Time-Resolved Synchronous Fluorescence for Biomedical Diagnosis
Abstract
:1. Introduction
1.1. Synchronous Fluorescence
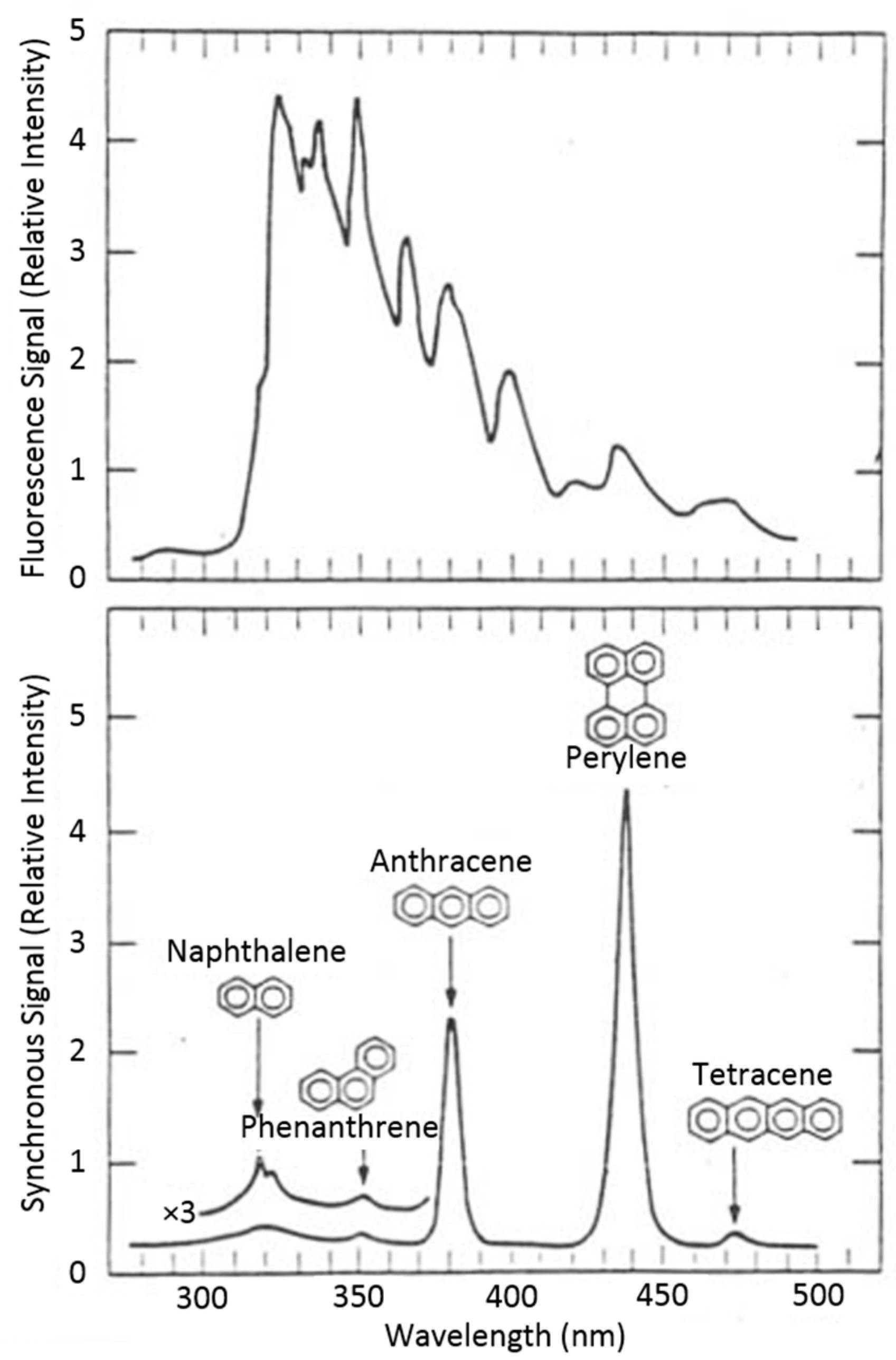
1.2. Synchronous Fluorescence Imaging
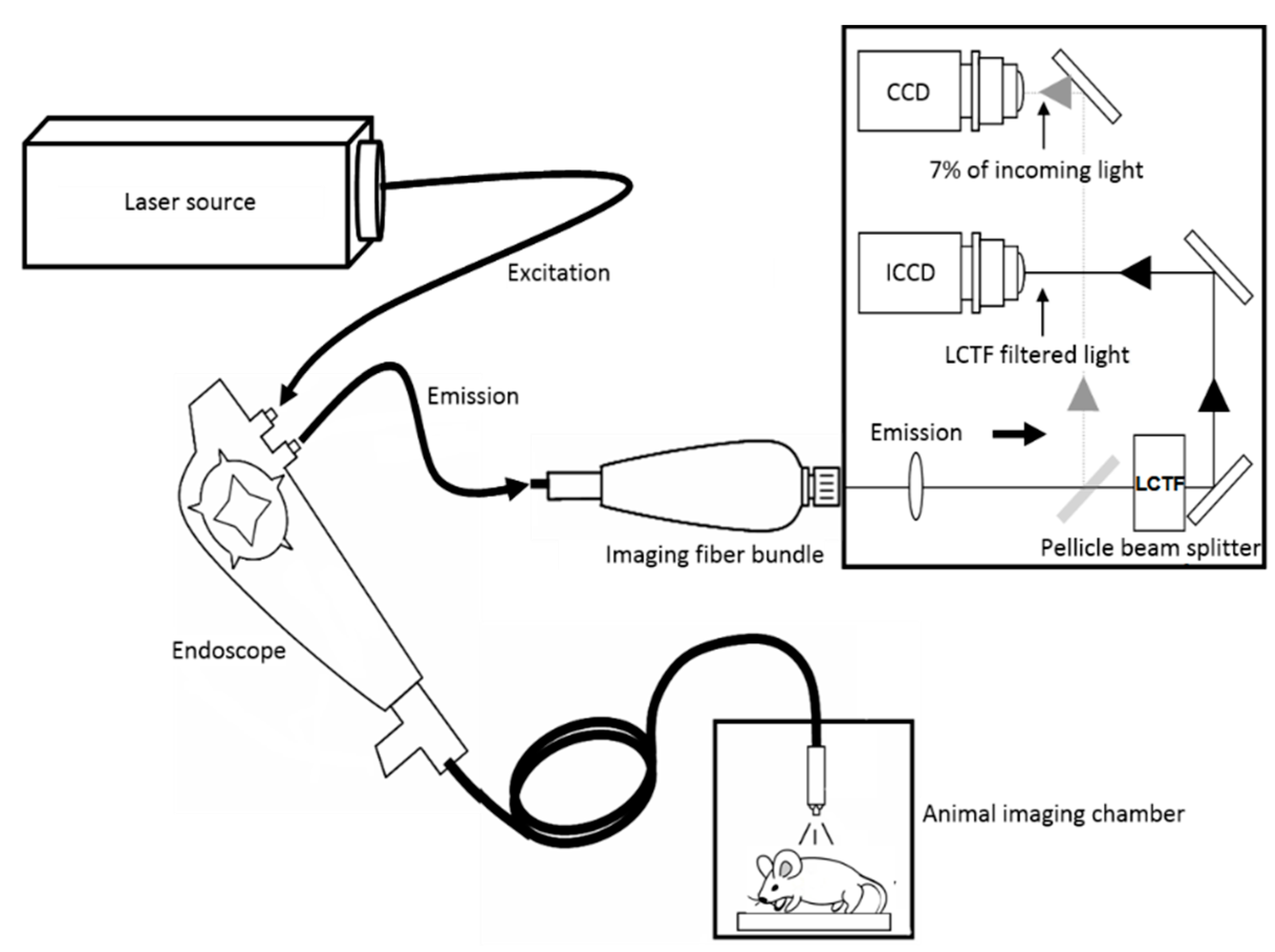
2. Methods
2.1. Time-Resolved Synchronous Fluorescence
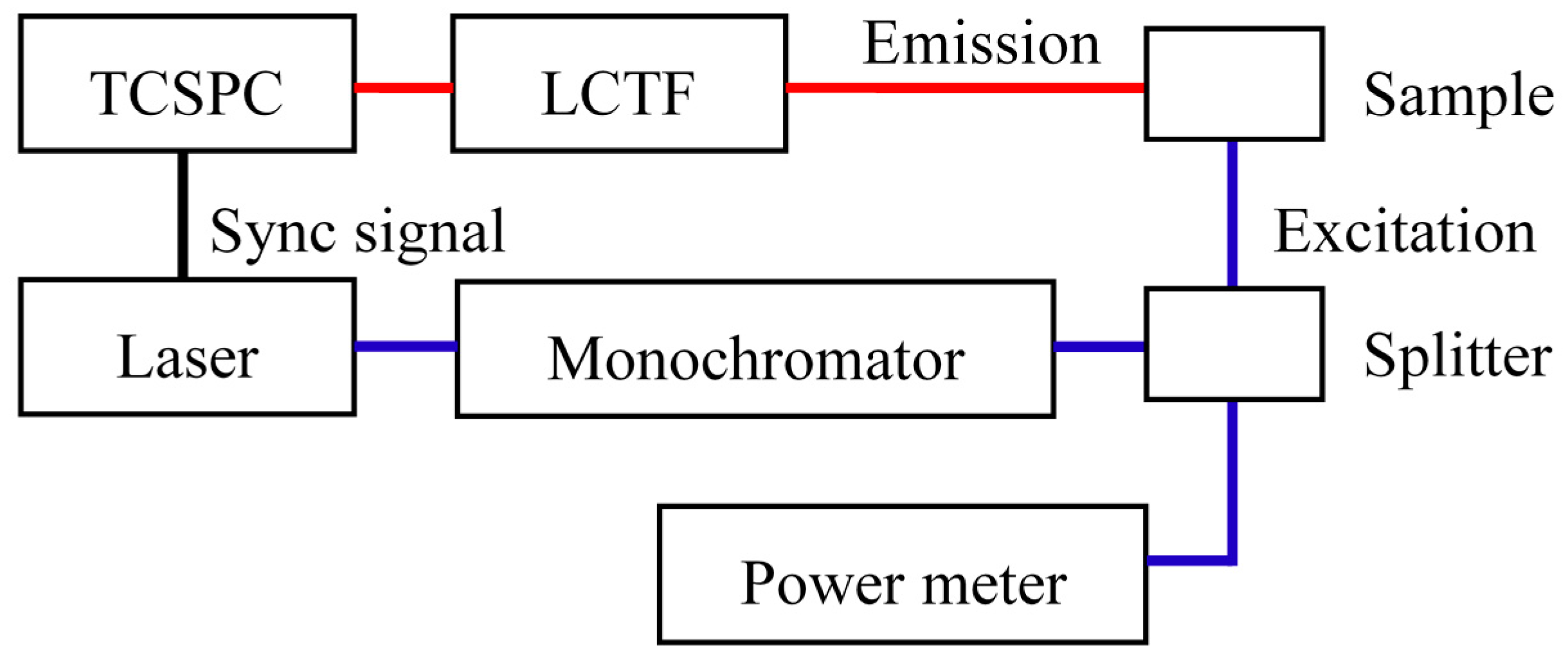
2.2. System Configuration
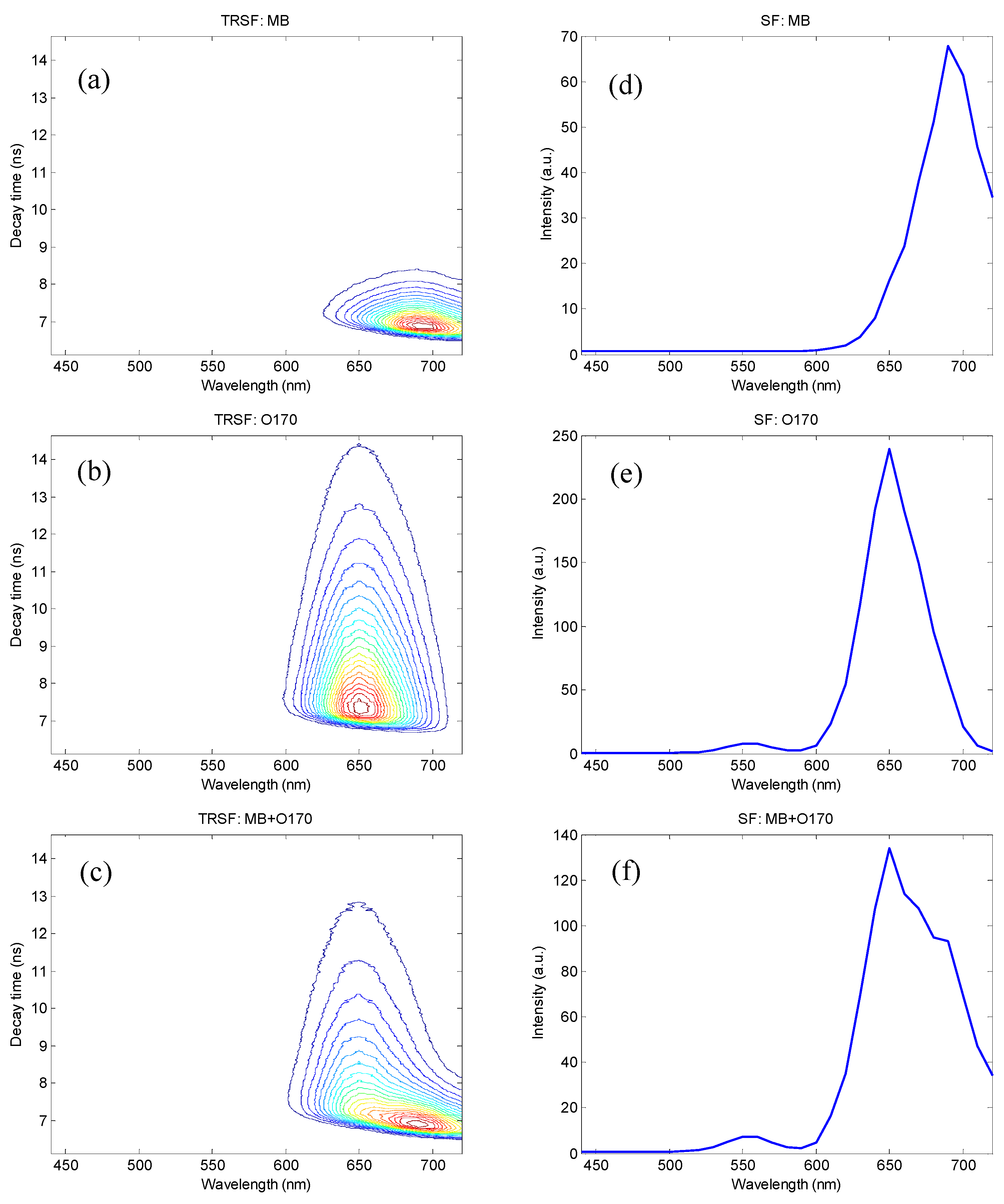
3. Results and Discussion

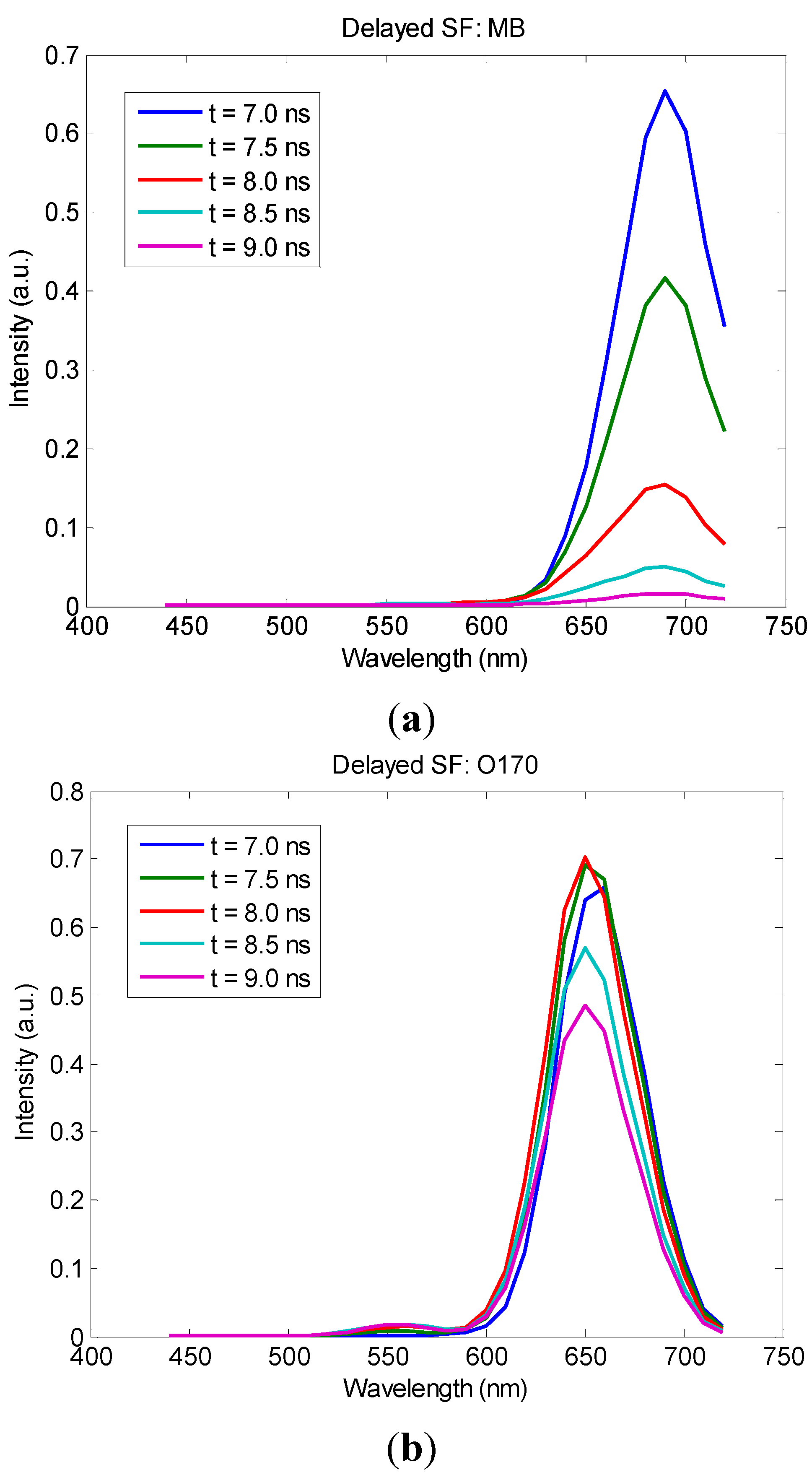
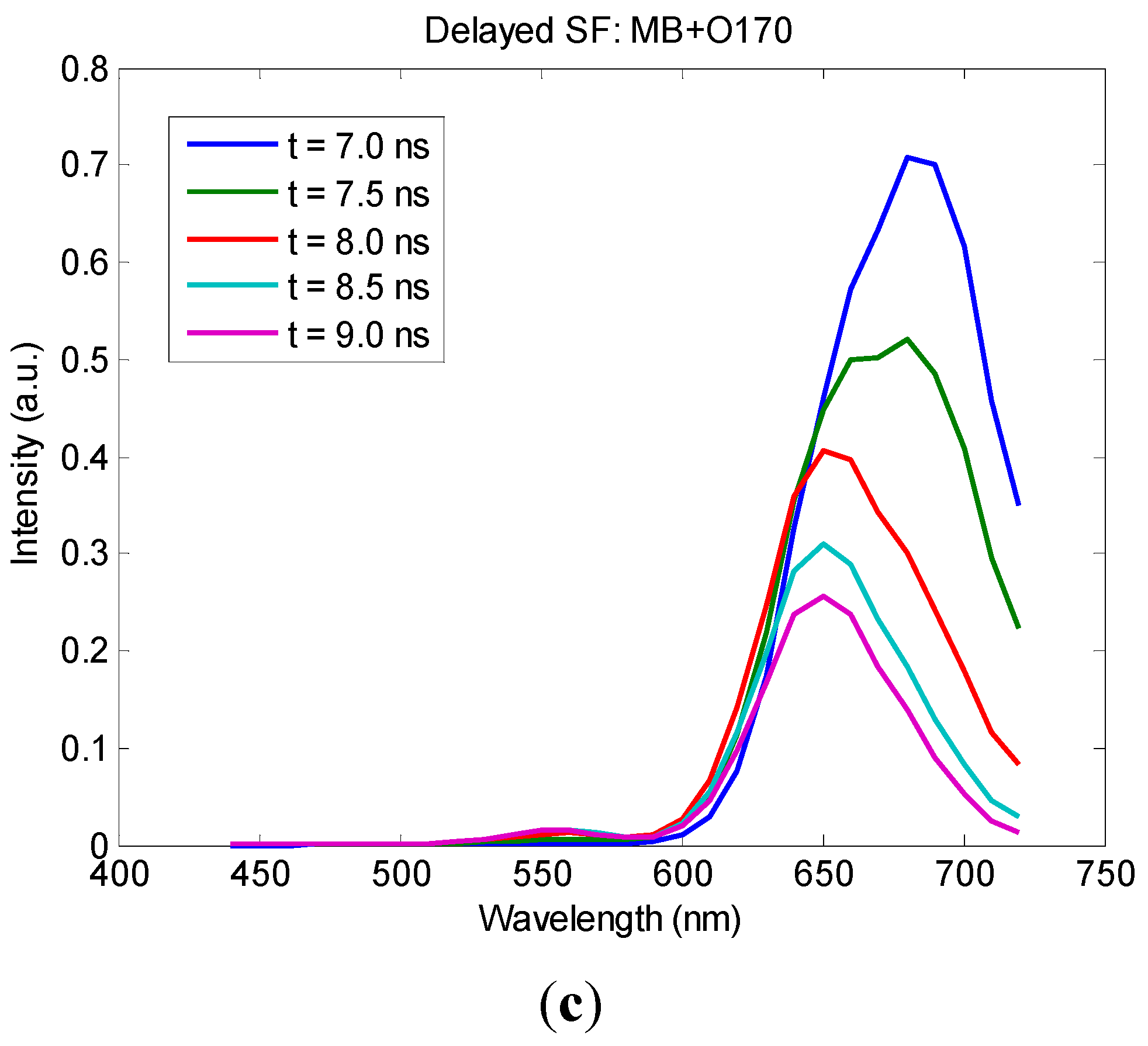
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Georgakoudi, I.; Feld, M.S. The combined use of fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in Barrett’s esophagus. Gastrointest. Endosc. Clin. North Am. 2004, 14, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia 2000, 2, 89–117. [Google Scholar] [CrossRef] [PubMed]
- Richards-Kortum, R.; Mitchell, M.F.; Ramanujam, N.; Mahadevan, A.; Thomsen, S. In vivo fluorescence spectroscopy: Potential for non-invasive, automated diagnosis of cervical intraepithelial neoplasia and use as a surrogate endpoint biomarker. J. Cell. Biochem. Suppl. 1994, 19, 111–119. [Google Scholar] [PubMed]
- Harris, D.M.; Werkhaven, J. Endogenous porphyrin fluorescence in tumors. Lasers Surg. Med. 1987, 7, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Panjehpour, M.; Overholt, B.F.; Farris, C.; Buckley, F.P., III; Sneed, R. In vivo cancer diagnosis of the esophagus using differential normalized fluorescence (DNF) indices. Lasers Surg. Med. 1995, 16, 41–47. [Google Scholar] [PubMed]
