A Simple, Low-Cost Platform for Real-Time Isothermal Nucleic Acid Amplification
Abstract
:1. Introduction
2. Experimental Section
2.1. Design of Microfluidic Chip
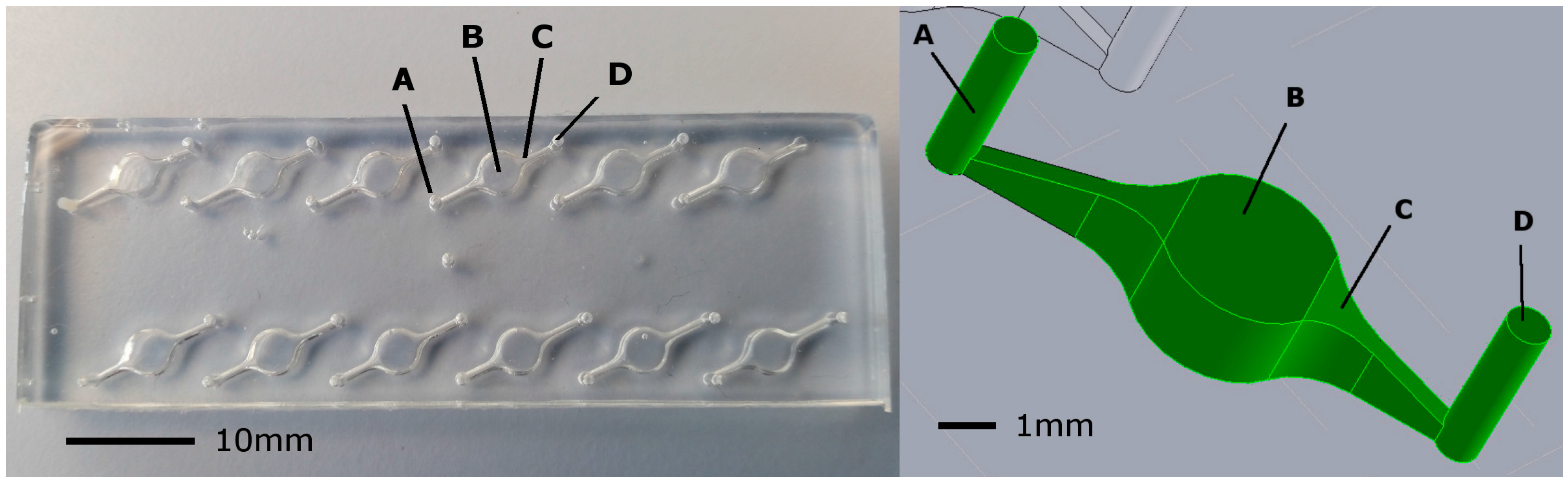
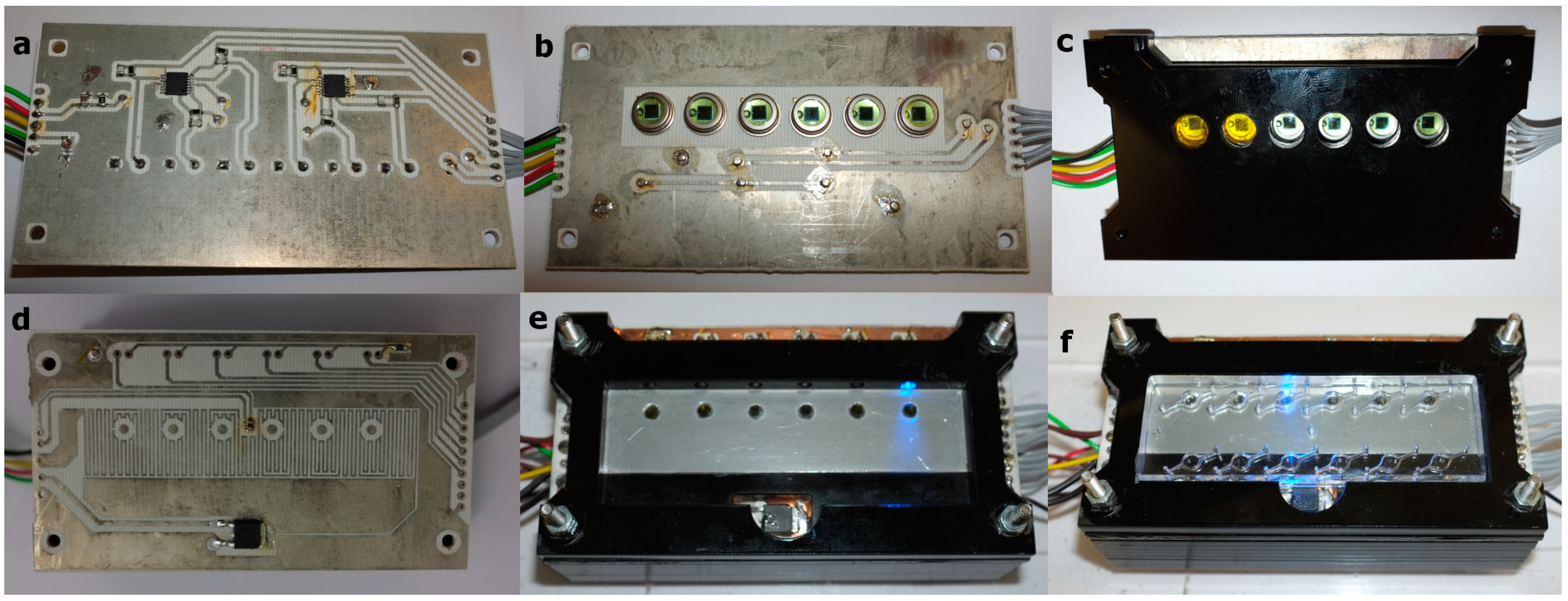
2.2. SCOB Heating Element
2.3. Fluorescence Detection
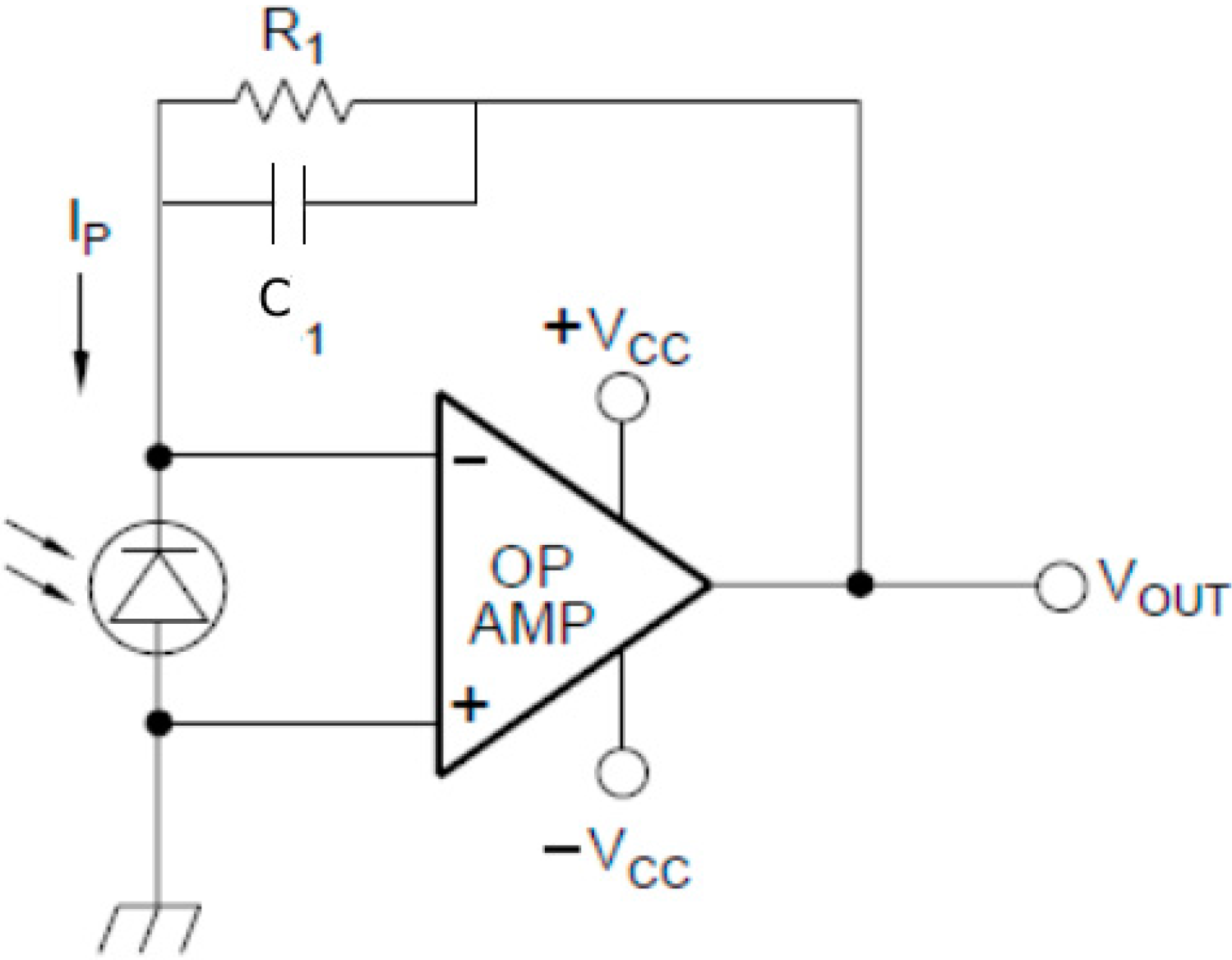

2.4. Control and Signal Acquisition
2.5. Helicase Dependent Amplifcation (HDA)
2.6. Platform Construction
| Item | Cost (GBP) | |
|---|---|---|
| Hardware | PMMA | 4.00 |
| Isothermal plate | 1.30 | |
| Fasteners | 0.40 | |
| Electronics | Operational Amplifier | 4.60 |
| MOSFET | 0.49 | |
| Passive components | 7.00 | |
| LEDs | 3.75 | |
| Photodiodes | 44.46 | |
| Arduino Mega2560 | 35.58 | |
| PCB | 2.28 | |
| Optical Components | Filter | 1.63 |
| Optical fibre | 0.73 | |
| Microfluidics | Microfluidic mold | 27.38 |
