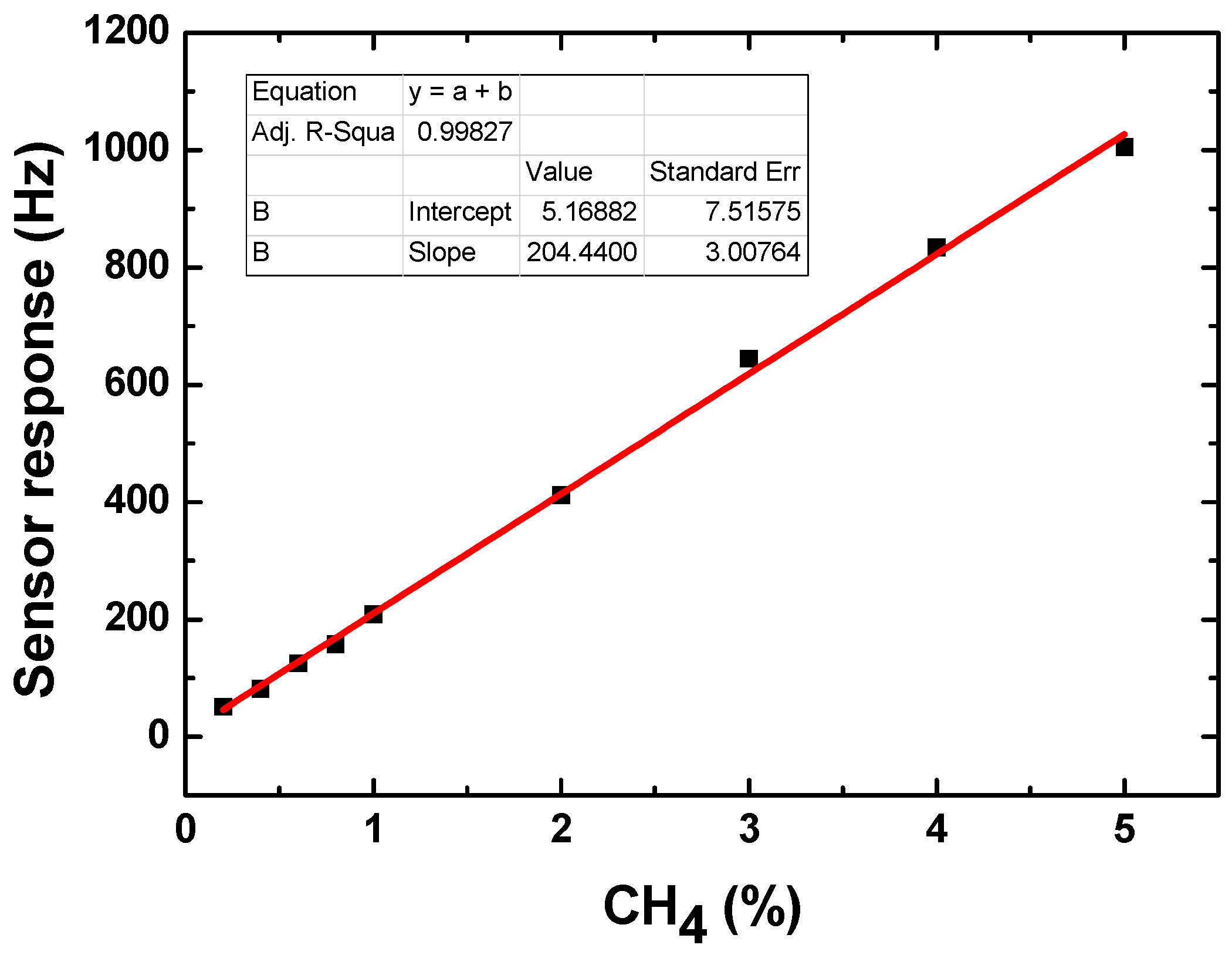
3.2. Sensor Performance Evaluation



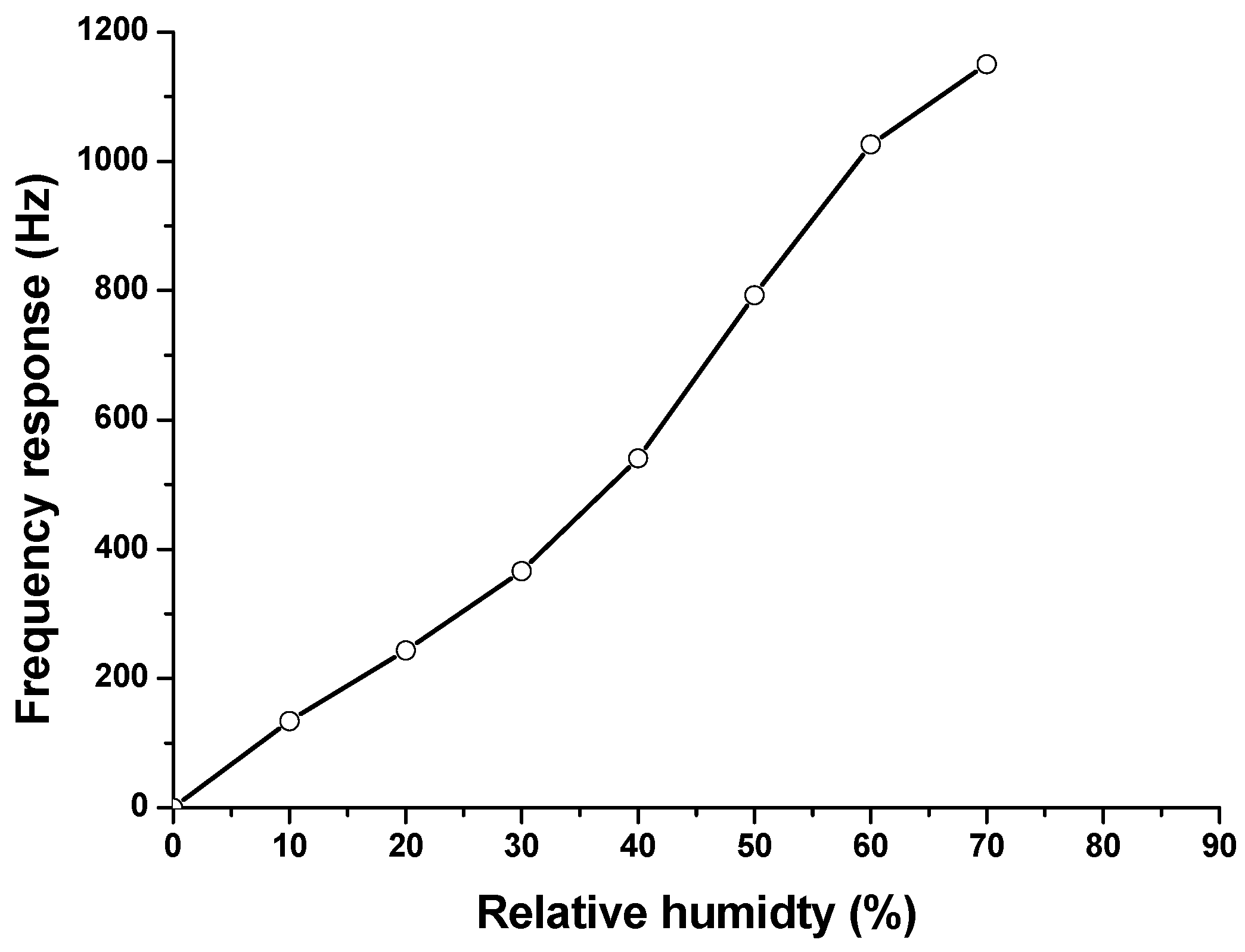
3.3. Humidity Effect Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacquinot, P.; Muller, B.; Wehrli, B.; Hauser, P.C. Determination of methane and other small hydrocarbons with a platinum nafion electrode by stripping voltammetry. Anal. Chem. Acta 2001, 432, 1–10. [Google Scholar] [CrossRef]
- Otagama, T.; Zaromb, S.; Stetter, J.R. A room-temperature electrochemical sensor and instrument for monitoring methane. Sens. Actuators 1985, 8, 65–88. [Google Scholar] [CrossRef]
- Tai, H.; Yamamoto, K.; Uchida, M.; Osawa, S.; Uehara, K. Long distance simultaneous detection of methane and acetylene by using diode lasers coupled with optical fibers. IEEE Photon. Technol. Lett. 1992, 4, 804–807. [Google Scholar] [CrossRef]
- Shemshad, J. Design of a fiber optic sequential multipoint sensor for methane detection using a single tunable diode laser near 1666 nm. Sens. Actuators B 2013, 186, 466–477. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.; Tao, C.; Li, X.; Chen, W. Sensitivity enhanced of transition mode long-period fiber grating as methane sensor using high refractive index polycarbonate/cryptophane a overlay deposition. Sens. Actuators B 2015, 207, 477–480. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, N.M.; Hieu, H. Ni2O3-decorated SnO2 particulate films for methane gas sensor. Sens. Actuators B 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Das, N.; Halder, A.K.; Sen, J.M.A. Sonochemically prepared tin-dioxide based composition for methane sensor. Mater. Lett. 2006, 60, 991–994. [Google Scholar] [CrossRef]