- Alfano, R.R. Advances in Optical Biopsy and Optical Mammography; Wiley: Hoboken, NJ, USA, 1997; pp. 1–203. [Google Scholar]
- Lam, S.; Palcic, B.; McLean, D.; Hung, J.; Korbelik, M.; Profio, A.E. Detection of early lung cancer using low dose Photofrin II. Chest 1990, 97, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T. Multicomponent analysis by synchronous luminescence spectrometry. Anal. Chem. 1978, 50, 396–401. [Google Scholar] [CrossRef]
- Vo-Dinh, T. Synchronous luminescence spectroscopy: Methodology and applicability. Appl. Spectrosc. 1982, 36, 576–581. [Google Scholar] [CrossRef]
- Alarie, J.P.; Vo-Dinh, T.; Miller, G.; Ericson, M.N.; Eastwood, D.; Lidberg, R.; Dominguez, M. Development of a battery-operated portable synchronous luminescence spectrofluorometer. Rev. Sci. Instrum. 1993, 64, 2541–2546. [Google Scholar] [CrossRef]
- Hueber, D.M.; Stevenson, C.L.; Vo-Dinh, T. Fast scanning synchronous luminescence spectrometer based on acousto-optic tunable filters. Appl. Spectrosc. 1995, 49, 1624–1631. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Uziel, M. Laser-induced room-temperature phosphorescence detection of benzo[a]pyrene-DNA adducts. Anal. Chem. 1987, 59, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Uziel, M.; Ward, R.J.; Vodinh, T. Synchronous fluorescence measurement of bap metabolites in human and animal urine. Anal. Lett. 1987, 20, 761–776. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Gammage, R.B. Singlet-Triplet energy difference as a parameter of selectivity in synchronous phosphorimetry. Anal. Chem. 1978, 50, 2054–2058. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, A.K. Analysis of dilute aqueous multifluorophoric mixtures using excitation-emission matrix fluorescence (EEMF) and total synchronous fluorescence (TSF) spectroscopy: A comparative evaluation. Talanta 2013, 117, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Habibul, N.; Liu, X.Y.; Sheng, G.P.; Yu, H.Q. FTIR and synchronous fluorescence heterospectral two-dimensional correlation analyses on the binding characteristics of copper onto sissolved organic matter. Environ. Sci. Technol. 2015, 49, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.E.; Frank, R.A.; Headley, J.V.; Peru, K.M.; Hewitt, L.M.; Dixon, D.G. Enhanced characterization of oil sands acid-extractable organics fractions using electrospray ionization-high resolution mass spectrometry and synchronous fluorescence spectroscopy. Environ. Toxicol. Chem. 2015, 34, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Lee, B.M.; Lee, T.H.; Park, D.H. Estimation of biological oxygen demand and chemical oxygen demand for combined sewer systems using synchronous fluorescence spectra. Sensors 2010, 10, 2460–2471. [Google Scholar] [CrossRef] [PubMed]
- Ziak, L.; Majek, P.; Hrobonova, K.; Cacho, F.; Sadecka, J. Simultaneous determination of caffeine, caramel and riboflavin in cola-type and energy drinks by synchronous fluorescence technique coupled with partial least squares. Food Chem. 2014, 159, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Sergiel, I.; Pohl, P.; Biesaga, M.; Mironczyk, A. Suitability of three-dimensional synchronous fluorescence spectroscopy for fingerprint analysis of