| Microscope slide | 0.14 | |
| PDMS | 0.93 | |
| Consumables | PCR tube/pipette tips | 0.85 |
| Total | 135.52 |
3. Results and Discussion
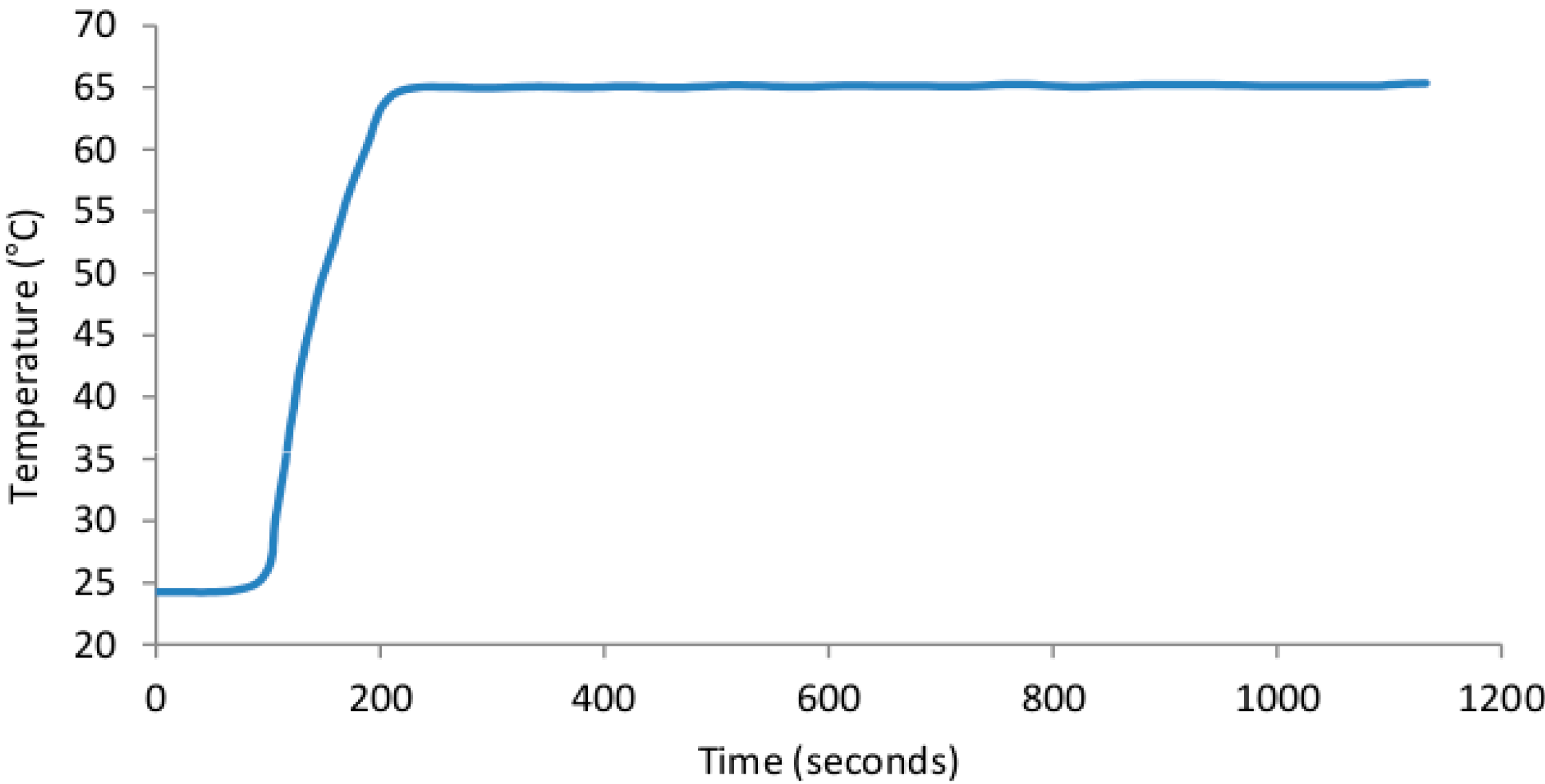
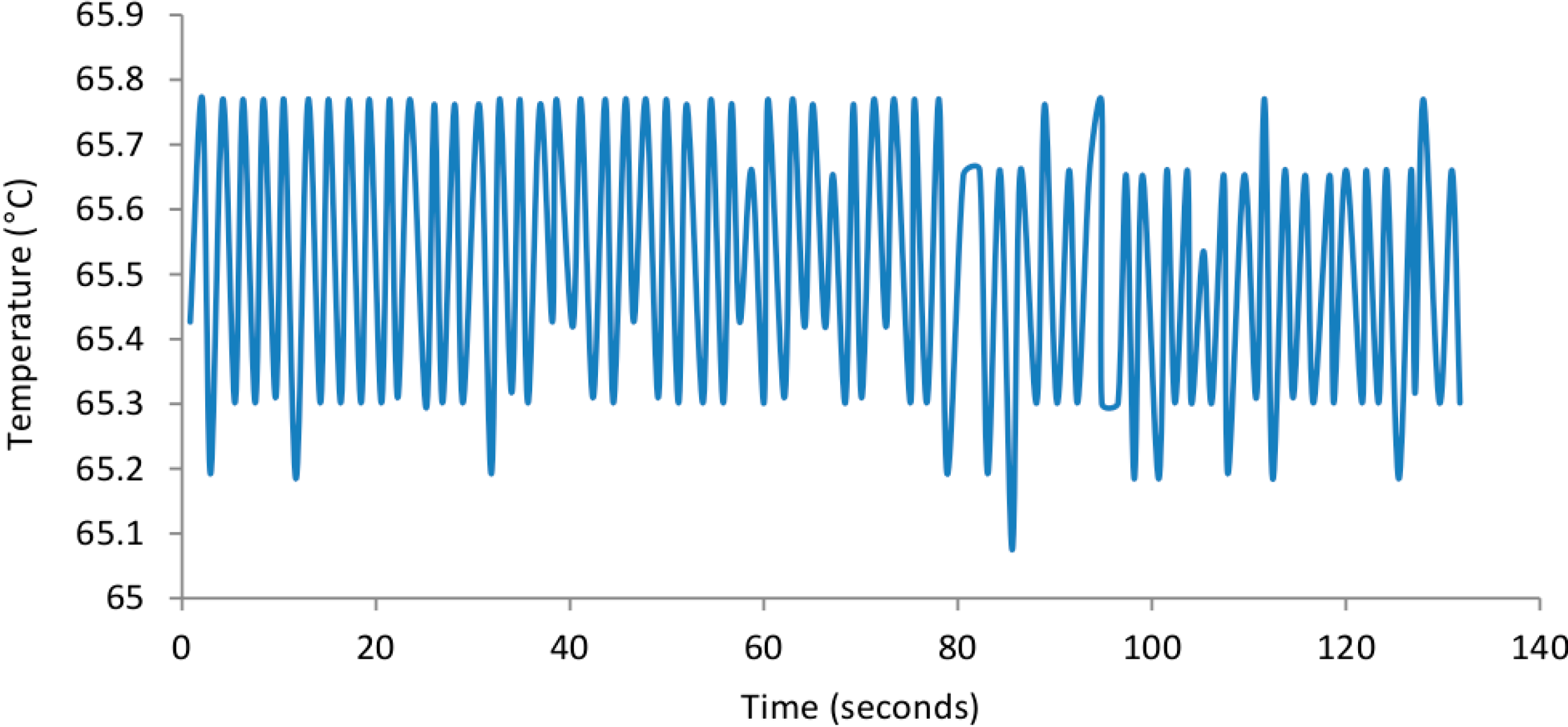
3.1. SCOB Heating Element Stability

3.2. Fluorescence Detection

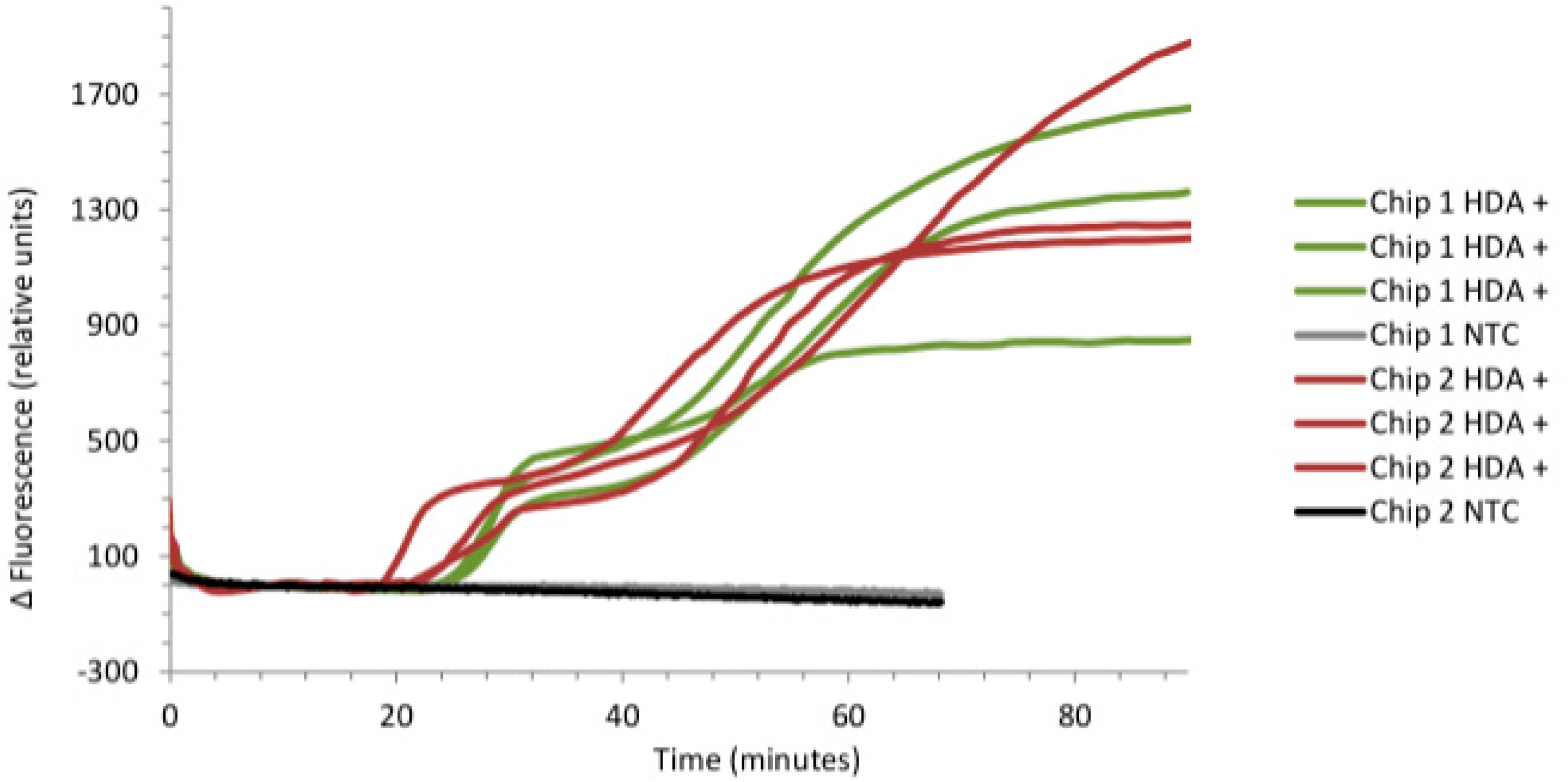
3.3. Real-Time Detection of pCNG1
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cepheid. Cepheid GeneXpert. Available online: http://www.cepheid.com/us/cepheid-solutions/systems/genexpert-systems/genexpert-iv (accessed on 11 August 2015).
- Saiki, R.; Scharf, S.; Faloona, F.; Mullis, K. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985, 230, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Craw, P.; Balachandran, W. Isothermal Nucleic Acid Amplification Technologies for Point-of-Care Diagnostics: A Critical Review. Lab Chip 2012, 12, 2469–2486. [Google Scholar] [CrossRef]
