A Wireless Optogenetic Headstage with Multichannel Electrophysiological Recording Capability
Abstract
:1. Introduction
2. System Overview and Design
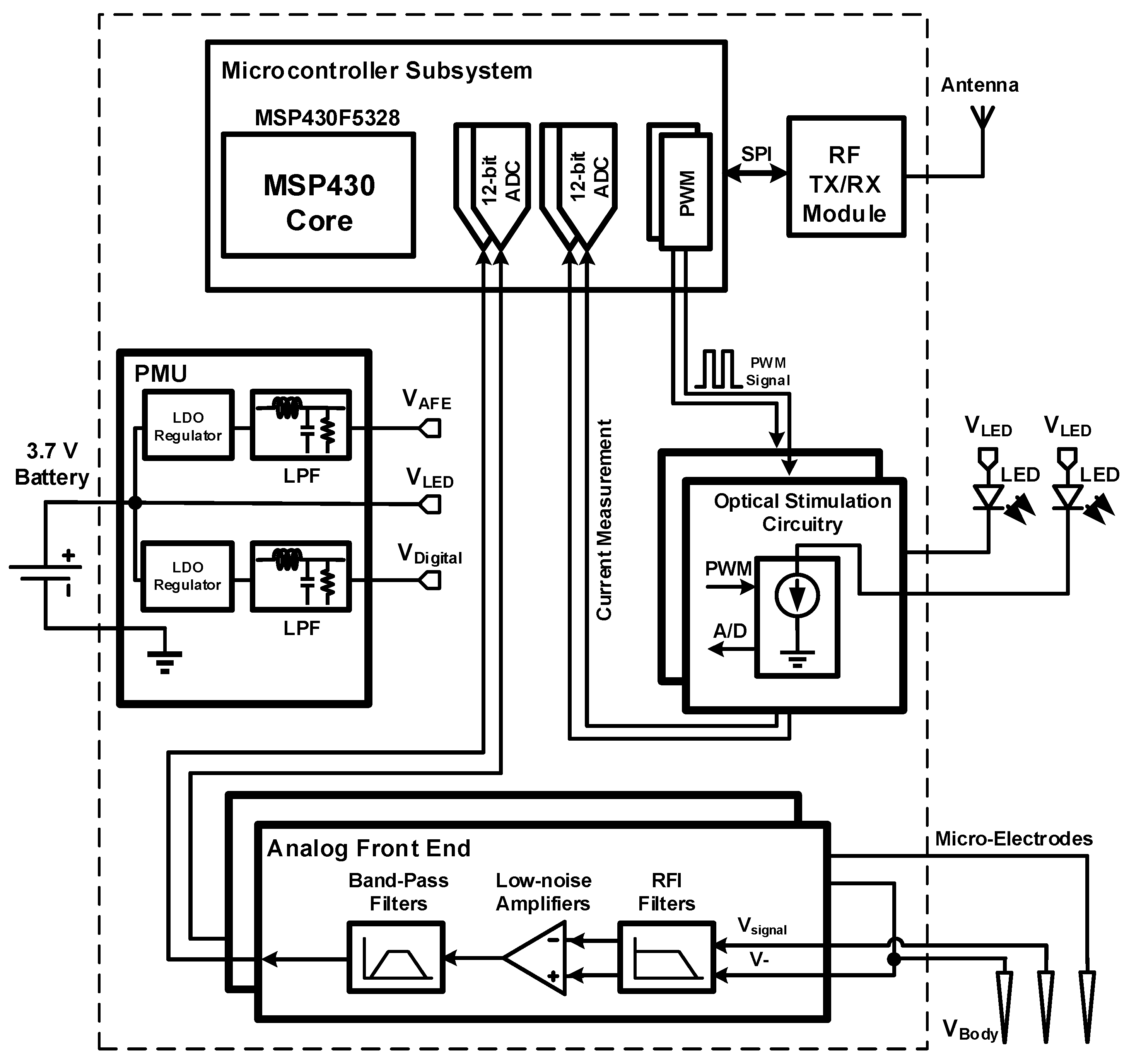
2.1. Implantable Module (LED and Microelectrodes)
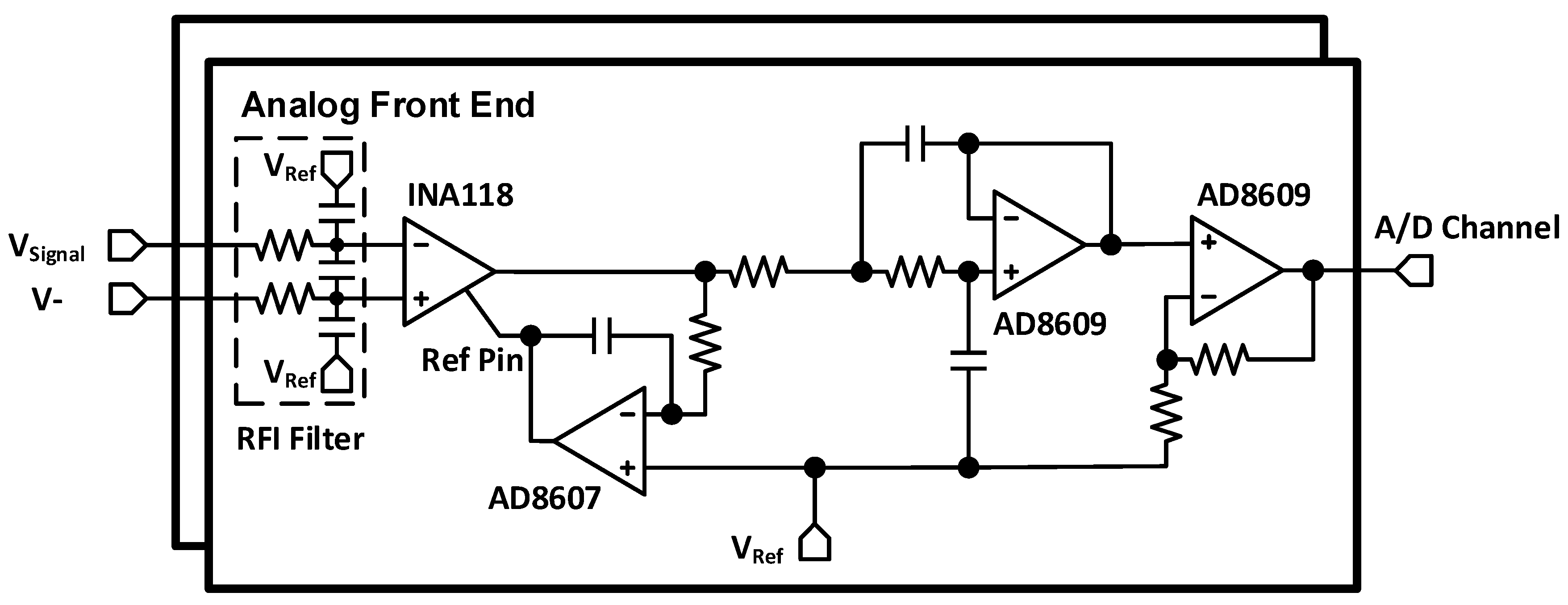
2.2. Analog Front End
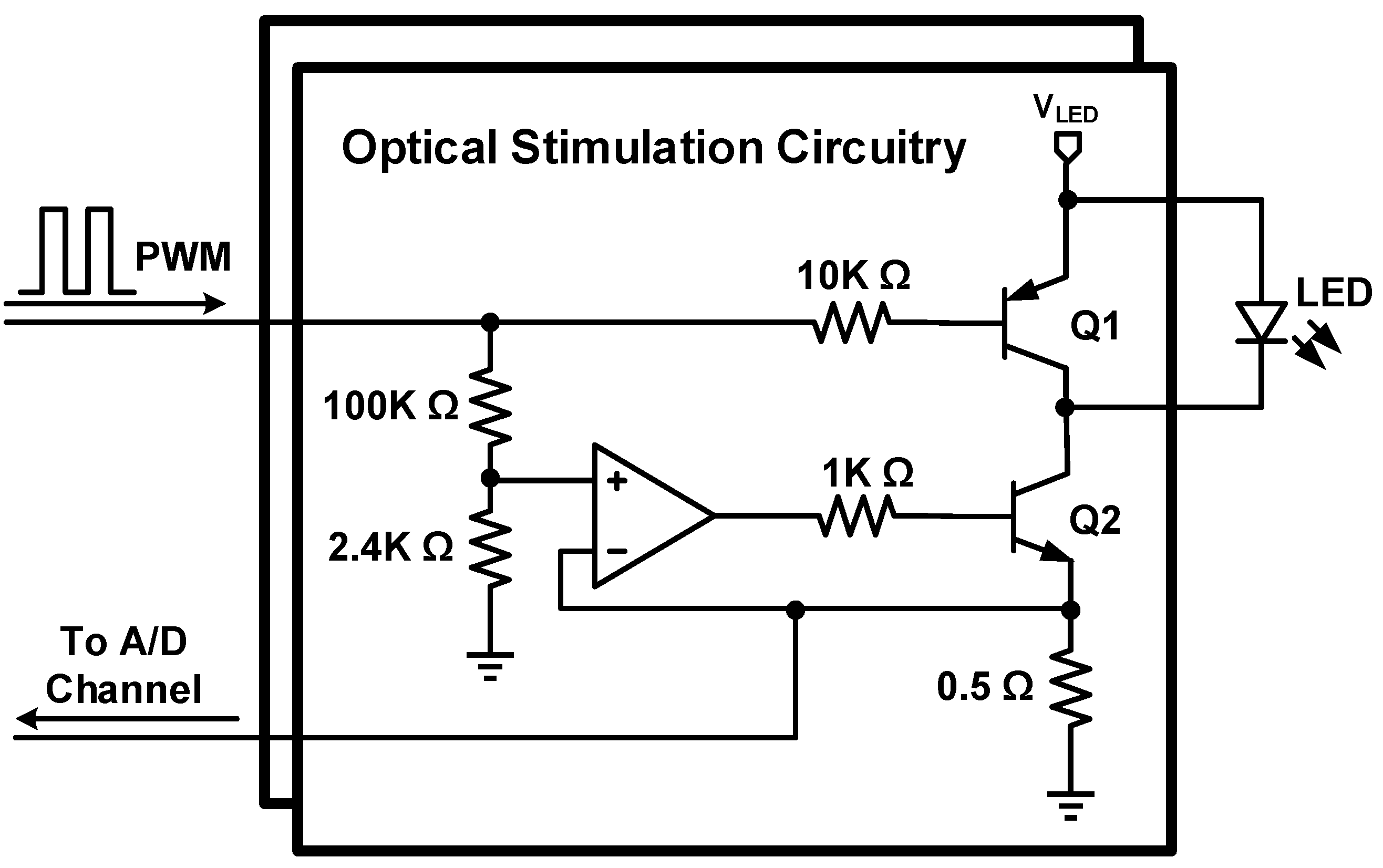
2.3. Optical Stimulation Circuitry
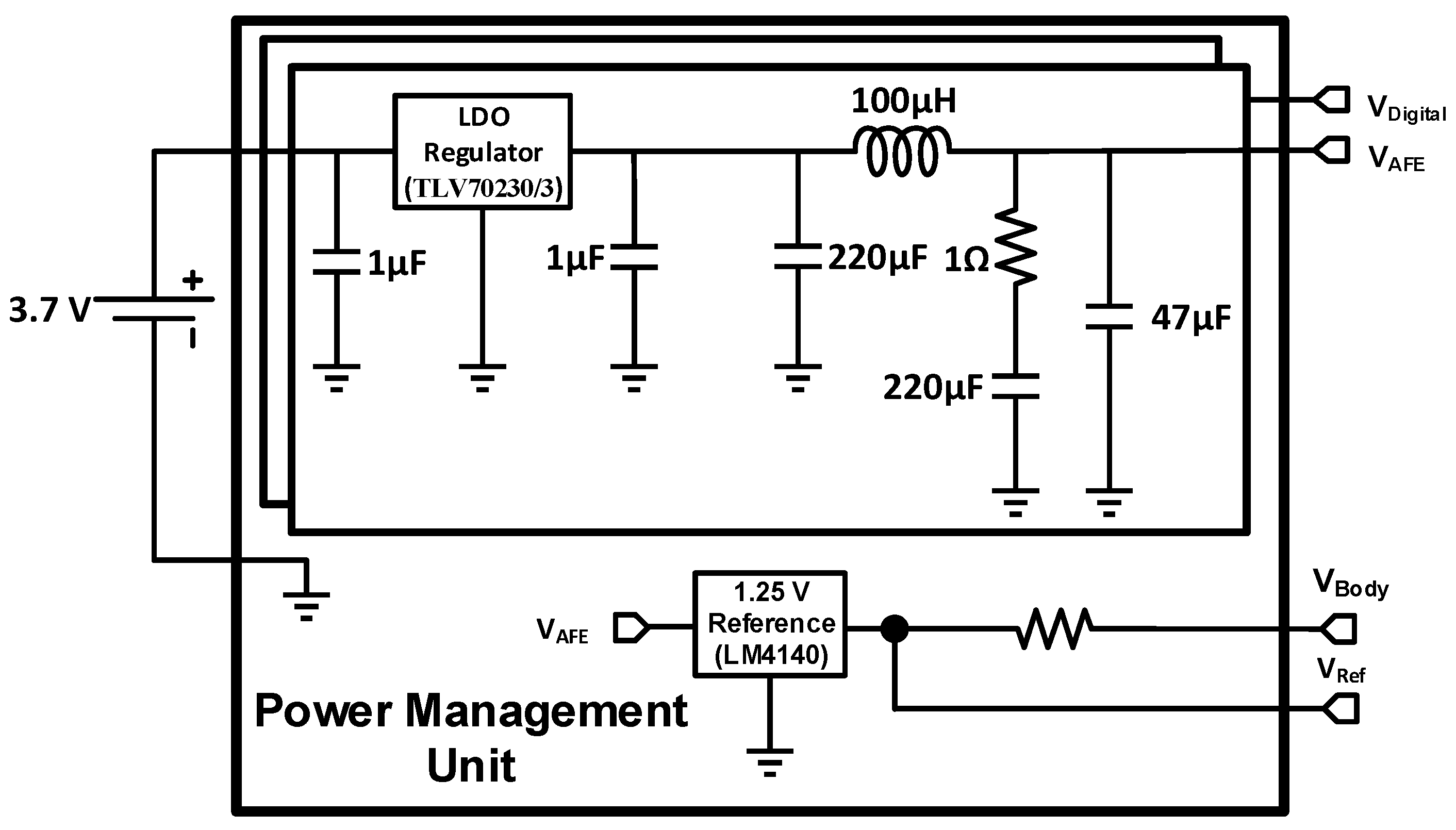
2.4. Power Management Unit
2.5. Wireless Transceiver
2.6. Mixed-Signal Control System
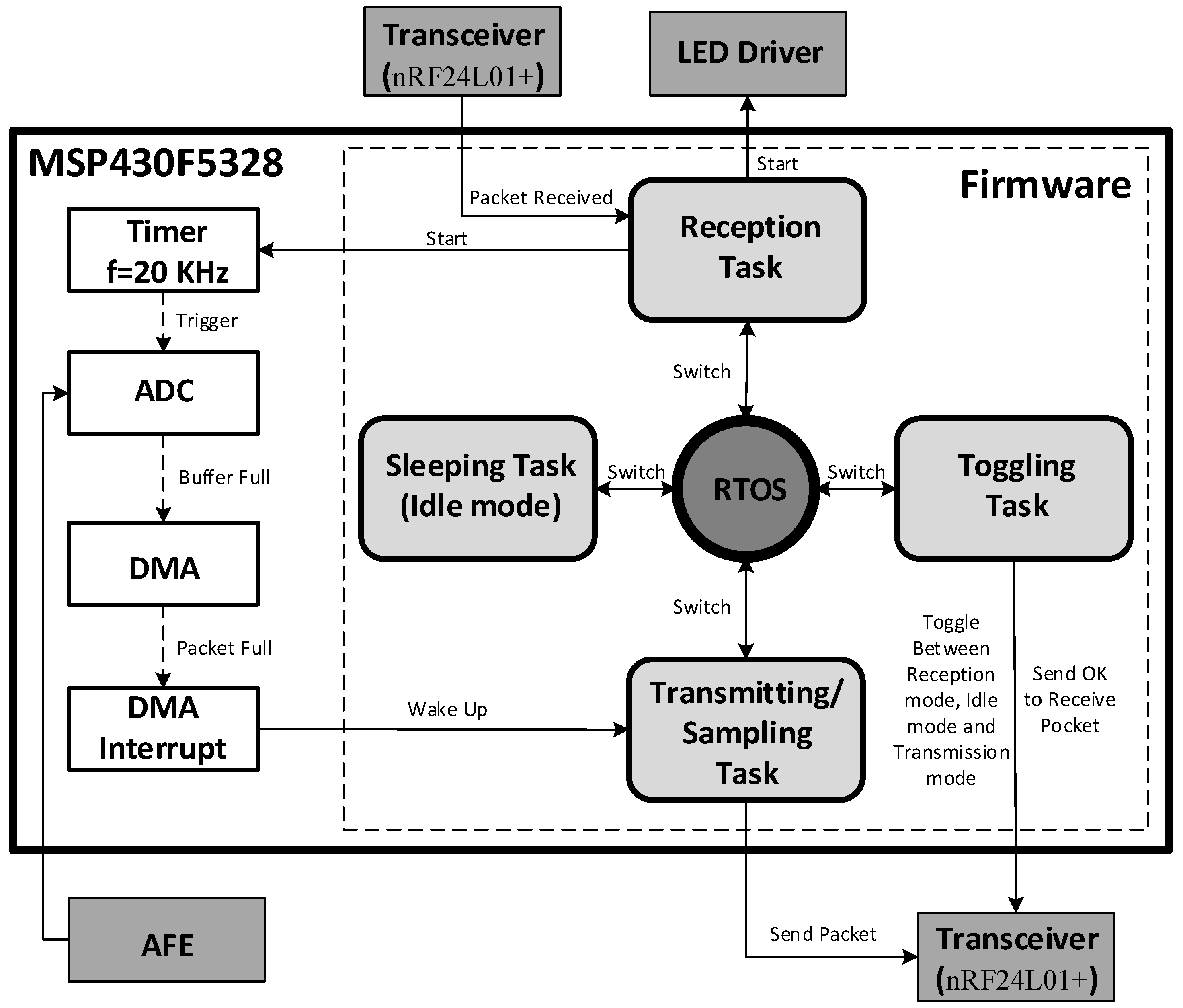
3. Experimental Results
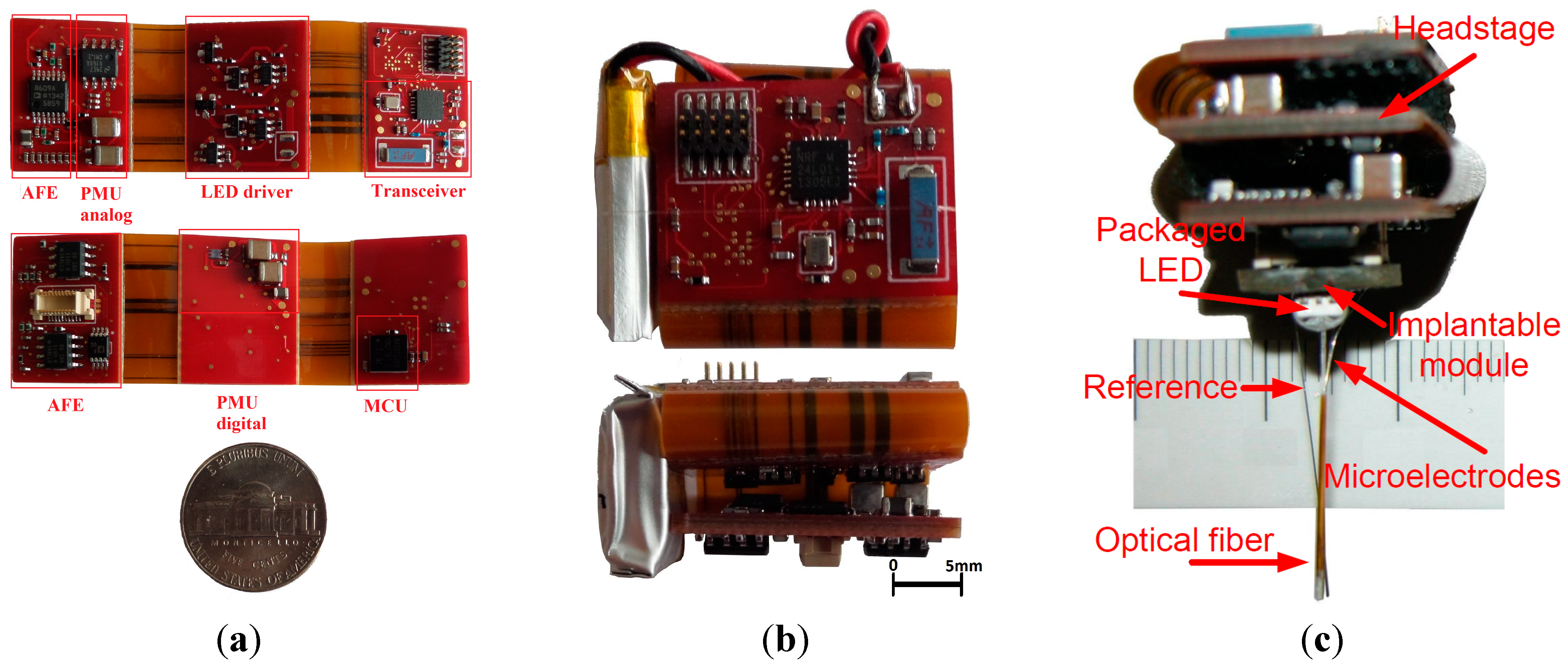
3.1. Rigid-Flex Printed Circuit Board Implementation
3.2. Measured System Characteristics