honey samples with reference to their phenolic profiles. Food Chem. 2014, 145, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Lenhardt, L.; Zekovic, I.; Dramicanin, T.; Dramicanin, M.D.; Bro, R. Determination of the botanical origin of honey by front-face synchronous fluorescence spectroscopy. Appl. Spectrosc. 2014, 68, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Liu, Y.; Luo, M.; Xiang, X.; Ji, X.; Zhou, G.; He, Z. Metal-organic framework-based molecular beacons for multiplexed DNA detection by synchronous fluorescence analysis. Analyst 2014, 139, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.P.; Ibanez, G.A. Second-order multivariate models for the processing of standard-addition synchronous fluorescence-pH data. Application to the analysis of salicylic acid and its major metabolite in human urine. Talanta 2014, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Madrakian, T.; Bagheri, H.; Afkhami, A. Determination of human albumin in serum and urine samples by constant-energy synchronous fluorescence method. Luminescence 2015, 30, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Schenone, A.V.; Culzoni, M.J.; Campiglia, A.D.; Goicoechea, H.C. Total synchronous fluorescence spectroscopic data modeled with first- and second-order algorithms for the determination of doxorubicin in human plasma. Anal. Bioanal. Chem. 2013, 405, 8515–8523. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Saini, S.; Singh, B.; Malik, A.K. Highly sensitive synchronous fluorescence measurement of danofloxacin in pharmaceutical and milk samples using aluminium (III) enhanced fluorescence. J. Fluoresc. 