Porous Silicon Structures as Optical Gas Sensors
Abstract
:1. Introduction
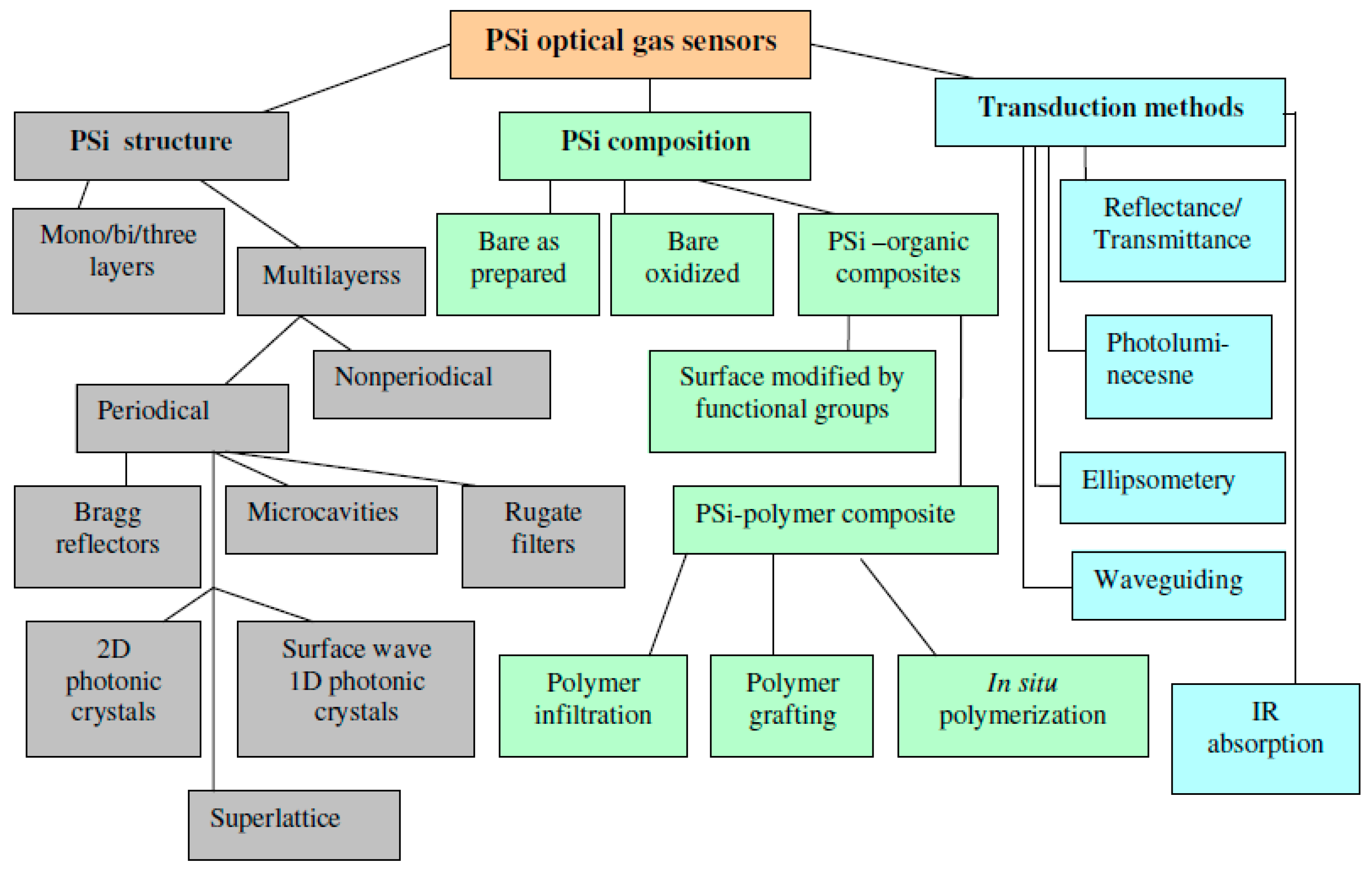
2. Early Studies and Mechanisms of Optical Gas Sensing
3. Recent Progress in the Improvement of Sensitivity and Specificity of Gas Sensors with Multilayered Psi Structures
3.1. Sensors with Modified PSi Surface
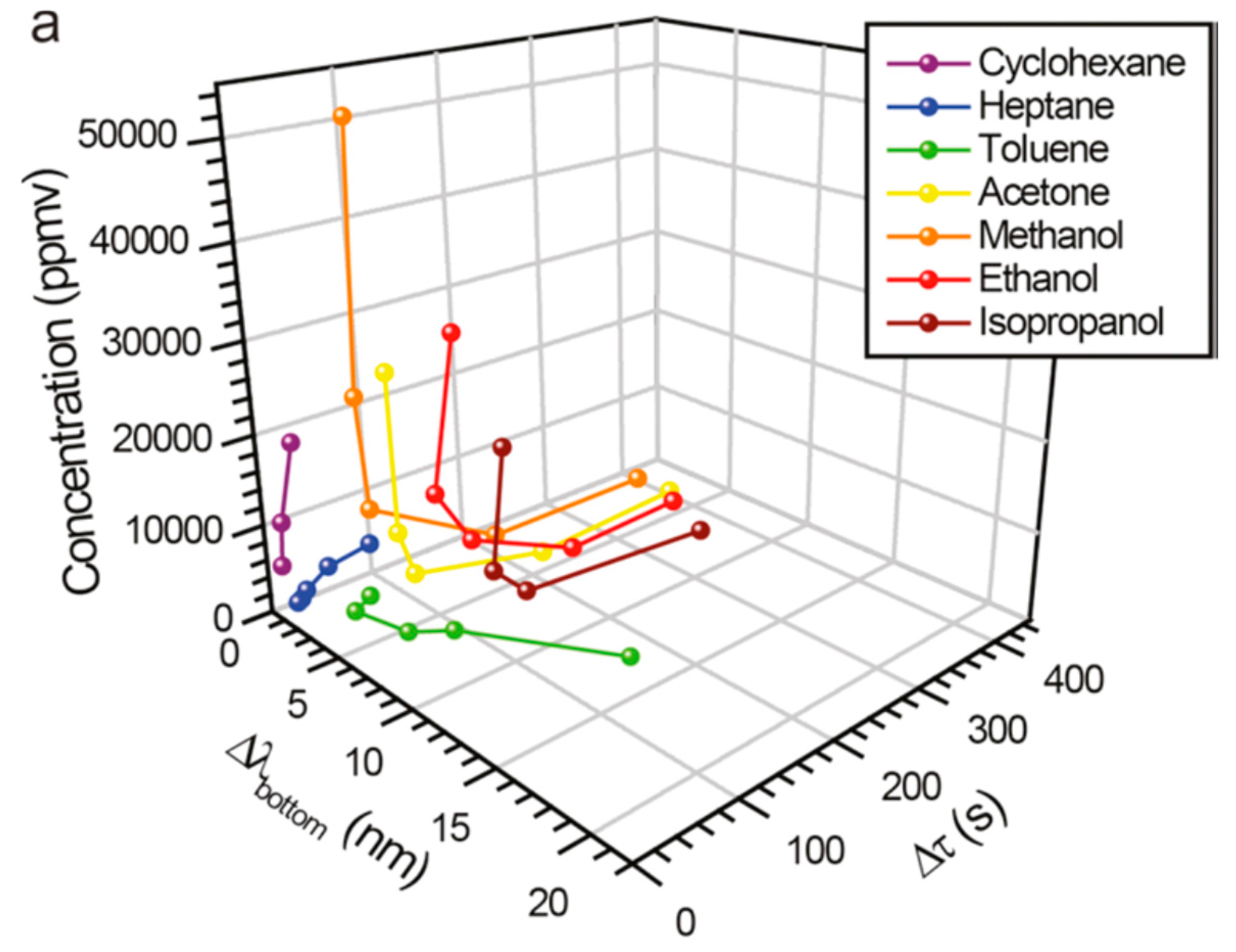
3.2. Sensors Based on PSi-Polymer Composites
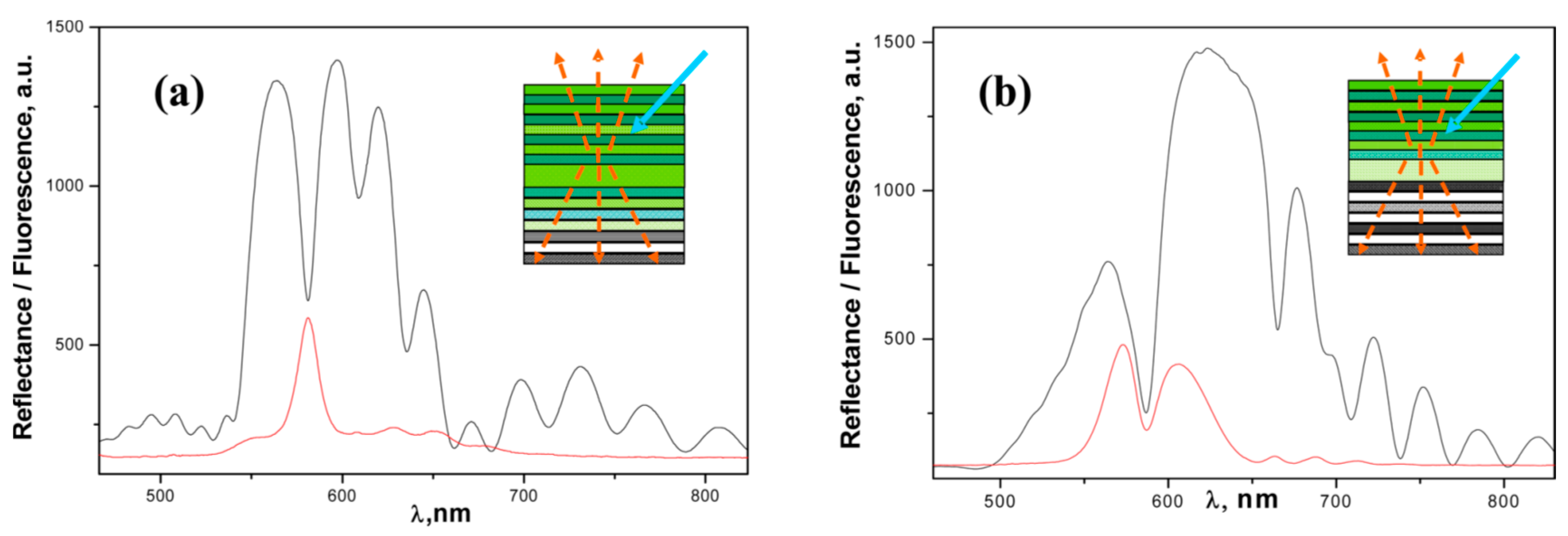
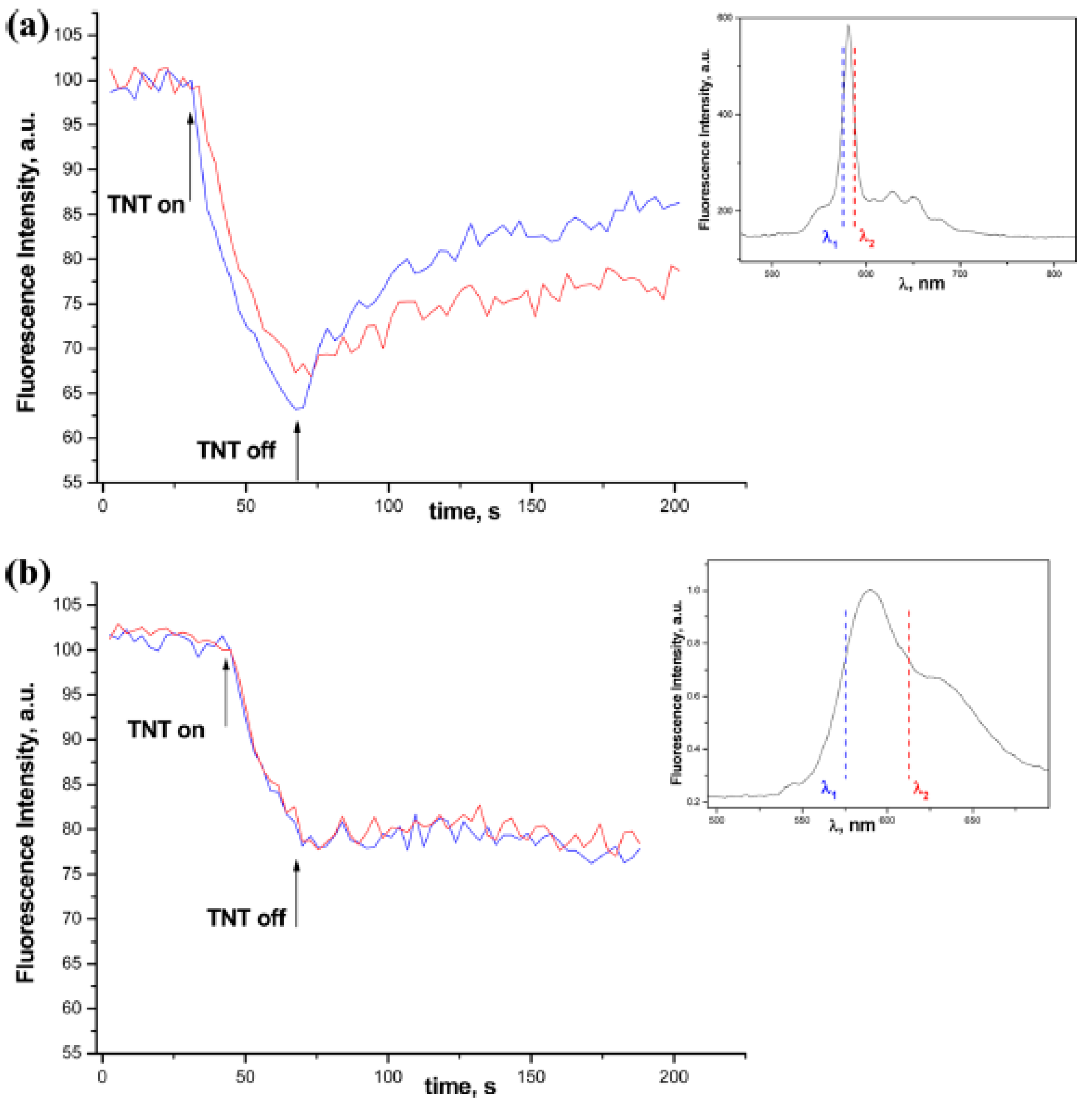
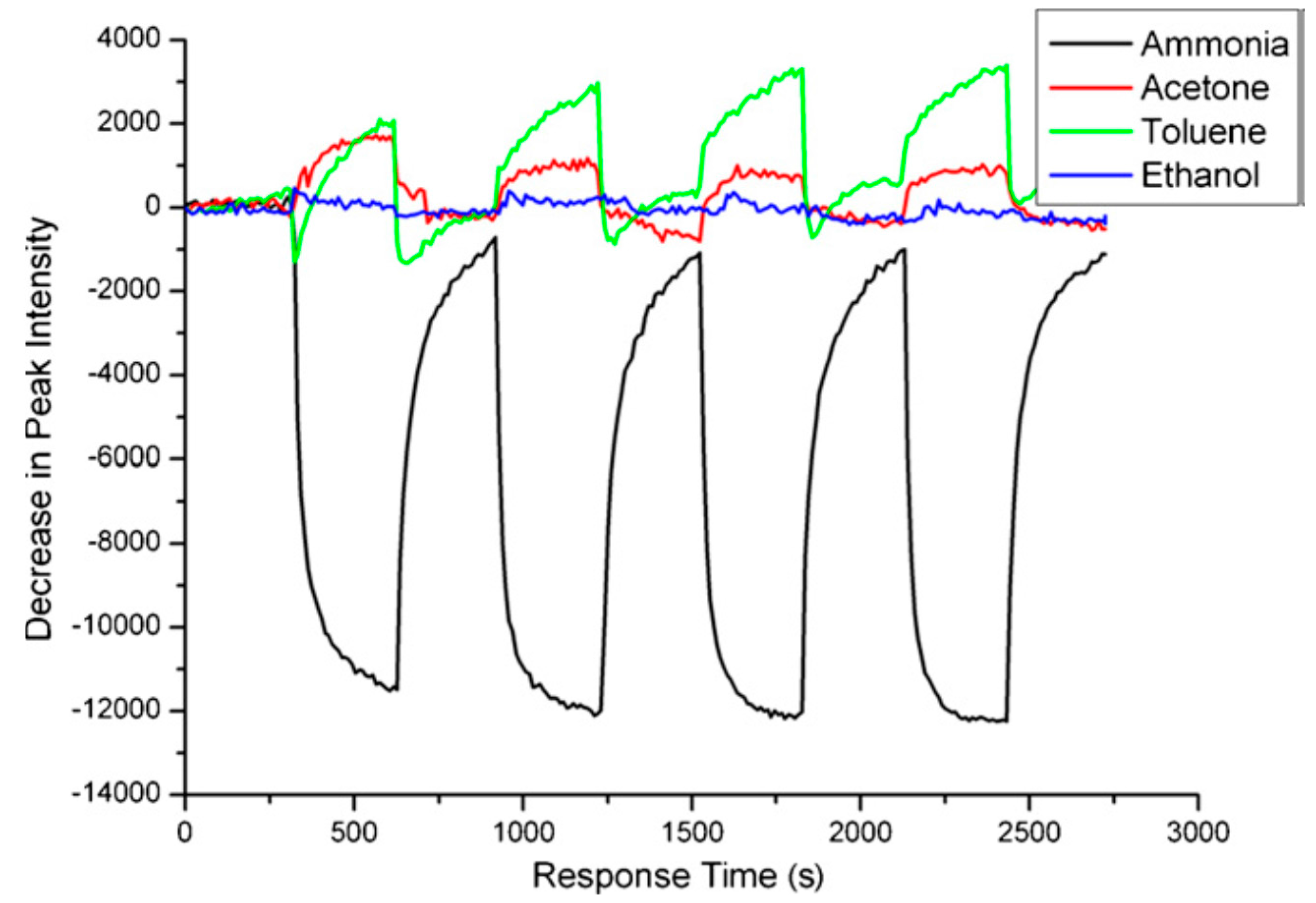
4. New Structures and Approaches
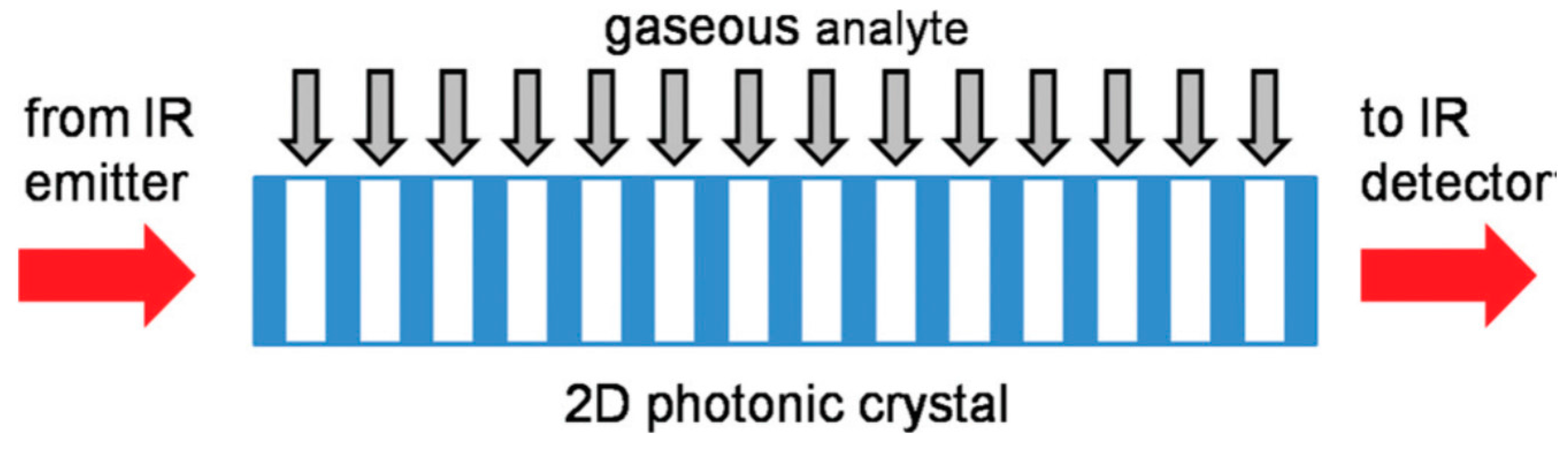
4.1. Nonperiodic, Superlattice, Surface Wave, and 2D Photonic Band Gap Structures
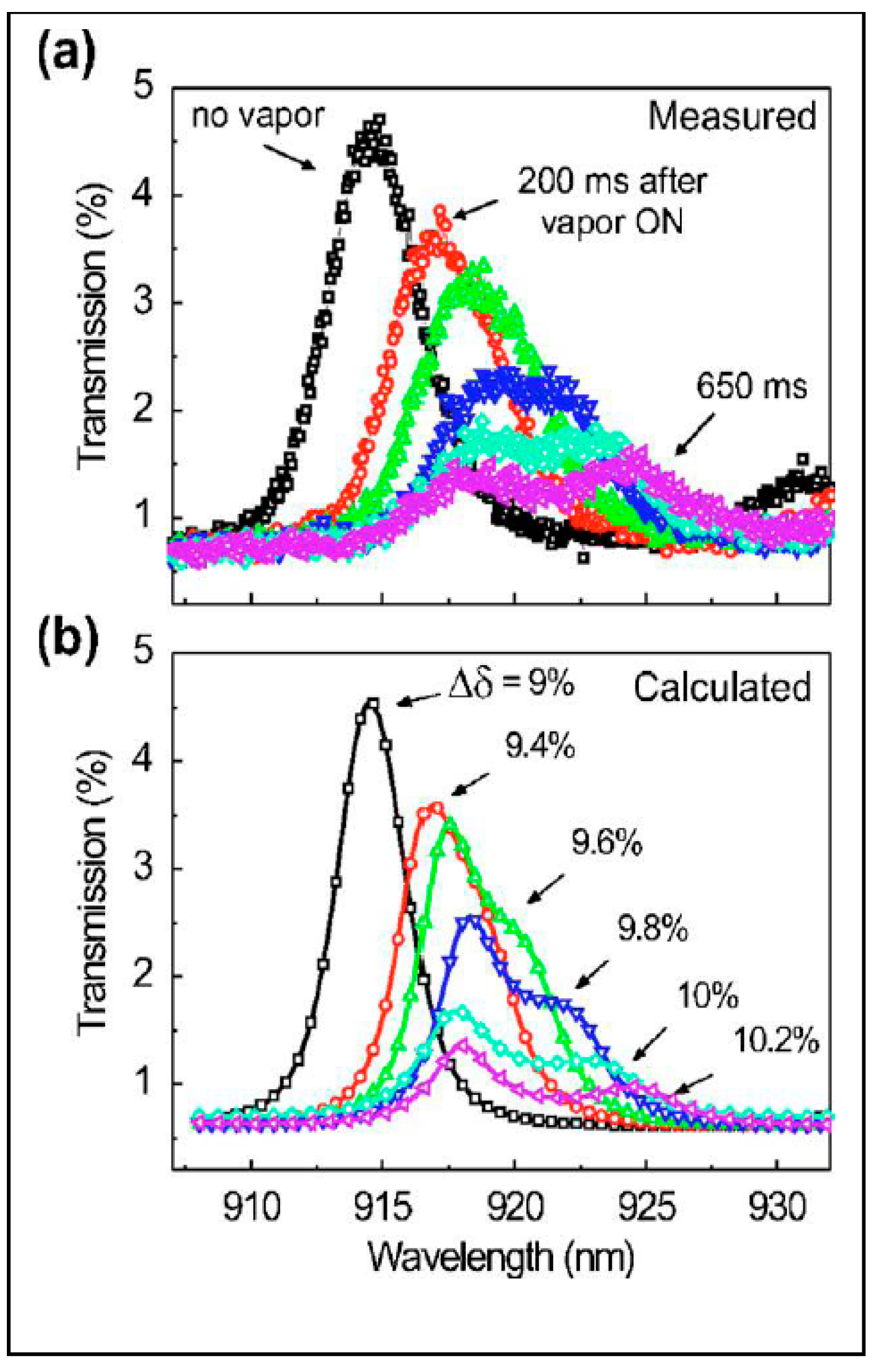
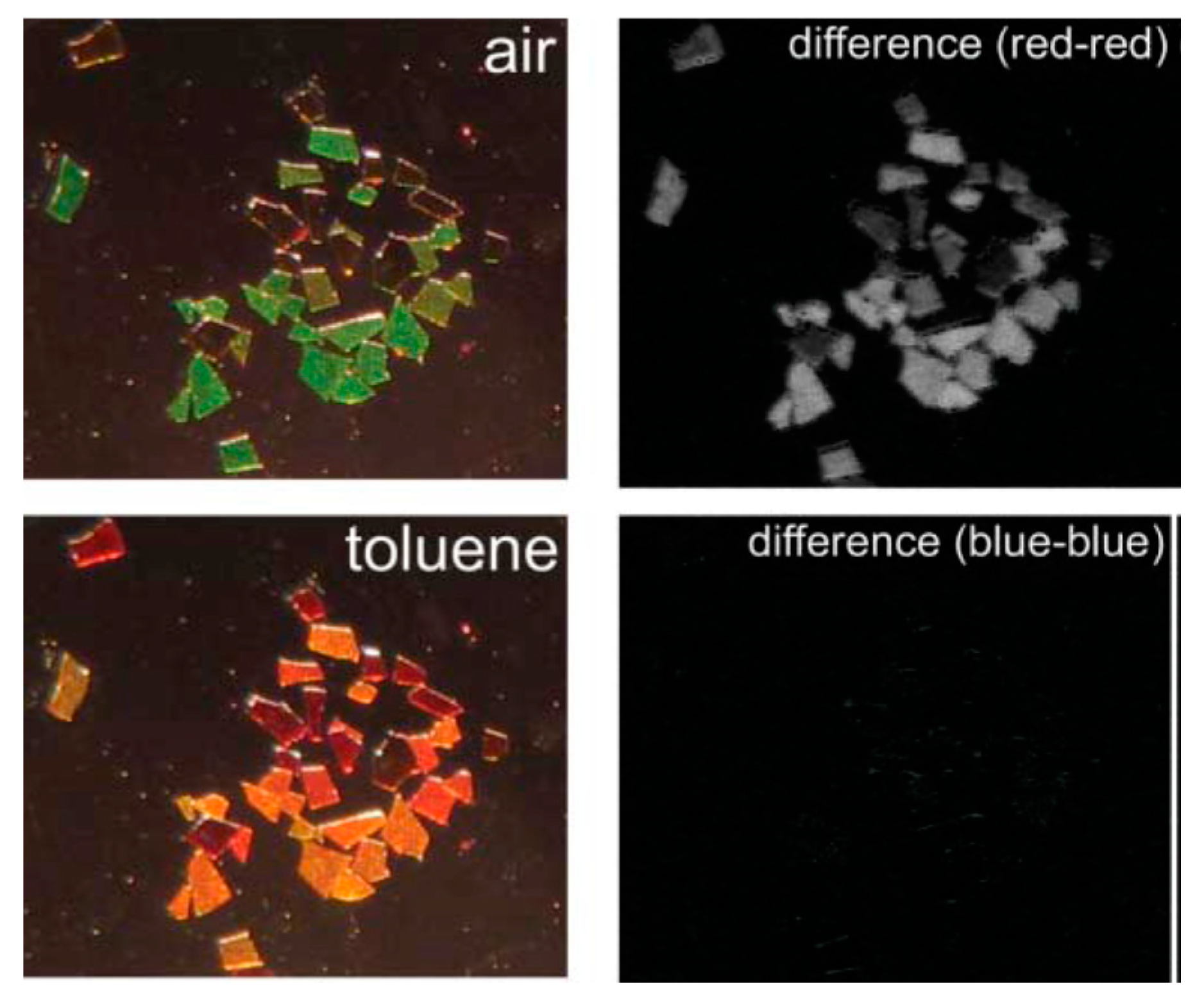
4.2. Colorometric, Standoff, and Fiber Optic Methodologies
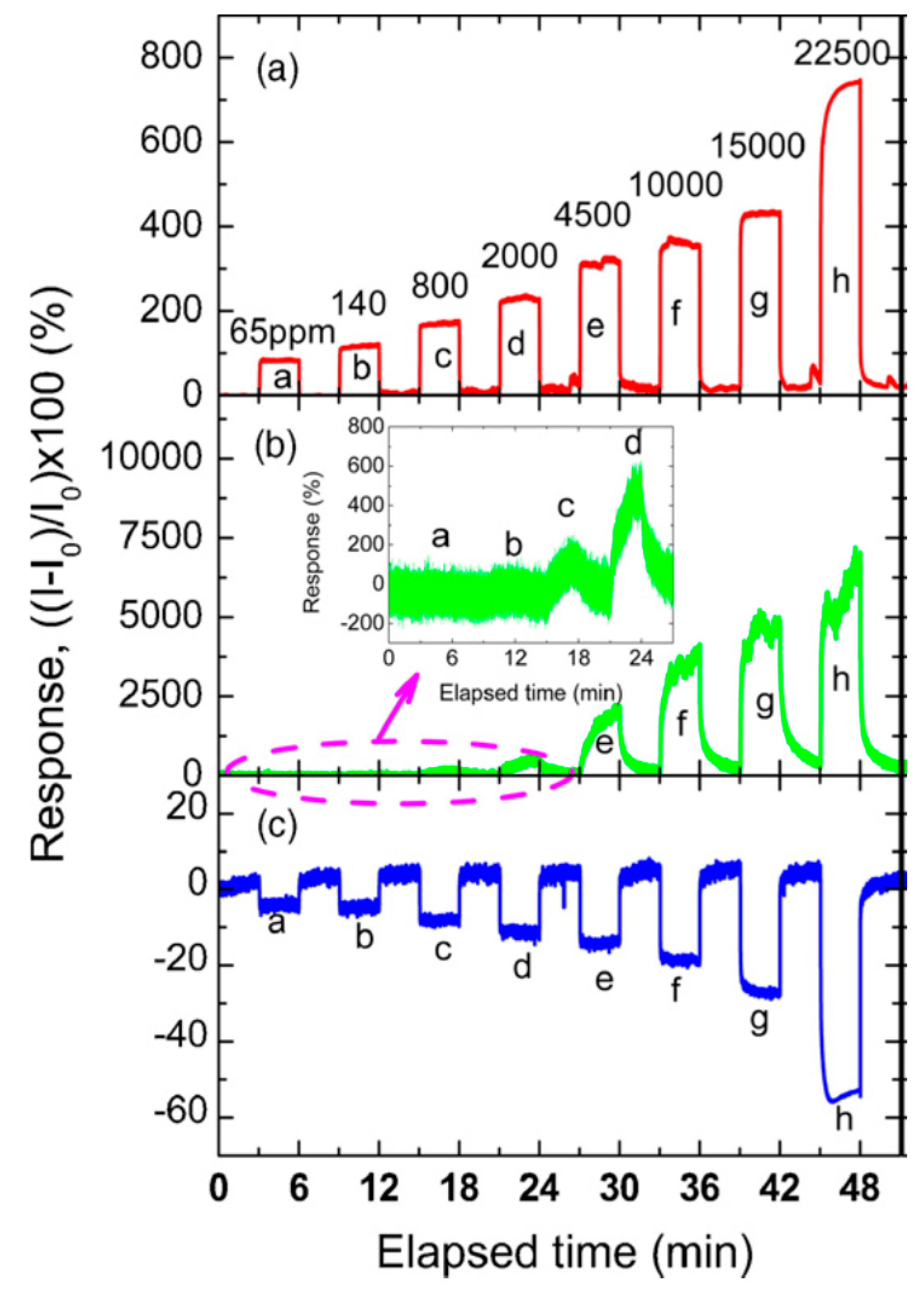
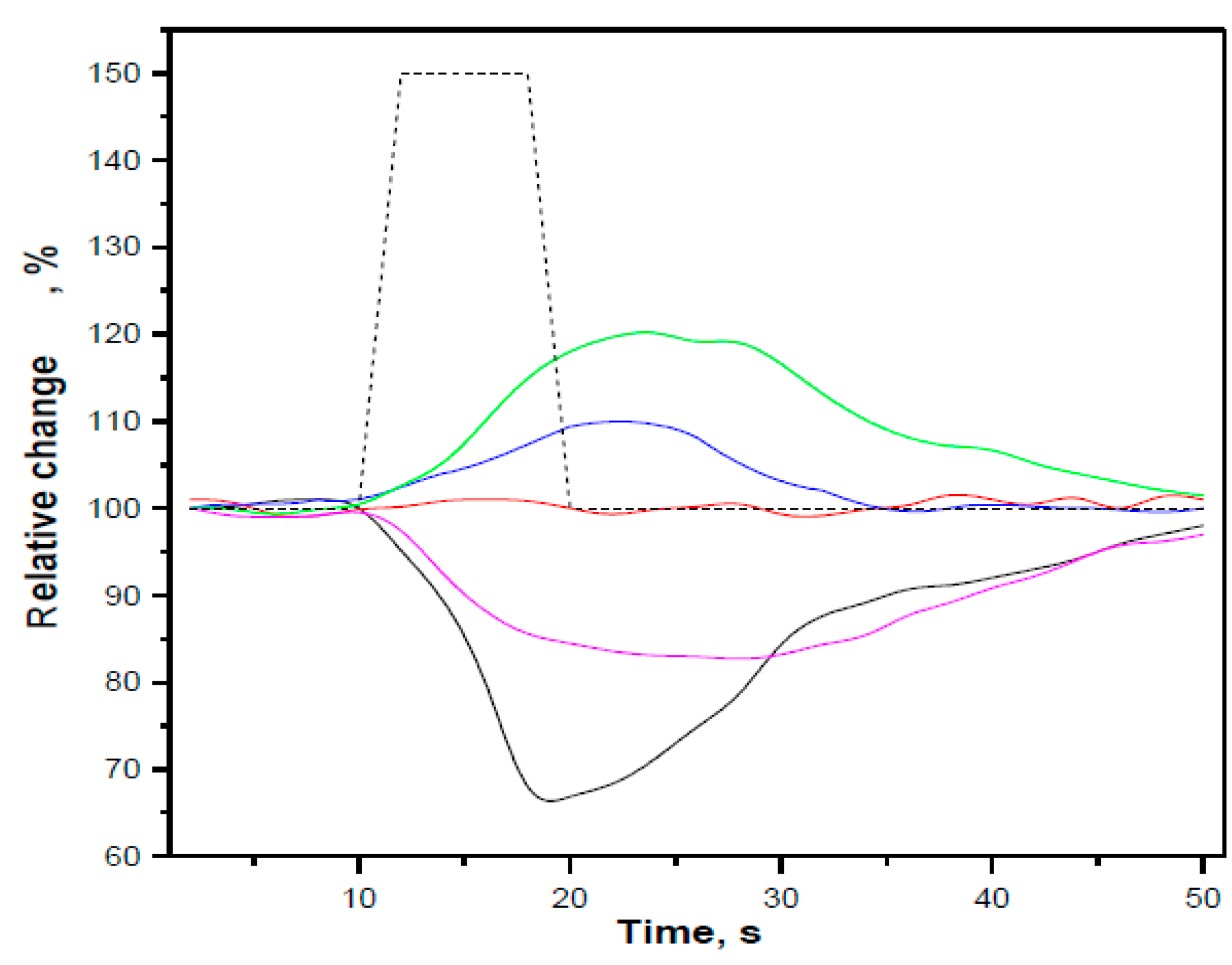
5. Sensors Arrays and Multiparametric Gas Sensing
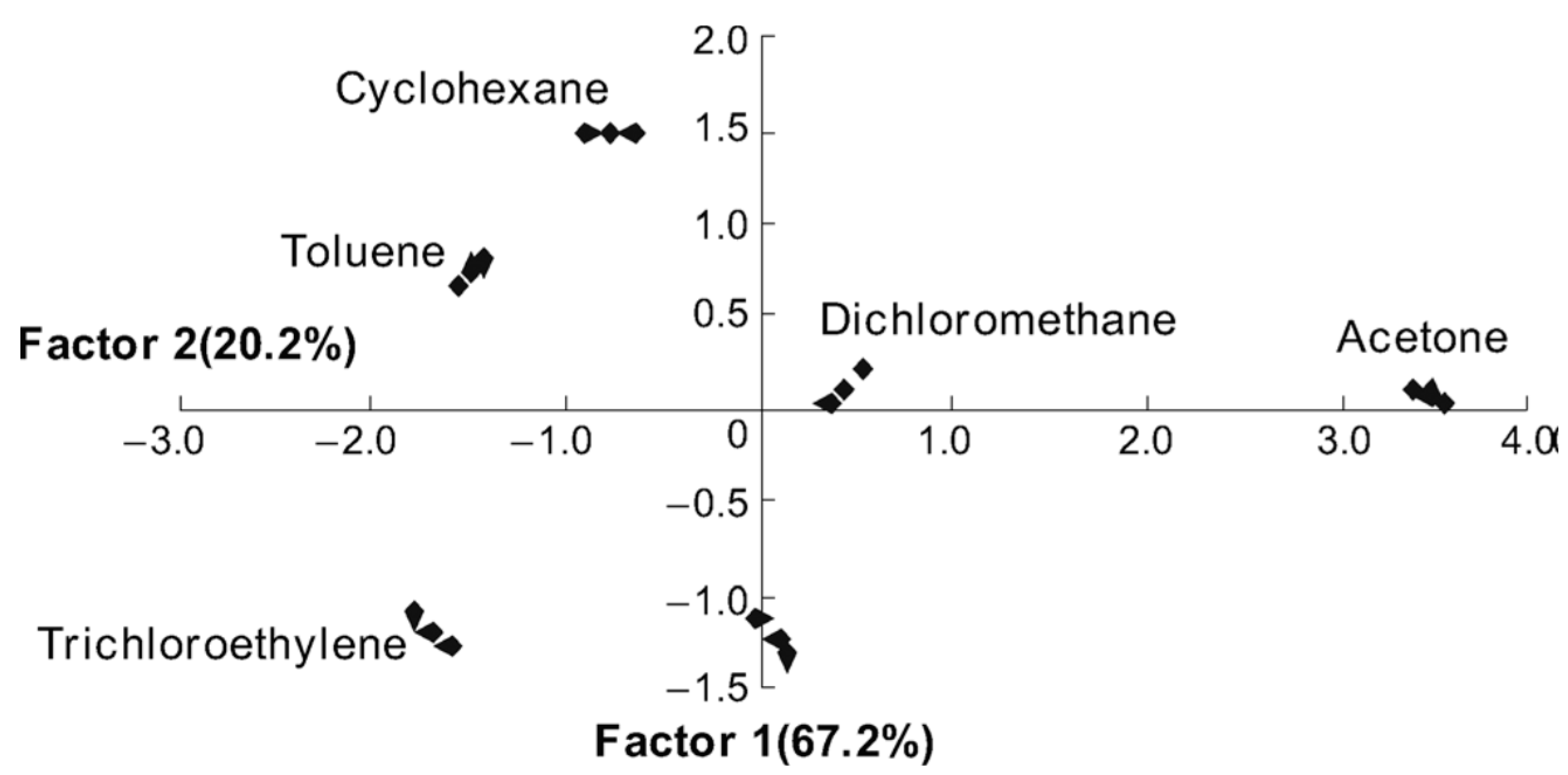
6. Conclusions
Conflicts of Interest
References
- Sailor, M.J. Porous Silicon in Practice; Wiley-VCH Verlag: Weinheim, Germany, 2012. [Google Scholar]
- Lauerhaas, J.M.; Gredo, G.M.; Heinrich, J.L.; Sailor, M.J. Reversible luminescence quenching of porous Si by solvents. J. Am. Chem. Soc. 1992, 114, 1911–1912. [Google Scholar] [CrossRef]
- Lauerhaas, J.M.; Sailor, M.J. Chemical modification of the photoluminescence quenching of porous silicon. Science 1993, 261, 1567–1568. [Google Scholar] [CrossRef] [PubMed]
- Harraz, F.A. Porous silicon chemical sensors and biosensors: A review. Sens. Actuators B Chem. 2014, 202, 897–912. [Google Scholar] [CrossRef]
- Pacholski, C. Photonic crystal sensors based on porous silicon. Sensors 2013, 13, 4694–4713. [Google Scholar] [CrossRef] [PubMed]
- Barrillaro, G. Porous silicon gas sensing. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 856–858. [Google Scholar]