| Parameter | Value |
|---|---|
| Gain | 2851 V/V (69.09 dB) |
| Low Cut-Off Frequency | 285 Hz |
| High Cut-Off Frequency | 6580 Hz |
| Input-Referred Noise | 2.1 µV (rms) |
| CMRR | 110 dB @ 1 KHz |
| Number of Analog Channels | 2 |
| Power Consumption | 1 mA @ 3.0 V (3 mW) |
| Precision | Selectable, 12 or 8 bits |
| Parameter | Value |
|---|---|
| Forward voltage | 3.275 V |
| Forward current | 150 mA |
| Frequency | 1 Hz to 100 Hz |
| Duty Cycle | 10% |
| Rise Time | 1.6 µsec |
| Fall Time | 5.1 µsec |
| LED input electrical power | 491 mW |
| Implanted fiber output optical power | 70 mW/mm2 |
| Parameter | Value |
|---|---|
| Frequency band | 2.4 GHz |
| Modulation | GFSK |
| In air data rate | 2 Mbps |
| Measured effective data rate | 700 Kbps |
| Transmission power | 0 dBm |
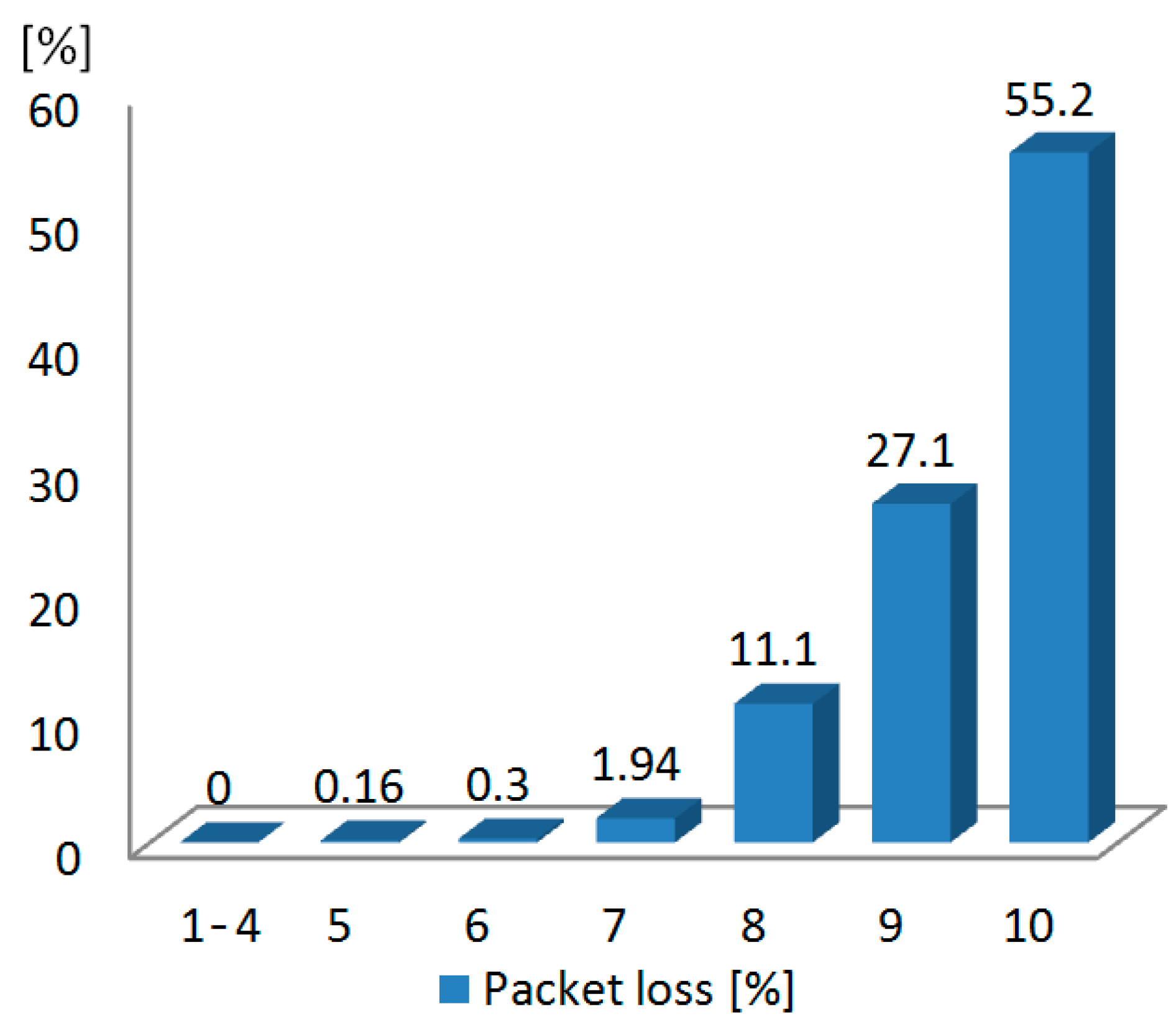
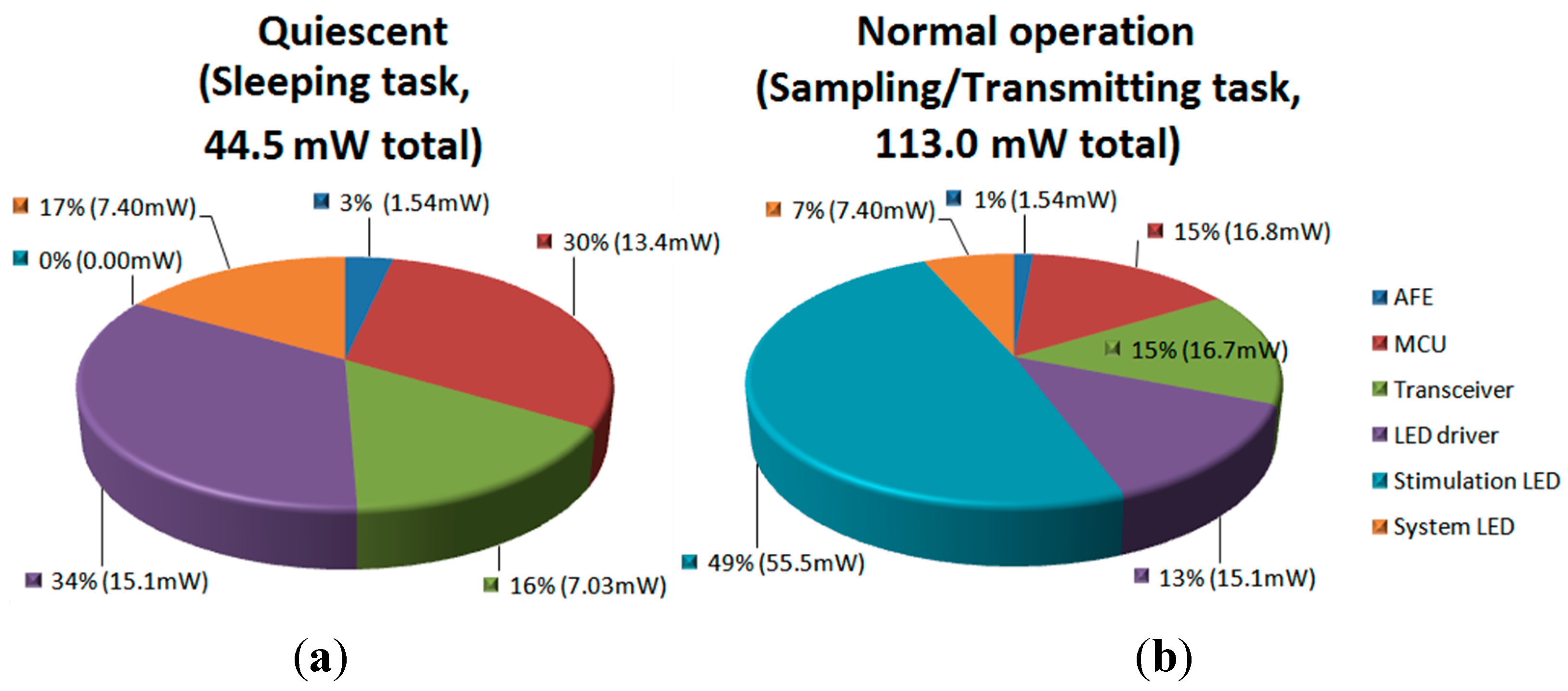
3.3. Test with a Synthetic Input Signal
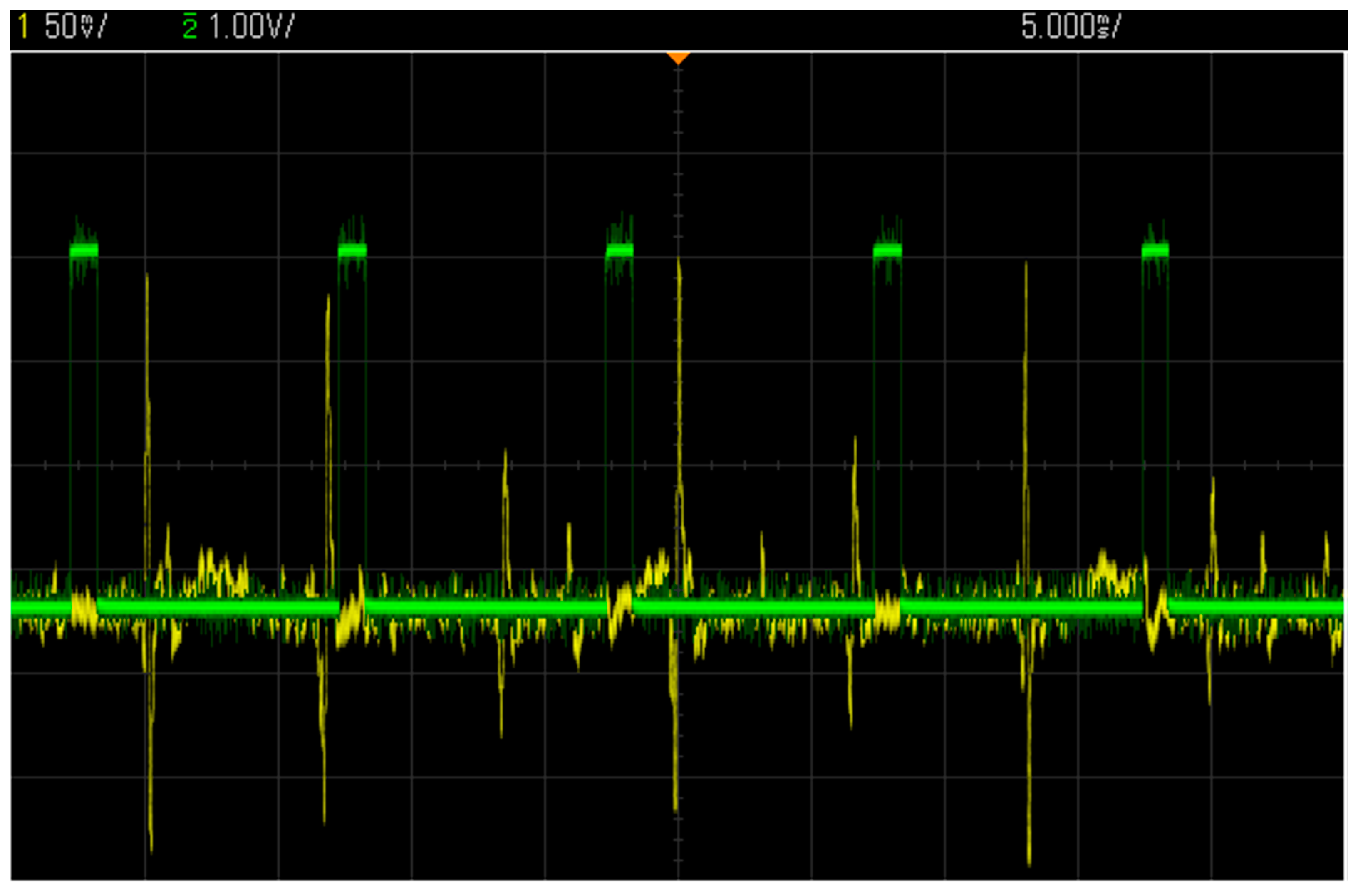
3.4. Comparison with Other Wireless COTS Systems
| Work | No. of Recording Channels | No. of Stimulation Channels | Ch. Sampling Rate (KSample/s) | Bits/Sample (Stim. or Rec.) | Weight (g) | Power Consumption (mW) |
|---|---|---|---|---|---|---|
| This work | 2 | 2 (opt. fiber) | 20,000 | 12 | 4.9 | 113 |
| [5] | 2 | 1 (opt. fiber) | 20,000 | 8 | 7.4 | 115-475 |
| [26] | 1 | 1 | 11,700 | N/A | 8.4 | 40-120 |
| [27] | 1 | 1 | 10,000 | N/A | N/A | N/A |