2012, 22, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.P.; Duarte, T.M.; Carrondo, M.J.; Alves, P.M. Synchronous fluorescence spectroscopy as a novel tool to enable PAT applications in bioprocesses. Biotechnol. Bioeng. 2011, 108, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Pulgarin, J.A.M.; Molina, A.A.; Robles, I.S.-F. Rapid simultaneous determination of four non-steroidal anti-inflammatory drugs by means of derivative nonlinear variable-angle synchronous fluorescence spectrometry. Appl. Spectrosc. 2010, 64, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, L.; Wan, Y.; Pan, P.; Feng, L. Simultaneous determination of three potential cancer biomarkers in rat urine by synchronous fluorescence spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Dramicanin, T.; Dimitrijevic, B.; Dramicanin, M.D. Application of supervised self-organizing maps in breast cancer diagnosis by total synchronous fluorescence spectroscopy. Appl. Spectrosc. 2011, 65, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ebenezar, J.; Aruna, P.; Ganesan, S. Synchronous fluorescence spectroscopy for the detection and characterization of cervical cancers in vitro. Photochem. Photobiol. 2010, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Gammage, R.B.; Martinez, P.R. Analysis of a workplace air particulate sample by synchronous luminescence and room-temperature phosphorescence. Anal. Chem. 1981, 53, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Vahakangas, K.; Trivers, G.; Rowe, M.; Harris, C.C. Benzo(a)pyrene diolepoxide-DNA adducts detected by synchronous fluorescence spectrophotometry. Environ. Health Perspect. 