- Bose, S.; Chakraborty, S.; Ghosh, B.K. Methane sensitivity of Fe-doped SnO2 thick films. Sens. Actuators B 2005, 105, 346–350. [Google Scholar] [CrossRef]
- Wang, X.D.; Wang, W.; Li, H.L. Development of a SnO2/CuO-coated surface acoustic wave-based H2S sensor with switch-like response and recovery. Sens. Actuators B 2012, 169, 10–16. [Google Scholar] [CrossRef]
- Asad, M.; Sheikhi, M.H. Surface acoustic wave based H2S gas sensors incorporating sensitive layers of single wall carbon nanotubes decorated with Cu nanoparticles. Sens. Actuators B 2014, 216, 237–242. [Google Scholar] [CrossRef]
- Souteyrand, E.; Nicolas, D.; Martin, J.R.; Chauvet, J.P.; Perez, H. Behaviour of cryptophane molecules in gas media. Sens. Actuators B 1996, 33, 182–187. [Google Scholar] [CrossRef]
- Garel, L.; Dutasta, J.P.; Collet, A. Complexation of methane and chlorofluorocarbons by cryptophane-A in organic solution. Angew. Chem. Int. Ed. Engl. 1993, 32, 1169–1171. [Google Scholar] [CrossRef]
- Sun, P.; Jiang, Y.D.; Xie, G.Z. A room temperature supramolecular quartz crystal microbalance (QCM) methane gas sensor. Sens. Actuators B 2009, 141, 104–108. [Google Scholar] [CrossRef]
- Khoshaman, A.H.; Li, P.C.H.; Merbouh, N. Highly sensitive supra-molecular thin films for gravimetric detection of methane. Sens. Actuators B 2012, 161, 954–960. [Google Scholar] [CrossRef]