| [28] | 1 | 1 | 12,000 | 12 | 20 | N/A |
| [29] | 1 | 0 | N/A | N/A | 0.5 | N/A |
| [30] | 0 | 4 (surface LED) | N/A | N/A | N/A | N/A |
| [8] | 0 | N/A (µ-ILEDs) | N/A | N/A | N/A | N/A |
| [31] | 15 | 0 | 20,000 | 12 | 4.0 | 142 |
| [32] | 16 | 0 | 25,000 | 10 | 16.5 | N/A |
| [8] | 16 | 0 | N/A | N/A | 3.0 | 2000 |
| [33] | 32 | 0 | 30,000 | 12 | N/A | 142 |
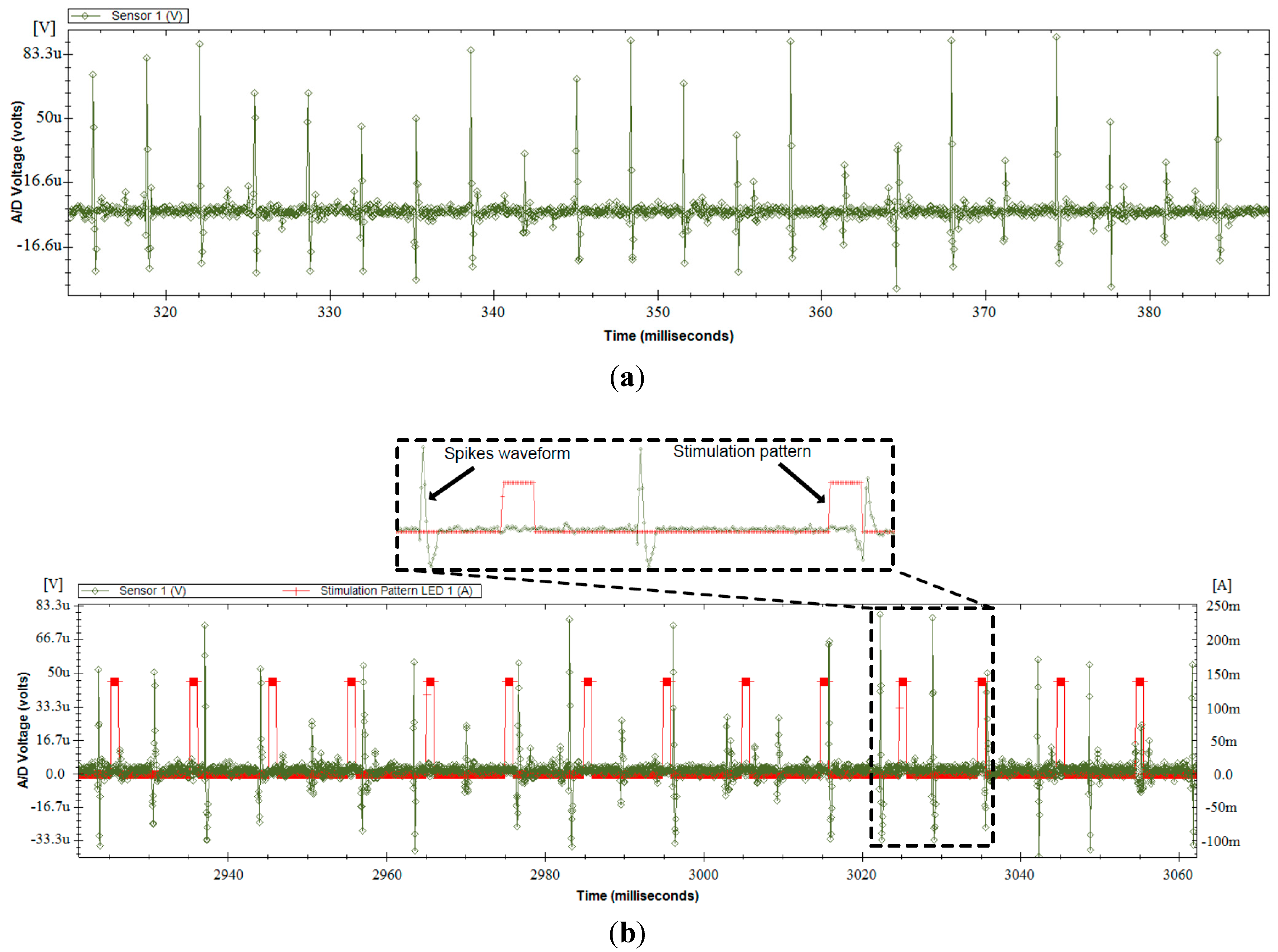
3.5. In-Vitro Validation

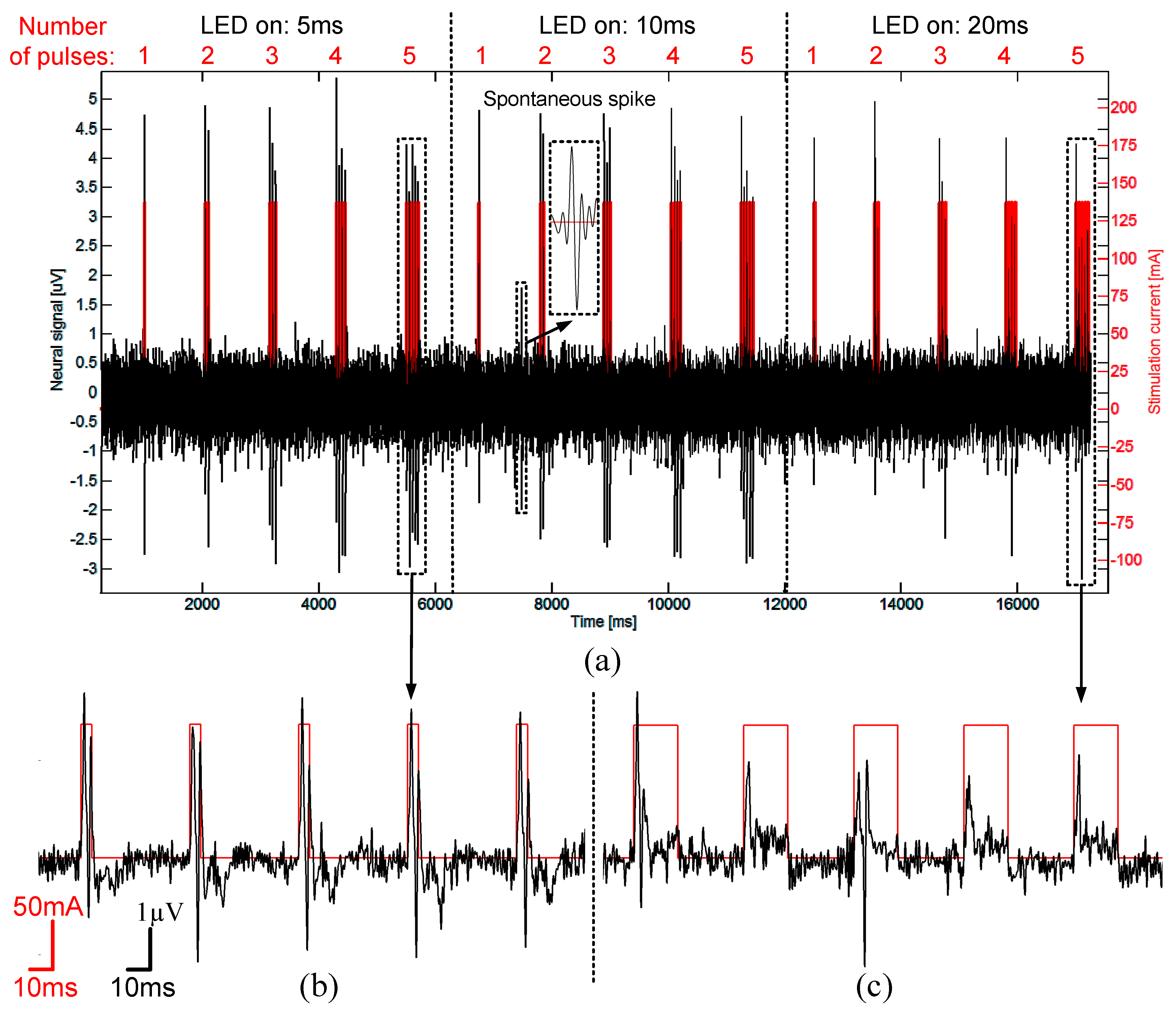
3.6. In-Vivo Validation
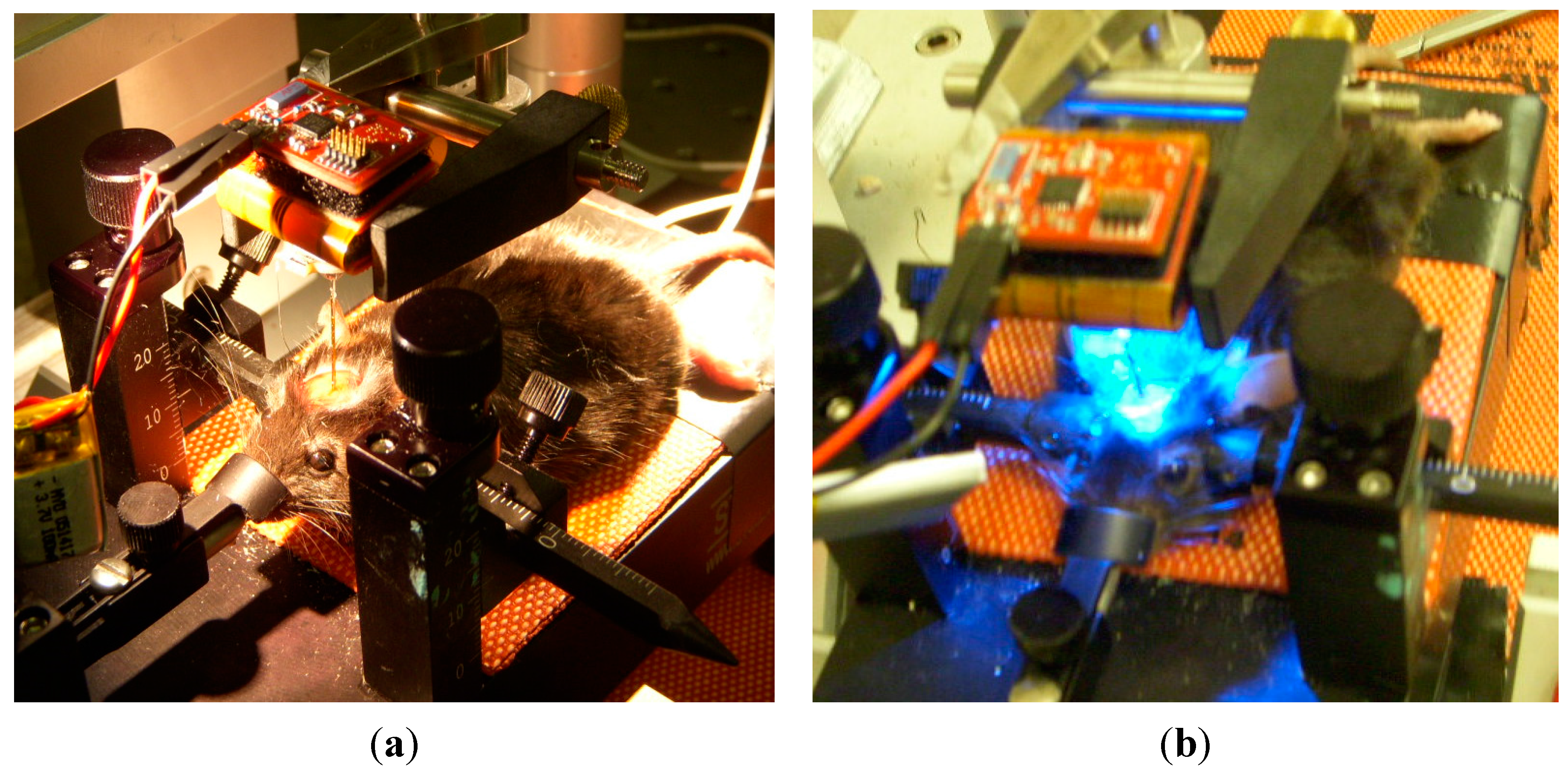
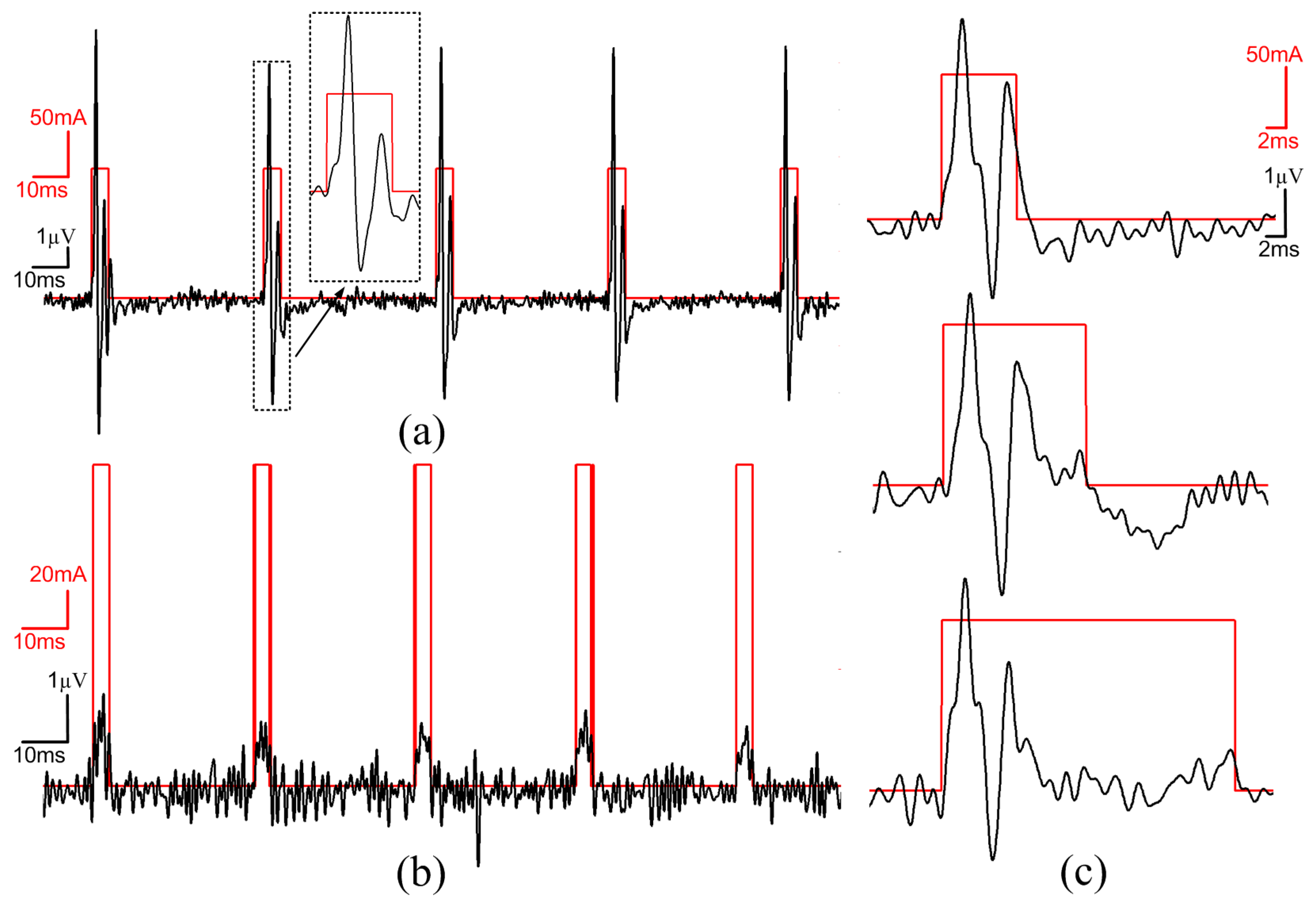
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mancuso, J.J.; Kim, J.; Lee, S.; Tsuda, S.; Chow, N.B.H.; Augustine, G.J. Optogenetic probing of functional brain circuitry. Exp. Physiol. 