- Mahalanabis, M.; Do, J.; ALMuayad, H.; Zhang, J.Y.; Klapperich, C.M. An integrated disposable device for DNA extraction and helicase dependent amplification. Biomed. Microdevices 2010, 12, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hataoka, Y.; Zhang, L.; Mori, Y.; Tomita, N.; Notomi, T.; Baba, Y. Analysis of specific gene by integration of isothermal amplification and electrophoresis on poly(methyl methacrylate) microchips. Anal. Chem. 2004, 76, 3689–3693. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Tong, Y.; Tang, W.; Quimson, L.; Cope, V.A.; Pan, X.; Motre, A.; Kong, R.; Hong, J.; Kohn, D.; et al. A rapid and simple isothermal nucleic acid amplification test for detection of herpes simplex virus types 1 and 2. J. Clin. Virol. 2011, 50, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kivlehan, F.; Mavré, F.; Talini, L.; Limoges, B.; Marchal, D. Real-time electrochemical monitoring of isothermal helicase-dependent amplification of nucleic acids. Analyst 2011, 136, 3635–3642. [Google Scholar] [CrossRef] [PubMed]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Myers, F.B.; Henrikson, R.H.; Bone, J.M.; Bone, J.; Lee, L.P. A handheld point-of-care genomic diagnostic system. PLoS ONE 2013, 8, e70266. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.M.; Kubota, R.; Dong, J.; Li, Y.; Higashiguchi, D. Handheld device for real-time, quantitative, LAMP-based detection of Salmonella enterica using assimilating probes. Biosens. Bioelectron. 2011, 30, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hoehl, M.M.; Bocholt, E.S.; Kloke, A.; Paust, N.; von Stetten, F.; Zengerle, R.; Steigert, J.; Slocum, A.H. A versatile-deployable bacterial