- Korotchenkov, G. Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings for Applications; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Bjorklund, R.B.; Zangooie, S.; Arwin, H. Color changes in thin porous silicon films caused by vapor exposure. Appl. Phys. Lett. 1996, 69, 3001–3003. [Google Scholar] [CrossRef]
- Zangooie, S.; Bjorklund, R.; Arwin, H. Vapor sensitivity of thin poprous silicon layers. Sens. Actuators B Chem. 1997, 43, 168–174. [Google Scholar] [CrossRef]
- Snow, P.A.; Squire, E.K.; Russell, P.S.J.; Canham, L.T. Vapor sensing ising the optical properties of porous silicon Bragg mirrors. J. Appl. Phys. 1999, 86, 1781–1784. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: Waltham, MA, USA, 1991. [Google Scholar]
- Gao, J.; Gao, T.; Li, Y.Y.; Sailor, M.J. Vapor sensors based on optical interferometry from oxidized microporous silicon films. Langmuir 2002, 18, 2229–2233. [Google Scholar] [CrossRef]
- Segal, E.; Krepker, M.A. Polymer-porous silicon composites. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 187–198. [Google Scholar]
- McGill, R.A.; Abraham, M.H.; Grate, J.W. Choosing polymer coating for chemical sensors. Chemtech 1994, 9, 27–37. [Google Scholar]
- Mulloni, V.; Pavesi, L. Porous silicon microcavities as optical chemical sensors. Appl. Phys. Lett. 2000, 76, 2523–2525. [Google Scholar] [CrossRef]
- Baratto, C.; Faglia, G.; Sberveglieri, G.; Gaburro, Z.; Pancheri, L.; Oton, C.; Pavesi, L. Multiparametric porous silicon sensors. Sensors 2002, 2, 121–126. [Google Scholar] [CrossRef]
- Heitmann, J.; Muller, F.; Yi, L.; Zacharias, M.; Kovalev, D.; Eichhorn, F. Excitons in Si nanocrystals: Confinement and migration effect. Phys. Rev. B 2004, 69, 195309–195316. [Google Scholar] [CrossRef]
