Insights on Capacitive Interdigitated Electrodes Coated with MOF Thin Films: Humidity and VOCs Sensing as a Case Study
Abstract
:1. Introduction
2. Sensing Element
2.1. Interdigitated Electrodes (IDEs)


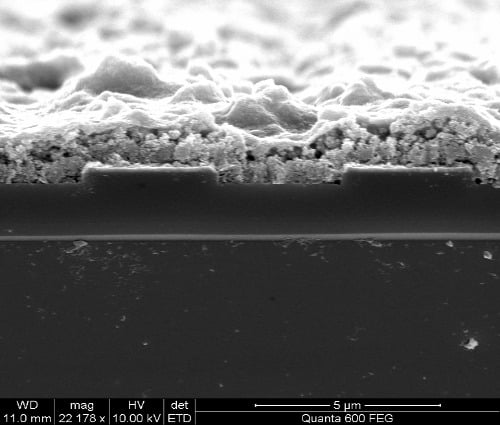
2.2. Cu(bdc)·xH2O MOFs Thin Film
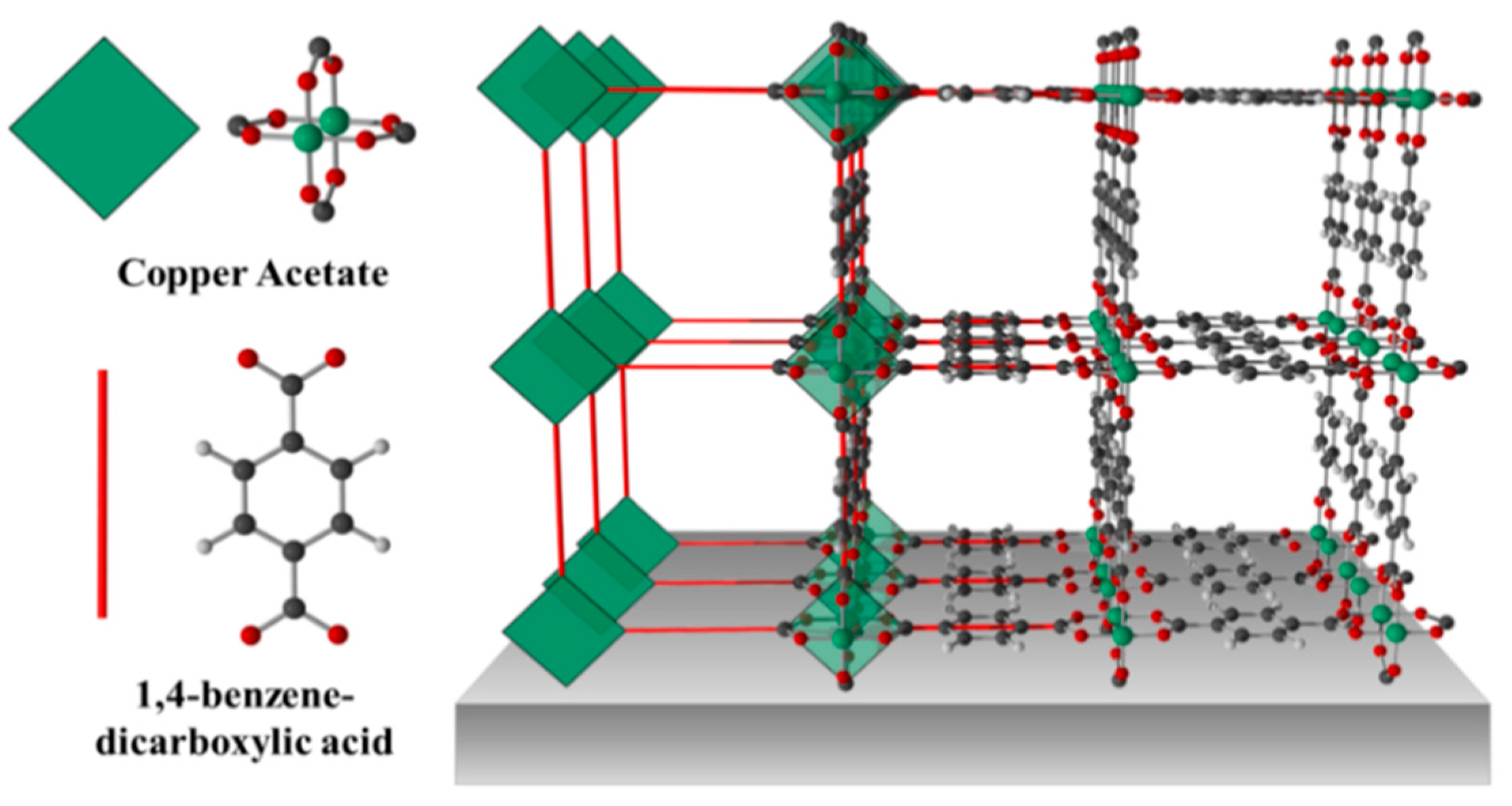
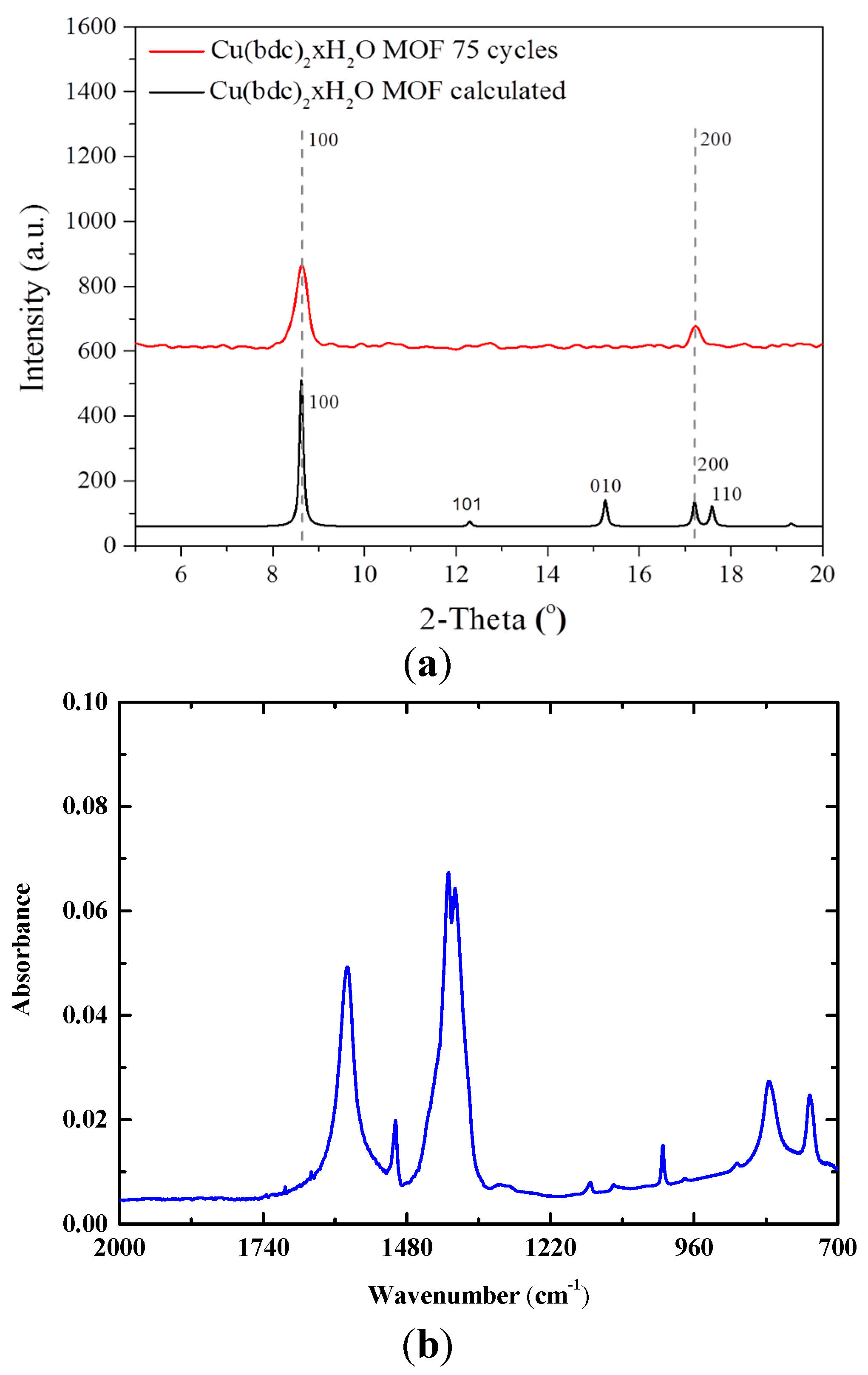
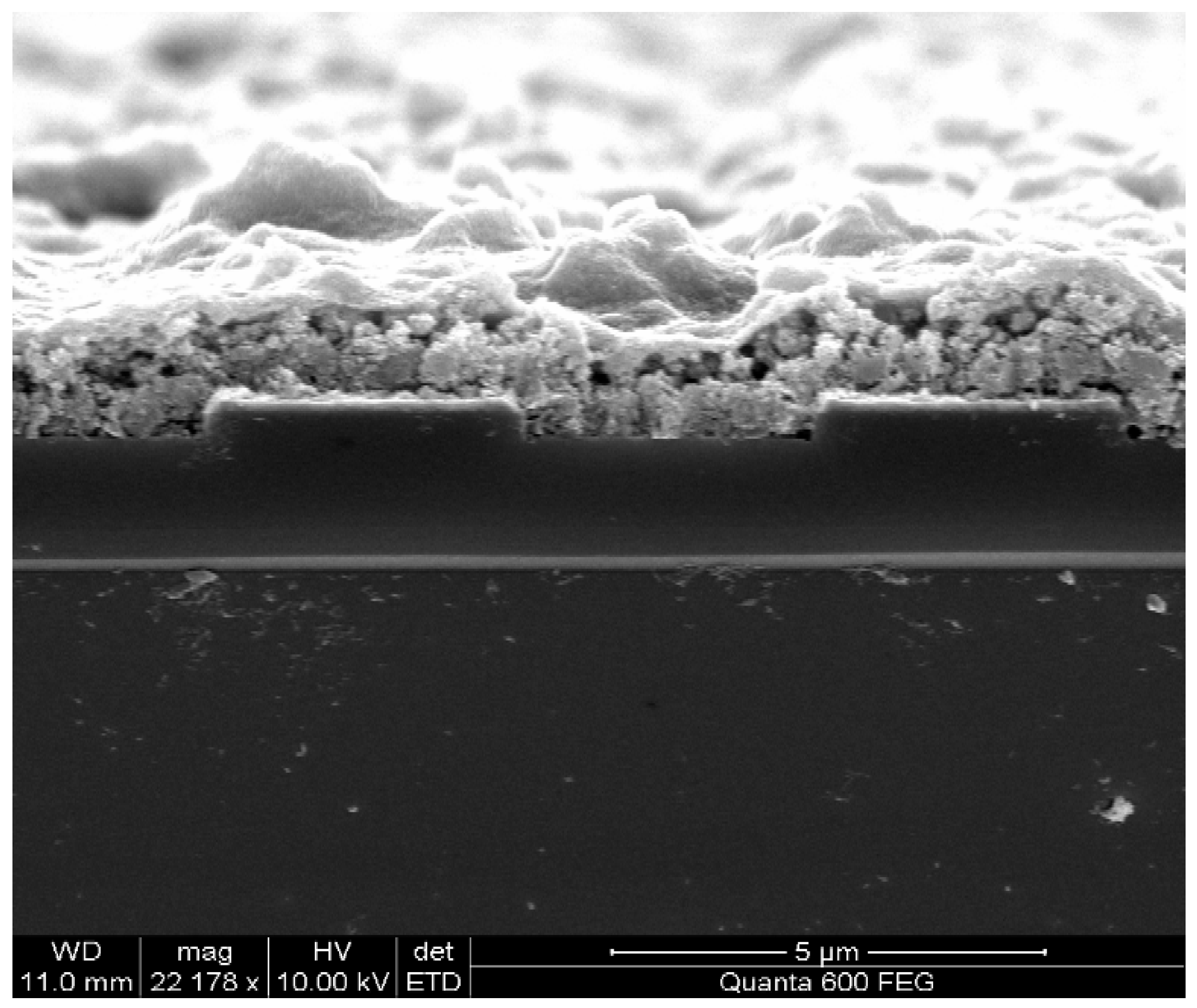
3. Gas Sensing Setup
3.1. Measurement System Implementation
- diluted VOC or toxic gas cylinders,
- dry N2 for diluting other gases/vapors in mixtures,