1985, 62, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Shields, P.G.; Kato, S.; Bowman, E.D.; Petruzzelli, S.; Cooper, D.P.; Povey, A.C.; Weston, A. Combined micropreparative techniques with synchronous fluorescence spectroscopy or 32P-postlabelling assay for carcinogen-DNA adduct determination. IARC Sci. Publ. 1993, 124, 243–254. [Google Scholar] [PubMed]
- Askari, M.D.; Vo-Dinh, T. Implication of mitochondrial involvement in apoptotic activity of fragile histidine triad gene: Application of synchronous luminescence spectroscopy. Biopolymers 2004, 73, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Watts, W.E.; Isola, N.R.; Frazier, D.; Vo-Dinh, T. Differentiation of normal and neoplastic cells by synchronous fluorescence: Rat liver epithelial and rat hepatoma cell models. Anal. Lett. 1999, 32, 2583–2594. [Google Scholar] [CrossRef]
- Viallet, P.M.; Vo-Dinh, T.; Vigo, J.; Salmon, J.M. Investigation of lysozyme-chitobioside interactions using synchronous luminescence and lifetime measurements. J. Fluoresc. 2002, 12, 57–63. [Google Scholar] [CrossRef]
- Baucel, F.; Salmon, J.M.; Vigo, J.; Vo-Dinh, T.; Viallet, F. Investigation of noncalcium interactions of fura-2 by classical and synchronous fluorescence spectroscopy. Anal. Biochem. 1992, 204, 231–238. [Google Scholar]
- Stevenson, C.L.; Johnson, R.W.; Vo-Dinh, T. Synchronous luminescence: A new detection technique for multiple fluorescent-probes used for DNA-sequencing. Biotechniques. 1994, 16, 1104–1111. [Google Scholar] [PubMed]