- Content, S.; Trogler, W.C.; Sailor, M.J. Detection of Nitrobenzene, DNT and TNT vapors by quenching of porous sililicone photoluminescence. Chem. Eur. J. 2000, 6, 2205–2213. [Google Scholar] [CrossRef]
- Levitsky, I.A.; Euler, W.B.; Tokranova, N.; Rose, A. Fluorescent Polymer Porous Silicon Microcavity Devices for Explosives Detection. Appl. Phys. Lett. 2007, 90, 041904–041907. [Google Scholar] [CrossRef]
- Ong, P.L.; Levitsky, I.A. Fluorescent Gas Sensors Based on Nanoporous Optical Resonators (Microcavities) Infiltrated with Sensory Emissive Polymers. Sensors 2011, 11, 2947–2951. [Google Scholar] [CrossRef]
- Tokranova, N.; Novak, S.W.; Castracane, J.; Levitsky, I.A. Deep Infiltration of Emissive Polymers into Mesoporous Silicon Microcavities: Nanoscale Confinement and Advanced Vapor Sensing. J. Phys. Chem. C 2013, 117, 22667–22676. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.Y.; Sailor, M.J. Tuning the response and stability of thin film mesoporous silicon vapor sensors by surface modification. Langmuir 2002, 18, 9953–9957. [Google Scholar] [CrossRef]
- Salonen, J.; Lehto, V.P.; Björkqvist, M.; Laine, E.; Niinistö, L. Studies of thermally carbonized porous silicon surface. Phys. Status Solidi A 2000, 182, 123–126. [Google Scholar] [CrossRef]
- Salonen, J.; Laine, E.; Niinistö, L. Thermal carbonization of porous silicon surface by acetylene. J. Appl. Phys. 2002, 91, 456–461. [Google Scholar] [CrossRef]
- Salonen, J.; Björkqvist, M.; Laine, E.; Niinistö, L. Stabilization of porous silicon surface by thermal decomposition of acetylene. Appl. Surf. Sci. 2004, 225, 389–394. [Google Scholar] [CrossRef]
- Jalkanen, T.; Torres-Costa, V.; Salonen, J.; Björkqvist, M.; Mokilo, E.; Martínez-Duart, J.M.; Lehto, V.P. Optical gas sensing properties of thermally hydrocarbonized porous silicon Bragg reflector. Opt. Express 2009, 17, 5446–5451. [Google Scholar] [CrossRef] [PubMed]