- Wang, W.; Xie, X.; He, S.T. Optimal design on polyaniline-coated surface acoustic wave based humidity sensor. Sensors 2013, 13, 16816–16828. [Google Scholar] [CrossRef]
- Caniceill, J.; Collet, A. Two step synthesis of D3 and C3h cryptophanes. J. Chem. Soc. Chem. Commun. 1988, 9, 582–584. [Google Scholar] [CrossRef]
- Rebière, D.; Déjous, C.; Pistré, J. Synthesis and evaluation of fluoropolyol isomers as saw microsensor coatings: Role of humidity and temperature. Sens. Actuators B 1998, 49, 139–145. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hu, H.; Liu, X.; He, S.; Pan, Y.; Zhang, C.; Dong, C. Development of a Room Temperature SAW Methane Gas Sensor Incorporating a Supramolecular Cryptophane A Coating. Sensors 2016, 16, 73. https://doi.org/10.3390/s16010073
Wang W, Hu H, Liu X, He S, Pan Y, Zhang C, Dong C. Development of a Room Temperature SAW Methane Gas Sensor Incorporating a Supramolecular Cryptophane A Coating. Sensors. 2016; 16(1):73. https://doi.org/10.3390/s16010073
Chicago/Turabian StyleWang, Wen, Haoliang Hu, Xinlu Liu, Shitang He, Yong Pan, Caihong Zhang, and Chuan Dong. 2016. "Development of a Room Temperature SAW Methane Gas Sensor Incorporating a Supramolecular Cryptophane A Coating" Sensors 16, no. 1: 73. https://doi.org/10.3390/s16010073
APA StyleWang, W., Hu, H., Liu, X., He, S., Pan, Y., Zhang, C., & Dong, C. (2016). Development of a Room Temperature SAW Methane Gas Sensor Incorporating a Supramolecular Cryptophane A Coating. Sensors, 16(1), 73. https://doi.org/10.3390/s16010073