- carrier gas to generate water vapor, and
- carrier gas to generate VOC vapor.
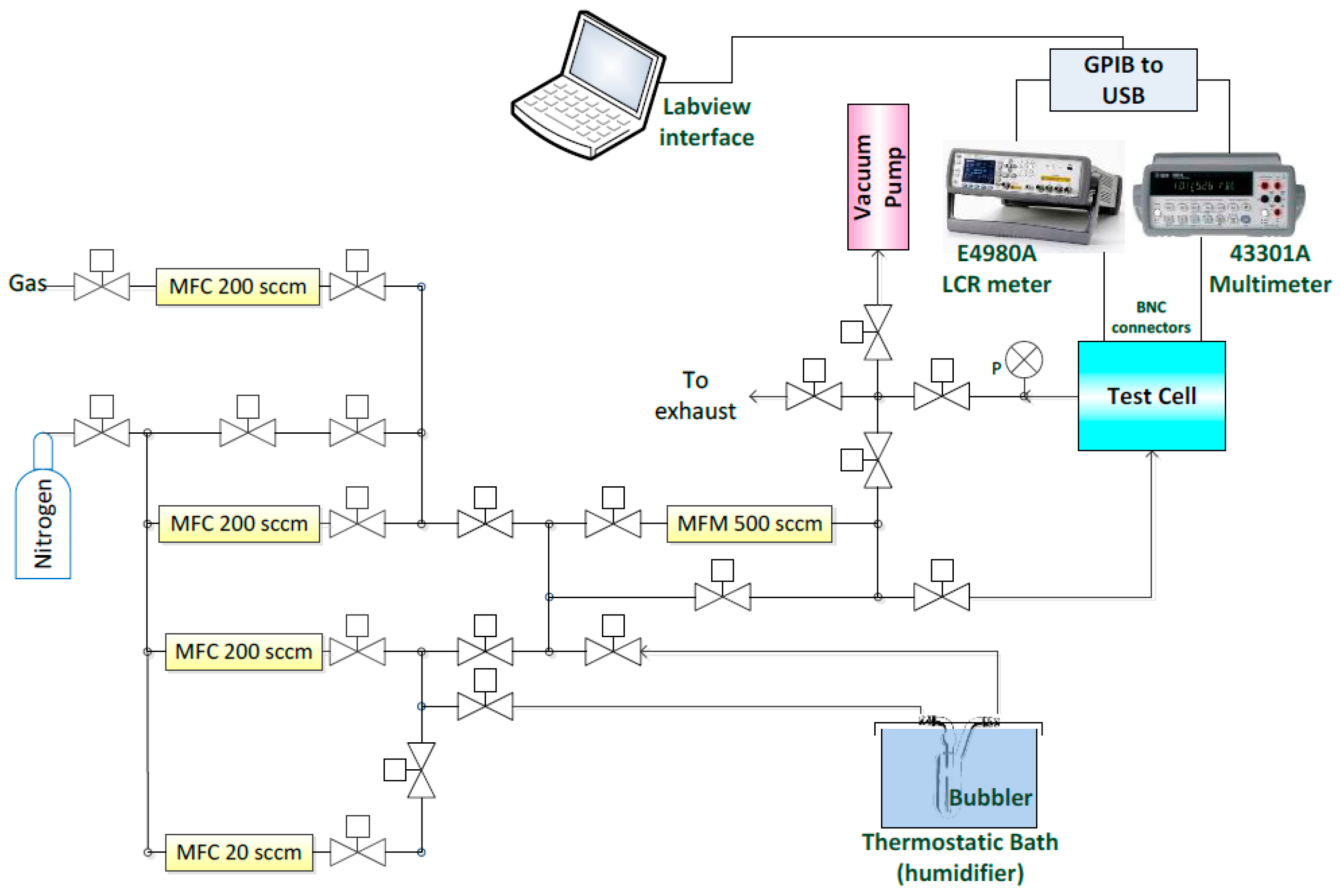
| MFC | Usage | Maximum Value (mL/min) |
|---|---|---|
| A | VOCs carrier | 20 |
| B | H2O carrier | 200 |
| C | Dry N2 carrier | 200 |
| D | Diluted gas cylinder | 200 |
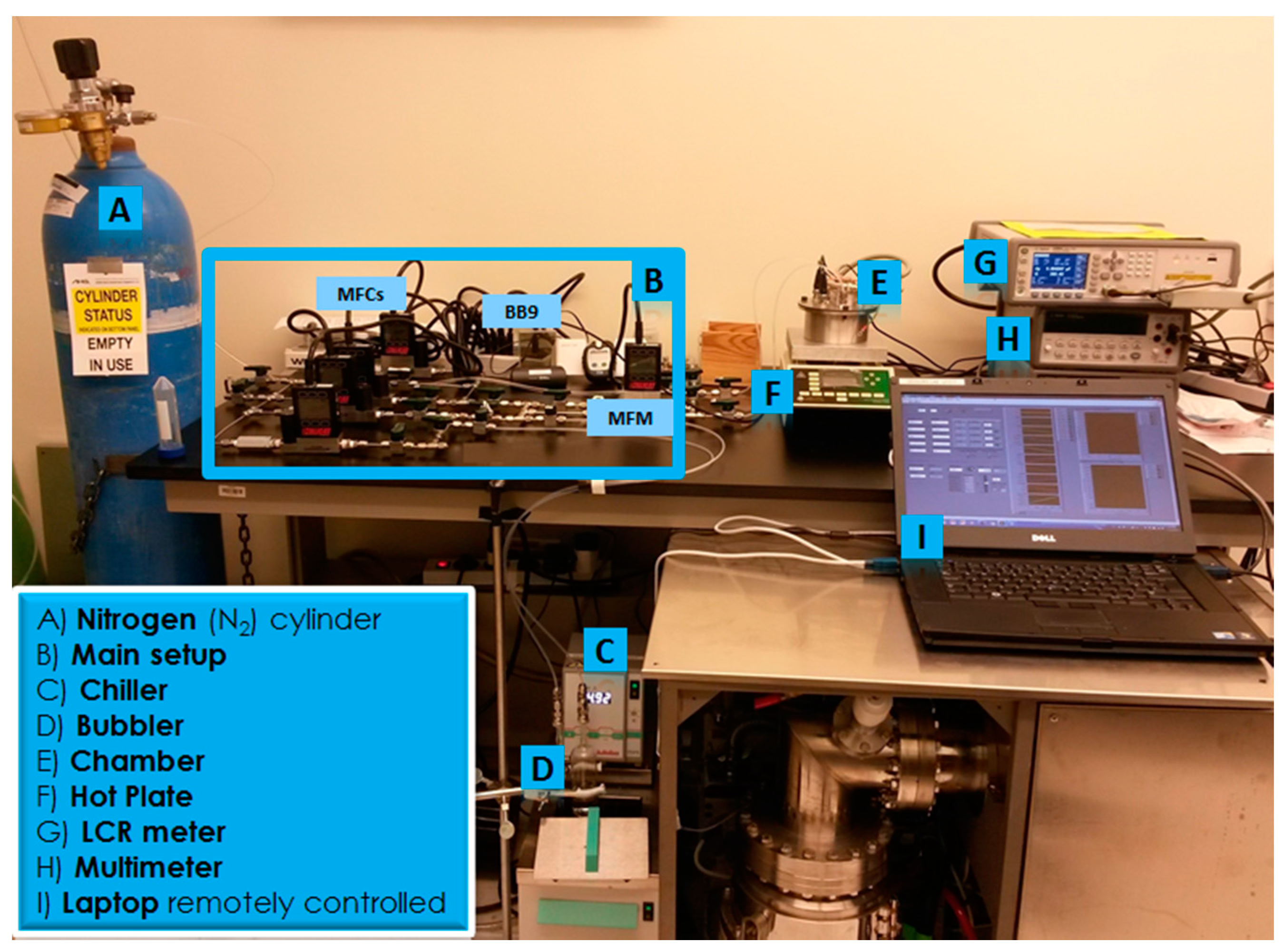
3.2. Experiment Automation
4. Results and Discussion
4.1. Humidity Experiments
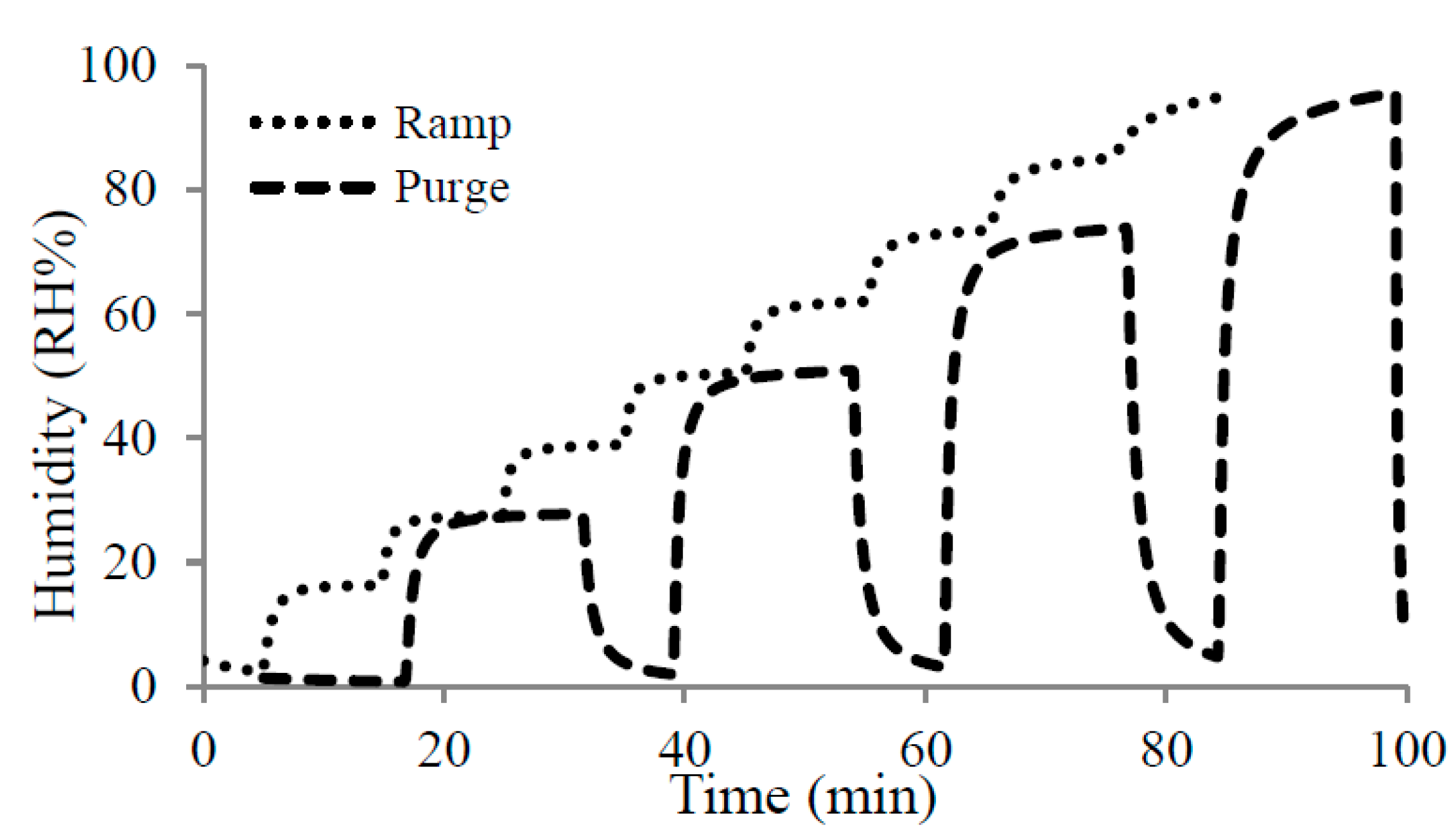
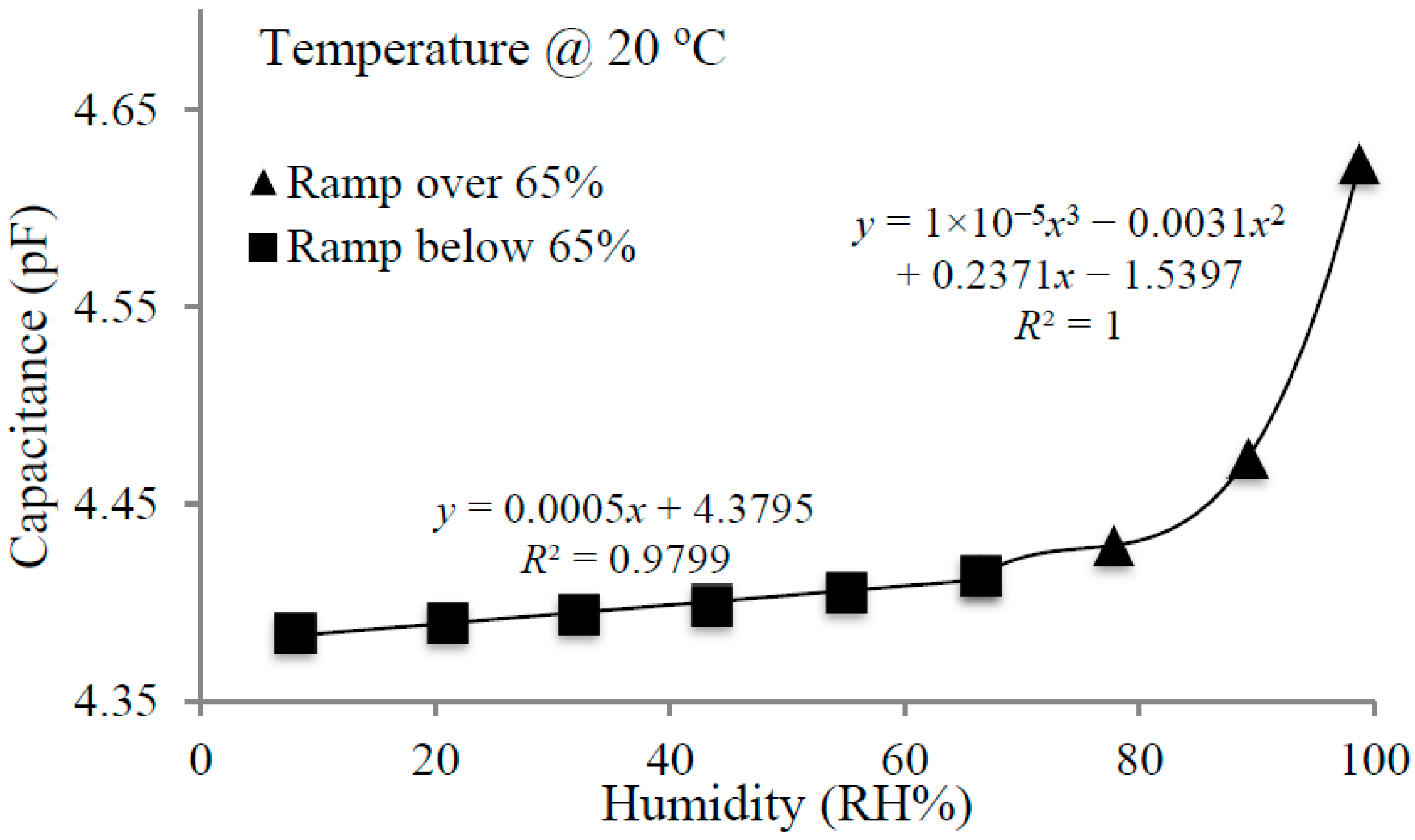