- Torres-Costa, V.; Salonen, J.; Jalkanen, T.; Lehto, V.P.; Martin Palma, R.J.; Martínez-Duart, J.M. Carbonization of porous silicon optical gas sensors for enhanced stability and sensitivity. Phys. Stat. Sol. A 2009, 206, 1306–1308. [Google Scholar] [CrossRef]
- Ruminski, A.M.; King, B.H.; Salonen, J.; Snyder, J.L.; Sailor, M.J. Porous silicon-based optical microsensors for volatile organic analytes: Effect of surface chemistry on stability and specificity. Adv. Funct. Mater. 2010, 20, 2874–2883. [Google Scholar] [CrossRef]
- Jalkanen, T.; Makila, E.; Suzuki, Y.-I.; Urata, T.; Fukami, K.; Sakka, T.; Salonen, J.; Ogata, Y.H. Studies on Chemical Modifiation of Porous Silicon-Based Graded-Index Optical Microcavities for Improved Stability Under Alkaline Conditions. Adv. Funct. Mater. 2012, 22, 3890–3898. [Google Scholar] [CrossRef]
- De Stefano, L.; Rendina, I.; Moretti, L.; Tundo, S.; Rossi, A.M. Smart optical sensors for chemical substances based on porous silicon technology. Appl. Opt. 2004, 43, 167–172. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, L.; Moretti, L.; Rendina, I.; Rossi, A.M. Porous silicon microcavities for optical hydrocarbons detection. Sens. Actuators A Phys. 2003, 104, 179–182. [Google Scholar] [CrossRef]
- De Stefano, L.; Moretti, L.; Rendina, I.; Rossi, A.M. Time-resolved sensing of chemical species in porous silicon optical microcavity. Sens. Actuators B Chem. 2004, 100, 168–172. [Google Scholar] [CrossRef]
- Letant, S.E.; Sailor, M.J. Molecular identification by time-resolved interferometry in a porous silicon film. Adv. Mater. 2001, 13, 335–338. [Google Scholar] [CrossRef]
- Letant, S.E.; Sailor, M.J. Detection of HF gas with a porous Si interferometer. Adv. Mater. 2000, 12, 355–359. [Google Scholar] [CrossRef]
- Sohn, H.; Letant, S.E.; Sailor, M.J.; Trogler, W.C. Detection of fluorophosphonate Chemical Warfare Agents by atalytic hydrolysis with porous silicon interferometer. J. Am. Chem. Soc. 2000, 122, 5399–5400. [Google Scholar] [CrossRef]