detection system for food and environmental safety based on LabTube-automated DNA purification, LabReader-integrated amplification, readout and analysis. Analyst 2014, 139, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Parloff, R. 2-Pound DNA-Reader Could Help Fight Ebola, TB, HIV. Available online: http://fortune.com/2015/07/28/cepheid-omni-portable-dna-reader-ebola-tb-hiv/ (accessed on 11 August 2015).
- Jiang, L.; Mancuso, M.; Lu, Z.; Akar, G.; Cesarman, E.; Erickson, D. Solar thermal polymerase chain reaction for smartphone-assisted molecular diagnostics. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Haubert, K.; Drier, T.; Beebe, D. PDMS bonding by means of a portable, low-cost corona system. Lab Chip 2006, 6, 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- BioHelix Corporation IsoAmp® III Universal tHDA Kit Manual. Available online: http://www.biohelix.com/pdf/h0120s_isoamp iii_bh_edatacard.pdf (accessed on 5 May 2014).
- Stedtfeld, R.D.; Tourlousse, D.M.; Seyrig, G.; Stedtfeld, T.M.; Kronlein, M.; Price, S.; Ahmad, F.; Gulari, E.; Tiedje, J.M.; Hashsham, S.A. Gene-Z: A device for point of care genetic testing using a smartphone. Lab Chip 2012, 12, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Mackay, R.E.; Garg, N.; Craw, P.; Ahern, J.C.; Balachandran, W. A Nucleic Acid Extraction Method for Point of Care Devices. In Proceedings of the 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Freiburg, Germany, 27–31 October 2013; pp. 1755–1757.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craw, P.; Mackay, R.E.; Naveenathayalan, A.; Hudson, C.; Branavan, M.; Sadiq, S.T.; Balachandran, W. A Simple, Low-Cost Platform for Real-Time Isothermal Nucleic Acid Amplification. Sensors 2015, 15, 23418-23430. https://doi.org/10.3390/s150923418
Craw P, Mackay RE, Naveenathayalan A, Hudson C, Branavan M, Sadiq ST, Balachandran W. A Simple, Low-Cost Platform for Real-Time Isothermal Nucleic Acid Amplification. Sensors. 2015; 15(9):23418-23430. https://doi.org/10.3390/s150923418
Chicago/Turabian StyleCraw, Pascal, Ruth E. Mackay, Angel Naveenathayalan, Chris Hudson, Manoharanehru Branavan, S. Tariq Sadiq, and Wamadeva Balachandran. 2015. "A Simple, Low-Cost Platform for Real-Time Isothermal Nucleic Acid Amplification" Sensors 15, no. 9: 23418-23430. https://doi.org/10.3390/s150923418
APA StyleCraw, P., Mackay, R. E., Naveenathayalan, A., Hudson, C., Branavan, M., Sadiq, S. T., & Balachandran, W. (2015). A Simple, Low-Cost Platform for Real-Time Isothermal Nucleic Acid Amplification. Sensors, 15(9), 23418-23430. https://doi.org/10.3390/s150923418