- Uma, L.; Sharma, Y.; Balasubramanian, D. Fluorescence properties of isolated intact normal human corneas. Photochem. Photobiol. 1996, 63, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Vengadesan, N.; Anbupalam, T.; Hemamalini, S.; Ebenezar, J.; Muthuvelu, K.; Koteeswaran, D.; Aruna, P.R.; Ganesan, S. Characterization of cervical normal and abnormal tissues by synchronous luminescence spectroscopy. Opt. Biopsy IV 2002, 4613, 13–17. [Google Scholar]
- Dramicanin, T.; Dramicanin, M.D.; Jokanovic, V.; Nikolic-Vukosavljevic, D.; Dimitrijevic, B. Three-dimensional total synchronous luminescence spectroscopy criteria for discrimination between normal and malignant breast tissues. Photochem. Photobiol. 2005, 81, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Diagaradjane, P.; Yaseen, M.A.; Yu, J.; Wong, M.S.; Anvari, B. Synchronous fluorescence spectroscopic characterization of DMBA-TPA-induced squamous cell carcinoma in mice. J. Biomed. Opt. 2006, 11. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T. Principle of synchronous luminescence (SL) technique for biomedical diagnostics. In Proceedings of the Biomedical Diagnostic, Guidance, and Surgical-Assist Systems II, San Jose, CA, USA, 3 May 2000; pp. 42–49.
- Liu, Q.; Chen, K.; Martin, M.; Wintenberg, A.; Lenarduzzi, R.; Panjehpour, M.; Overholt, B.F.; Vo-Dinh, T. Development of a synchronous fluorescence imaging system and data analysis methods. Opt. Express 2007, 15, 12583–12594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Grant, G.; Vo-Dinh, T. Investigation of synchronous fluorescence method in multi-component analysis in tissue. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 927–940. [Google Scholar]