4.2. Volatile Organic Compounds Experiments
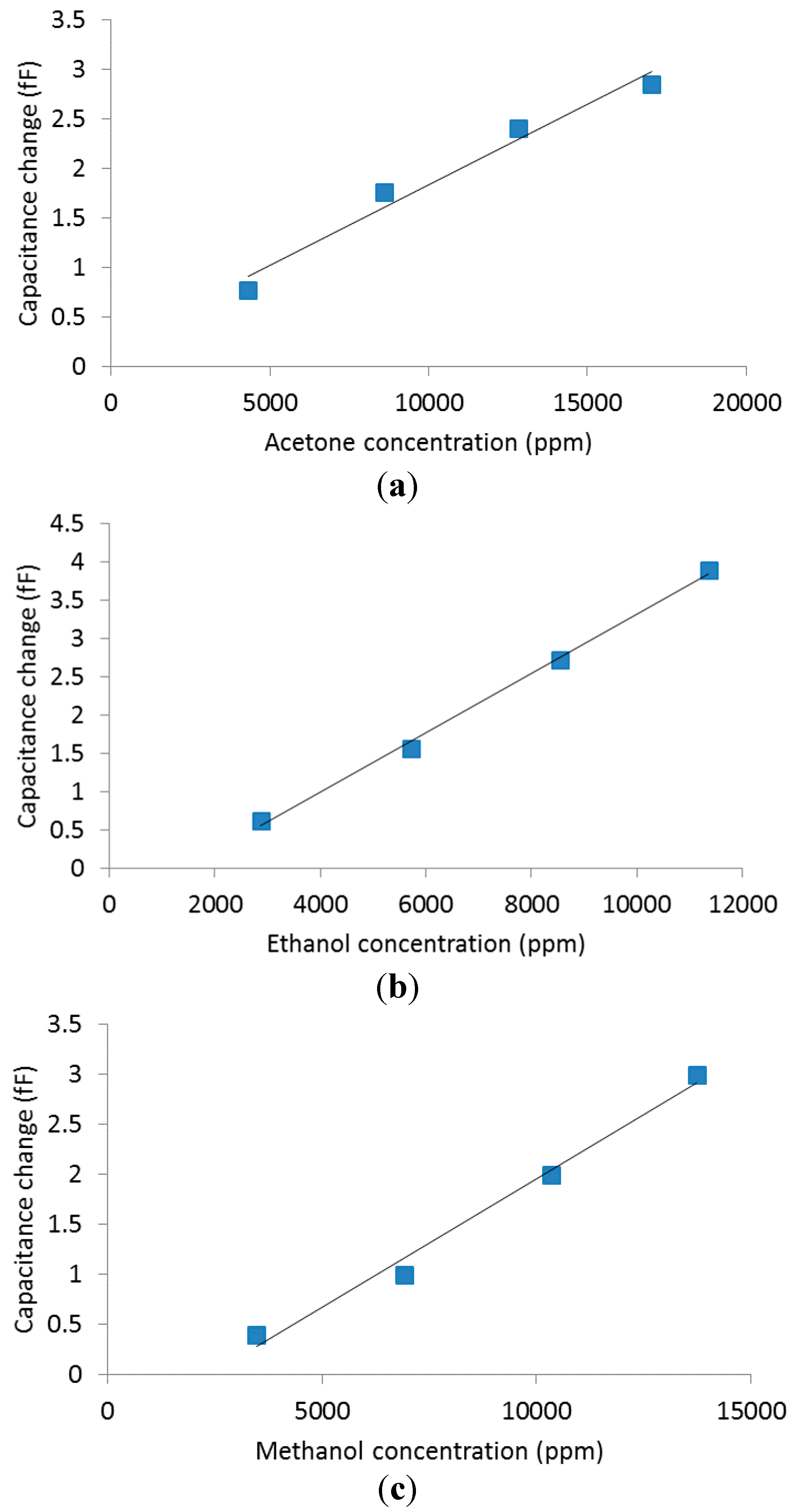
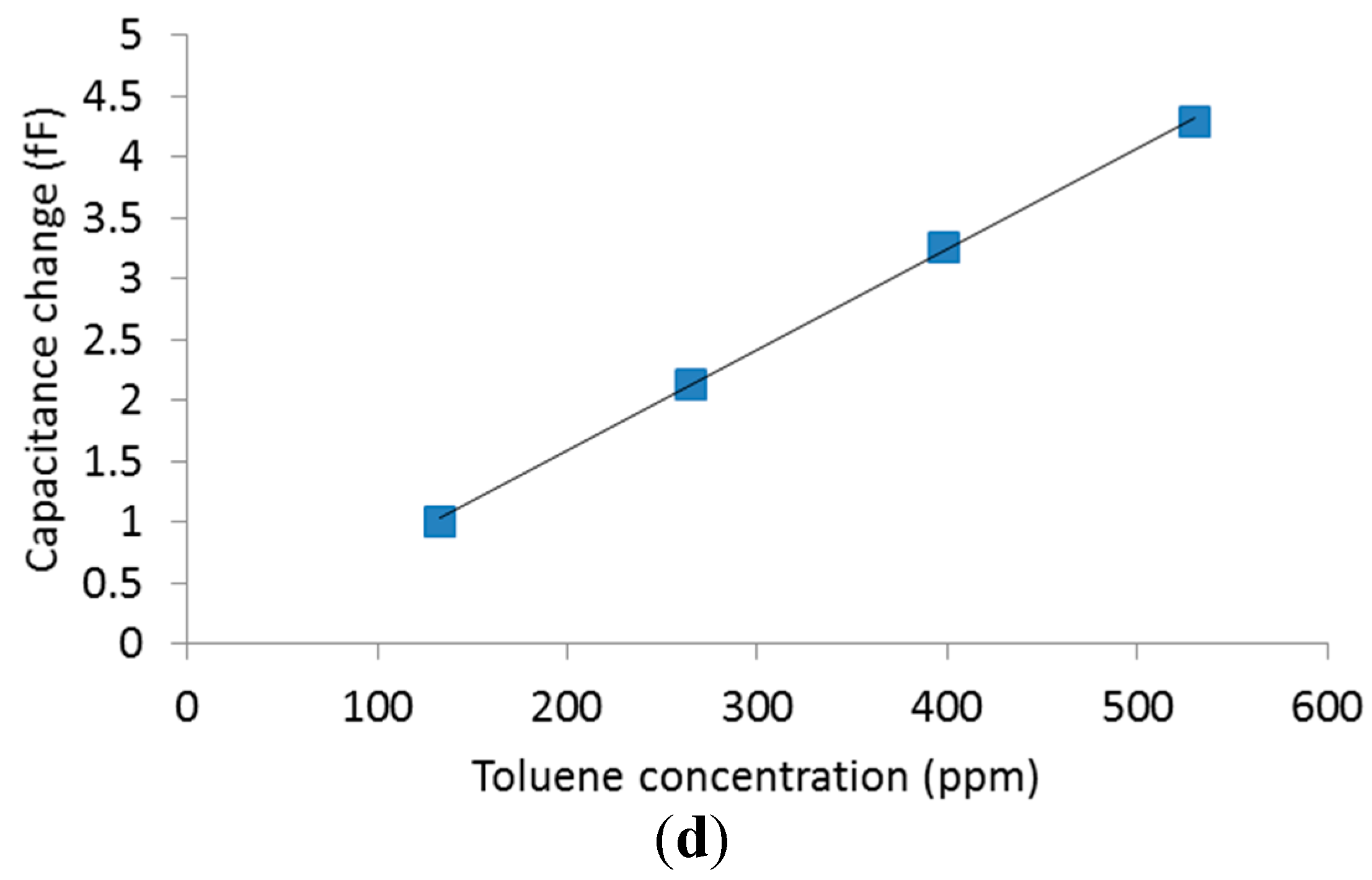
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Escuder-Gilabert, L. A 21st century technique for food control: Electronic noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si: WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.H.; Song, H.S.; Park, T.H. Recent advances in electronic and bioelectronic noses and their biomedical applications. Enzyme Microb. Technol. 