- King, B.H.; Ruminski, A.M.; Snyder, J.L.; Sailor, M.J. Optical-fiber-mounted porous silicon photonic crystals for sensing organic vapor breakthrough in activated carbon. Adv. Mater. 2007, 19, 4530–4534. [Google Scholar] [CrossRef]
- Cnan, D.Y.; Sega, A.G.; Lee, J.Y.; Gao, T.; Cunin, F.; Di Renzo, F.; Sailor, M.J. Optical detection of C2 hydrocarbons ethane, ethylene, and acetylene with a photonic crystal made from carbonized porous silicon. Inor. Chim. Acta 2014, 422, 21–29. [Google Scholar]
- King, B.H.; Gramada, A.; Link, J.R.; Sailor, M.J. Internally referenced ammonia sensor based on an electrochemically prepared porous SiO2 photonic crystal. Adv. Mater. 2007, 19, 4044–4048. [Google Scholar] [CrossRef]
- Ruminski, A.M.; Barillaro, G.; Chaffin, C.; Sailor, M.J. Internally referenced remote sensors for HF and Cl2 using reactive porous silicon photonic crystals. Adv. Funct. Mater. 2011, 21, 1511–1525. [Google Scholar] [CrossRef]
- Sweetman, M.J.; Voelcker, N.H. Chemically patterned porous silicon pnotonic crystal towards internally references organic vapour sensors. RSC Adv. 2012, 2, 4620–4622. [Google Scholar] [CrossRef]
- Hutter, T.; Ruschin, S. Non-imaging optical method for multi-sensing of gases based on porous silicon. IEEE Sens. J. 2010, 10, 97–103. [Google Scholar] [CrossRef]
- Ruminski, A.M.; Moore, M.M.; Sailor, M.J. Humidity-compensating sensor for volatile organic compounds using stacked porous silicon photonic crystals. Adv. Funct. Mater. 2008, 18, 3418–3426. [Google Scholar] [CrossRef]
- Jalkanen, T.; Salonen, J.; Torres-Costa, V.; Fukami, K.; Sakka, T.; Ogata, Y.H. Structural consideration multistopband mesoporous silicon rugate filter prepared for gas sensing purposes. Opt. Express 2011, 19, 13291–13305. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.S.; Dailor, M.J.; Fukami, K.; Sakka, T.; Ogata, Y.H. Sensitivity of porous silicon rugate filters for chemical vapor detection. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Li, S.; Hu, D.; Huang, G.; Cai, L. Optical sensing nanostructure for porous silicon rugare filters. Nanoscale Res. Lett. 2012, 7, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.L.; Sega, A.G.; Sailor, M.J. Identification and quantification of organic vapors by time-resolved diffusion in stacked mesoporous photonic crystals. Nano Lett. 2011, 11, 3169–3173. [Google Scholar] [CrossRef] [PubMed]
- King, B.H.; Wong, T.; Sailor, M.J. Detection of pure chemical vapors in a thermally cycled porous silica photonic crystal. Langmuir 2011, 27, 8576–8585. [Google Scholar] [CrossRef] [PubMed]
- Sailor, M.J. Color Me Sensitive: Amplification and Discrimination in Photonic Silicon Nanostructures. ACS Nano 2007, 1, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Jane, A.; Dronov, R.V.; Hodges, A.; Voelcker, N.H. Porous Silicon Biosensors on the Advance. Trends Biotechnol. 2009, 27, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Shtenberg, G.; Segal, E. Porous Si optical biosensors. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 869–885. [Google Scholar]
- Thomas, S.W., III; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescence conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, X.; Xu, E.; Tong, C.; Wu, J. Optical ammonia gas sensor based on a porous silicon rugate filter coated with polymer-supported dye. Anal. Chim. Acta 2011, 685, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Minko, S. Grafting on solid surfaces: “grafting” to and “grafting from” methods. In Polymer Surfaces and Interfaces; Springer: Berlin/Heidelberg, Germany, 2008; pp. 215–234. [Google Scholar]
- Bakker, J.W.P.; Arwin, H.; Wang, G.; Jarrendahl, K. Improvement of porous silicon based gas sensors by polymer modification. Phys. Stat. Sol. A. 2003, 197, 378–381. [Google Scholar] [CrossRef]