2011, 96, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Stark, E.; Berényi, A.; Khodagholy, D.; Kipke, D.R.; Yoon, E.; Wise, K.D. Tools for Probing Local Circuits: High-Density Silicon Probes Combined with Optogenetics. Neuron 2015, 86, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Ameli, R.; Mirbozorgi, A.; Neron, J.-L.; Lechasseur, Y.; Gosselin, B. A wireless and batteryless neural headstage with optical stimulation and electrophysiological recording. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 5662–5665.
- Triangle Biosystems Inc. Available online: http://www.trianglebiosystems.com/ (accessed on 17 February 2015).
- Blackrock Microsystems. Available online: http://www.blackrockmicro.com/ (accessed on 17 February 2015).
- Kim, T.I.; McCall, J.G.; Jung, Y.H.; Huang, X.; Siuda, E.R.; Li, Y.; Song, J.; Song, Y.M.; Pao, H.A.; Kim, R.-H.; et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 2013, 340, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Wentz, C.T.; Bernstein, J.G.; Monahan, P.; Guerra, A.; Rodriguez, A.; Boyden, E.S. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J. Neural Eng. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.K.; Long, M.A.; Fee, M.S.; Sarpeshkar, R. Wireless Neural Stimulation in Freely Behaving Small Animals. J. Neurophysiol. 2009, 102, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; de Koninck, Y. Optrodes for combined optogenetics and electrophysiology in live animals. Neurophotonics 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Rich, D.; Holtzman, T.; Ruther, P.; Dalley, J.W.; Lopez, A.; Rossi, M.A.; Barter, J.W.; Salas-Meza, D.; Herwik, S.; et al. A wireless multi-channel recording system for freely behaving mice and rats. PLoS ONE 2011, 6, e22033. [Google Scholar] [CrossRef] [PubMed]
- Ruther, P.; Holzhammer, T.; Herwik, S.; Rich, P.D.; Dalley, J.W.; Paul, O.; Holtzman, T. Compact wireless neural recording system for small animals using silicon-based probe arrays. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2284–2287.