- Patra, D.; Mishra, A.K. Recent developments in multi-component synchronous fluorescence scan analysis. TrAC Trends Anal. Chem. 2002, 21, 787–798. [Google Scholar] [CrossRef]
- Stevenson, C.L.; Vo-Dinh, T. Analysis of polynuclear aromatic compounds using laser-excited synchronous fluorescence. Anal. Chim. Acta 1995, 303, 247–253. [Google Scholar] [CrossRef]
- Wendel, W.B. The control of methemoglobinemia with methylene blue. J. Clin. Investig. 1939, 18, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hyun, H.; Ashitate, Y.; Wada, H.; Park, G.; Lee, J.H.; Njiojob, C.; Henary, M.; Frangioni, J.V.; Choi, H.S. Prototype nerve-specific near-infrared fluorophores. Theranostics 2014, 4, 823–833. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fales, A.; Vo-Dinh, T. Time-Resolved Synchronous Fluorescence for Biomedical Diagnosis. Sensors 2015, 15, 21746-21759. https://doi.org/10.3390/s150921746
Zhang X, Fales A, Vo-Dinh T. Time-Resolved Synchronous Fluorescence for Biomedical Diagnosis. Sensors. 2015; 15(9):21746-21759. https://doi.org/10.3390/s150921746
Chicago/Turabian StyleZhang, Xiaofeng, Andrew Fales, and Tuan Vo-Dinh. 2015. "Time-Resolved Synchronous Fluorescence for Biomedical Diagnosis" Sensors 15, no. 9: 21746-21759. https://doi.org/10.3390/s150921746
APA StyleZhang, X., Fales, A., & Vo-Dinh, T. (2015). Time-Resolved Synchronous Fluorescence for Biomedical Diagnosis. Sensors, 15(9), 21746-21759. https://doi.org/10.3390/s150921746