- Yang, J.-S.; Swager, T.M. Fluorescent Porous Polymer Films as TNT Chemosensors. J. Am. Chem. Soc. 1998, 120, 11864–11873. [Google Scholar] [CrossRef]
- Sohn, H.; Sailor, M.J.; Magde, D.; Trogler, W.C. Detection of Nitroaromatic Explosives Based on Photoluminescent Polymers Containing Metalloles. J. Am. Chem. Soc. 2003, 125, 3821–3286. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-P.; Chao, C.-Y.; Huang, J.H.; Li, A.-K.; Hsu, C.-S.; Lin, M.-S.; Hsieh, B.R.; Su, A.-C. Fluorescent Conjugated Polymer Films as TNT Chemosensors. Synth. Met. 2004, 144, 297–301. [Google Scholar] [CrossRef]
- Dian, J.; Konecny, M.; Broncova, G.; Krondak, M.; Matolinova, I. Electrochemical fabrication and characterization of porous silicon/polypyrrole composites and chemical sensing of organic vapors. Int. J. Electrochem. Sci. 2013, 8, 1559–1572. [Google Scholar]
- De Stefano, L.; Rotiroti, L.; Rea, I.; De Tommasi, E.; Canciello, M.; Maglio, G.; Palumbo, R. A nanostructured hybrid material based on polymer infiltrated porous silicon layer. Appl. Phys. A Mater. Sci. Process. 2010, 98, 525–530. [Google Scholar] [CrossRef]
- De Stefano, L.; Rotiroti, L.; Rea, I.; De Tommasi, E.; Rendina, I.; Canciello, M.; Maglio, G.; Palumbo, R. Hybrid polymer-porous silicon photonic crystals for optical sensing. J. App. Phys. 2009, 106, 023109–023114. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Hu, R.; Li, P.; Gao, L.; Heng, L.; Tang, B.Z.; Jiang, L. A visual and organic vapor sensitive photonic crystal sensor consisting of polymer-infiltrated SiO2 inverse opal. Phys. Chem. Chem. Phys. 2015, 17, 9651–9658. [Google Scholar] [CrossRef] [PubMed]
- Estevez, J.O.; Agraval, V. Porous Si photonic crystals. In Handbook of Porous Silicon; Canham, L., Ed.; Springer International Publishing: Cham, Switzerland; pp. 805–814.