- Mirbozorgi, S.A.; Sawan, M.; Gosselin, B. Multicoil resonance-based parallel array for smart wireless power delivery. In Proceedings of the 2013 35th Annual International Conference of the Engineering in Medicine and Biology Society, Osaka, Japan, 3–7 July 2013; pp. 751–754.
- Mirbozorgi, S.A.; Bahrami, H.; Sawan, M.; Gosselin, B. A Smart Multicoil Inductively Coupled Array for Wireless Power Transmission. IEEE Trans. Ind. Electron. 2014, 61, 6061–6070. [Google Scholar] [CrossRef]
- Gosselin, B. Recent advances in neural recording microsystems. Sensors 2011, 11, 4572–4597. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Pedreira, C.; Ison, M.J.; Quiroga, R.Q. Realistic simulation of extracellular recordings. J. Neurosci. Methods 2009, 184, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Perelman, Y.; Ginosar, R. An integrated system for multichannel neuronal recording with spike/LFP separation, integrated A/D conversion and threshold detection. IEEE Trans. Biomed. Eng. 2007, 54, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.S.; Yang, Z.; Yuce, M.R.; Hoang, L.; Liu, W. A 128-channel 6 mW wireless neural recording IC with spike feature extraction and UWB transmitter. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 312–321. [Google Scholar] [CrossRef] [PubMed]
- OSRAM-Opto-SemiConductor. LED LB G6SP. Available online: http://www.osram-os.com/ (accessed on 4 April 2015).
- Kitchin, C. Biasing and Decoupling Op Amps in Single Supply Applications. In Analog Device Application Note; Analog Devices, Inc.: Norwood, MA, USA, 2002; pp. 1–8. [Google Scholar]
- Texas-Instruments. MSP430F5328 Mixed Signal Microcontroller. Available online: http://www.ti.com/product/msp430f5328 (accessed on 10 April 2015).
- FunkOS. Available online: http://funkos.sourceforge.net/ (accessed on 10 April 2015).
- TinyOS. Available online: http://www.tinyos.net/ (accessed on 10 April 2015).
- Molex-Company. Molex SlimStack Connector 0537480208. Available online: http://www.molex.com/ (accessed on 10 April 2015).
- Mavoori, J.; Jackson, A.; Diorio, C.; Fetz, E. An autonomous implantable computer for neural recording and stimulation in unrestrained primates. J. Neurosci. Methods 2005, 148, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ativanichayaphong, T.; He, J.W.; Hagains, C.E.; Peng, Y.B.; Chiao, J.-C. A combined wireless neural stimulating and recording system for study of pain processing. J. Neurosci. Methods 2008, 170, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, P.; Liu, J.; Zhang, S.; Jiang, J.; Wang, Q.; Chen, W.; Zheng, X. A portable telemetry system for brain stimulation and neuronal activity recording in freely behaving small animals. J. Neurosci. Methods 2008, 174, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Schregardus, D.S.; Pieneman, A.W.; Maat, A.T.; Jansen, R.F.; Brouwer, T.J.; Gahr, M.L. A lightweight telemetry system for recording neuronal activity in freely behaving small animals. J. Neurosci. Methods 2006, 155, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Kwon, K.-Y.; Li, W.; Ghovanloo, M. A Wireless Implantable Switched-Capacitor Based Optogenetic Stimulating System. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014.
- Roy, S.; Wang, X. Wireless multi-channel single unit recording in freely moving and vocalizing primates. J. Neurosci. Methods 2012, 203, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Hampson, R.E.; Collins, V.; Deadwyler, S.A. A wireless recording system that utilizes Bluetooth technology to transmit neural activity in freely moving animals. J. Neurosci. Methods 2009, 182, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.; Gilja, V.; Chestek, C.A.; Shenoy, K.; Meng, T.H. HermesD: A High-Rate Long-Range Wireless Transmission System for Simultaneous Multichannel Neural Recording Applications. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Qian, X.; Bernstein, J.G.; Zhou, H.H.; Franzesi, G.T.; Stern, P.; Bronson, R.T.; Graybiel, A.M.; Desimone, R.; Boyden, E.S. Millisecond-Timescale Optical Control of Neural Dynamics in the Nonhuman Primate Brain. Neuron 2009, 62, 191–198. [Google Scholar]
- Han, X. Optogenetics in the nonhuman primate. Prog. Brain Res. 2012, 196, 215–233. [Google Scholar]
- Arenkiel, B.R.; Peca, J.; Davison, I.G.; Feliciano, C.; Deisseroth, K.; Augustine, G.J.; Ehlers, M.D.; Feng, G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 2007, 54, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.M.; Huang, L.; Murphey, D.K.; Garcia, I.; Arenkiel, B.R. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin-2. eLIFE 2014, 3, e01481. [Google Scholar]
- LeChasseur, Y.; Dufour, S.; Lavertu, G.; Bories, C.; Deschênes, M.; Vallée, R.; de Koninck, Y. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat. Methods 2011, 8, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, B.; Sawan, M. A low-power integrated neural interface with digital spike detection and extraction. Analog Integr. Circuits Signal Process. 2010, 64, 3–11. [Google Scholar] [CrossRef]
- Gosselin, B.; Sawan, M.; Kerherve, E. Linear-Phase Delay Filters for Ultra-Low-Power Signal Processing in Neural Recording Implants. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 171–180. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagnon-Turcotte, G.; Kisomi, A.A.; Ameli, R.; Camaro, C.-O.D.; LeChasseur, Y.; Néron, J.-L.; Bareil, P.B.; Fortier, P.; Bories, C.; De Koninck, Y.; et al. A Wireless Optogenetic Headstage with Multichannel Electrophysiological Recording Capability. Sensors 2015, 15, 22776-22797. https://doi.org/10.3390/s150922776
Gagnon-Turcotte G, Kisomi AA, Ameli R, Camaro C-OD, LeChasseur Y, Néron J-L, Bareil PB, Fortier P, Bories C, De Koninck Y, et al. A Wireless Optogenetic Headstage with Multichannel Electrophysiological Recording Capability. Sensors. 2015; 15(9):22776-22797. https://doi.org/10.3390/s150922776
Chicago/Turabian StyleGagnon-Turcotte, Gabriel, Alireza Avakh Kisomi, Reza Ameli, Charles-Olivier Dufresne Camaro, Yoan LeChasseur, Jean-Luc Néron, Paul Brule Bareil, Paul Fortier, Cyril Bories, Yves De Koninck, and et al. 2015. "A Wireless Optogenetic Headstage with Multichannel Electrophysiological Recording Capability" Sensors 15, no. 9: 22776-22797. https://doi.org/10.3390/s150922776
APA StyleGagnon-Turcotte, G., Kisomi, A. A., Ameli, R., Camaro, C.-O. D., LeChasseur, Y., Néron, J.-L., Bareil, P. B., Fortier, P., Bories, C., De Koninck, Y., & Gosselin, B. (2015). A Wireless Optogenetic Headstage with Multichannel Electrophysiological Recording Capability. Sensors, 15(9), 22776-22797. https://doi.org/10.3390/s150922776