- Moretti, L.; Rea, I.; de Stefano, L.; Rendina, I. Periodic versus aperiodic: Enhancing the sensitivity of porous silicon based optical sensors. Appl. Phys. Lett. 2007, 90, 191112–191115. [Google Scholar] [CrossRef]
- Casanova, F.; Chiang, C.E.; Ruminski, A.M.; Sailor, M.J.; Schuller, I.K. Controlling the Role of Nanopore Morphology in Capillary Condensation. Langmuir 2012, 28, 6832–6838. [Google Scholar] [CrossRef] [PubMed]
- Escorcia-Garcia, J.; Gaggero-Sager, L.M.; Palestino-Escobedo, A.G.; Agarwal, V. Optical properties of Cantor nanostructures made from porous silicon: A sensing application. Photonics Nanostruct. Fundam. Appl. 2012, 10, 452–458. [Google Scholar] [CrossRef]
- Ghulinyan, M.; Gaburro, Z.; Wiersma, D.S.; Pavesi, L. Tuning of resonant Zener tunneling by vapor diffusion and condensation in porous optical superlattices. Phys. Rev. B 2006, 74, 045118–045123. [Google Scholar] [CrossRef]
- Ghulinyan, M.; Oton, C.J.; Gaburro, Z.; Pavesi, L.; Toninelli, C.; Wiersma, D.S. Zener tunneling of light waves in an optical superlattice. Phys. Rev. Lett. 2005, 94, 127401–127405. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Fauchet, P.M. Two-dimensional silicon photonic crystal based biosensing platform for protein detection. Opt. Express 2007, 15, 4530–4535. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Fauchet, P.M. Biosensing using porous silicon photonic bandgap structures. In Photonic Crystals and Photonic Crystal Fibers for Sensing Applications; Du, H.H., Ed.; SPIE: Bellingham, WA, USA, 2005; pp. 1–15. [Google Scholar]
- Wehrspohn, R.B.; Schweizer, S.L.; Sandoghdar, V. Linear and non-linear experiments based on macroporous silicon photonic crystals. In Nanophotonic Materials: Photonic crystals, Plasmonics and Metamaterials; Wiley-VCH: Weinheim, Germany, 2008; pp. 157–182. [Google Scholar]
- Pergande, D.; Geppert, T.M.; von Rhein, A.; Schweizer, S.L.; Ralf, B.; Wehrspohn, R.B.; Moretton, S.; Lambrecht, A. Miniature infrared gas sensor using photonic crystals. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Korotchenkov, G. Photonic crystals. In Handbook of Gas Sensor Materials: Properties, Advantages and Shortcomings; Springer Science & Business Media: Berlin, Germany, 2014; pp. 111–120. [Google Scholar]
- Descrovi, E.; Frascella, F.; Sciacca, B.; Geobaldo, F.; Dominici, L.; Michelotti, F. Coupling of surface waves in highly defined one-dimensional porous silicon photonic crystals for gas sensing applications. Appl. Phys. Lett. 2007, 91, 24109–24113. [Google Scholar] [CrossRef]
- Michelotti, F.; Sciacca, B.; Dominici, L.; Quaglio, M.; Descrovi, E.; Giorgis, F.; Geobaldo, F. Fast optical vapour sensing by Bloch surface waves on porous silicon membranes. Phys. Chem. Chem. Phys. 2010, 12, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Guillermain, E.; Lysenko, V.; Orobtchouk, R.; Benyattoub, T.; Roux, S.; Pillonnet, A.; Perriat, P. Bragg surface wave device based on porous silicon and its application for sensing. Appl. Phys. Lett. 2007, 90, 241116–241120. [Google Scholar] [CrossRef]
- Rea, I.; Iodice, M.; Coppola, G.; Rendina, I.; Marino, A.; de Stefano, L. A porous silicon-based Bragg grating waveguide sensor for chemical monitoring. Sens. Actuators B Chem. 2009, 139, 39–43. [Google Scholar] [CrossRef]
- Arrand, H.F.; Benson, T.M.; Loni, A.; Arens-Fischer, R.; Krueger, M.G.; Thoenissen, M.; Lueth, H.; Kershaw, S.; Vorozov, N.N. Solvent detection using porous silicon optical waveguides. J. Lumin. 1999, 80, 119–123. [Google Scholar] [CrossRef]
- Schmedake, T.A.; Cunin, F.; Link, J.R.; Sailor, M.J. Standoff detection of chemicals using porous silicon “smart dust” particles. Adv. Mater. 2002, 14, 1270–1272. [Google Scholar] [CrossRef]
- Sailor, M.J.; Link, J.R. “Smart dust”: Nanostructured devices in a grain of sand. Chem. Commun. 2005, 11, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Seo, D.; Kim, Y.-Y.; Lee, K.-W. Organic vapor detection using a color-difference image technique for distributed Bragg reflector structured porous silicon. Sens. Actuators B Chem. 2010, 147, 775–779. [Google Scholar] [CrossRef]
- Epstein, J.R.; Walt, D.R. Fluorescence-based fiber optic arrays: A universal platform for sensing. Chem. Soc. Rev. 2003, 32, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Lee, K.-W.; Kim, J.-W.; Kim, D.-H. Rugate structured free-standing porous silicon-based fiber-optic sensors for the simultaneous detection of pressure and organic gases. Sens. Actuators B Chem. 2013, 183, 428–433. [Google Scholar] [CrossRef]
- Karacali, T.; Hasar, U.C.; Ozbek, I.Y.; Oral, E.A.; Efeoglu, H. Novel design of porous silicon based sensor for reliable and feasible chemical gas vapor detection. J. Lightwave Tech. 2013, 31, 295–305. [Google Scholar] [CrossRef]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Sitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-Reactive Chemical Sensor Arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef] [PubMed]
- Rock, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Jalkanena, T.; Tuuraa, J.; Mäkiläa, E.; Salonena, J. Electro-optical porous silicon gas sensor with enhanced selectivity. Sens. Actuators B Chem. 2010, 147, 100–104. [Google Scholar] [CrossRef]
- Oton, C.J.; Pancheri, L.; Gaburro, Z.; Pavesi, L.; Baratto, C.; Faglia, G.; Sberveglieri, G. Multiparametric porous silicon gas sensors with improved quality and sensitivity. Phys. Stat. Sol. A 2003, 197, 523–527. [Google Scholar] [CrossRef]
- Ben-Chorin, M.; Kux, A. Adsorbate effects on photoluminescence and electrical conductivity of porous silicon. Appl. Phys. Lett. 1994, 64, 481–483. [Google Scholar] [CrossRef]
- Levitsky, I.A. Unpublished work. 2015.
- Hutter, T.; Horesh, M.; Ruschin, S. Metod for increasing reliability in gas detection based on indicator gradient in a sensor array. Sens. Actuators B Chem. 2011, 152, 29–36. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, H.; Wang, X.; Wu, J. An optical olfactory sensor based on porous silicon infiltrated with room-temperature ionic liquid arrays. Chem. Eur. J. 2011, 17, 13400–13404. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levitsky, I.A. Porous Silicon Structures as Optical Gas Sensors. Sensors 2015, 15, 19968-19991. https://doi.org/10.3390/s150819968
Levitsky IA. Porous Silicon Structures as Optical Gas Sensors. Sensors. 2015; 15(8):19968-19991. https://doi.org/10.3390/s150819968
Chicago/Turabian StyleLevitsky, Igor A. 2015. "Porous Silicon Structures as Optical Gas Sensors" Sensors 15, no. 8: 19968-19991. https://doi.org/10.3390/s150819968
APA StyleLevitsky, I. A. (2015). Porous Silicon Structures as Optical Gas Sensors. Sensors, 15(8), 19968-19991. https://doi.org/10.3390/s150819968