2011, 48, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Schwartzberg, A.; Stavila, V.; Talin, A.A. A roadmap to implementing metal–organic frameworks in electronic devices: Challenges and critical directions. Chem. Eur. J. 2011, 17, 11372–11388. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal‐Organic Frameworks: A Rapidly Growing Class of Versatile Nanoporous Materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, A.; Petaccia, L.; Lizzit, S.; Larciprete, R. Sensing gases with carbon nanotubes: A review of the actual situation. J. Phys. Condensed Matter: Inst. Phys. J. 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Potyrailo, R.A.; Surman, C.; Nagraj, N.; Burns, A. Materials and transducers toward selective wireless gas sensing. Chem. Rev. 2011, 111, 7315–7354. [Google Scholar] [CrossRef] [PubMed]
- Ratinac, K.R.; Yang, W.; Ringer, S.P.; Braet, F. Toward Ubiquitous Environmental Gas Sensors—Capitalizing on the Promise of Graphene. Environ. Sci. Technol. 2010, 44, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Khoshaman, A.H.; Bahreyni, B. Application of metal organic framework crystals for sensing of volatile organic gases. Sens. Actuators B Chem. 2012, 162, 114–119. [Google Scholar] [CrossRef]
- Davydovskaya, P.; Pohle, R.; Tawil, A.; Fleischer, M. Work function based gas sensing with Cu-BTC metal-organic framework for selective aldehyde detection. Sens. Actuators B Chem. 2013, 187, 142–146. [Google Scholar] [CrossRef]
- Wan, X.; Song, H.; Zhao, D.; Zhang, L.; Lv, Y. A Y-doped metal-organic framework-based cataluminescence gas sensor for isobutanol. Sens. Actuators B Chem. 2014, 201, 413–419. [Google Scholar] [CrossRef]
- Achmann, S.; Hagen, G.; Kita, J.; Malkowsky, I.M.; Kiener, C.; Moos, R. Metal-organic frameworks for sensing applications in the gas phase. Sensors 2009, 9, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.K.; Shekhah, O.; Wieland, D.F.; Paulus, M.; Sternemann, C.; Schroer, M.A.; Tiemeyer, S.; Tolan, M.; Fischer, A.R.; Wöll, C. Intercalation in Layered Metal–Organic Frameworks: Reversible Inclusion of an Extended π-System. J. Am. Chem. Soc. 2011, 133, 8158–8161. [Google Scholar] [CrossRef] [PubMed]
- Sancet, M.P.A.; Hanke, M.; Wang, Z.; Bauer, S.; Azucena, C.; Arslan, H.K.; Heinle, M.; Gliemann, H.; Wöll, C.; Rosenhahn, A. Surface anchored metal-organic frameworks as stimulus responsive antifouling coatings. Biointerphases 2013, 8. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ha, S.C.; Yang, H.; Kim, Y.T. Gas sensor measurement system capable of sampling volatile organic compounds (VOCs) in wide concentration range. Sens. Actuators B Chem. 2007, 122, 211–218. [Google Scholar] [CrossRef]
- Araki, H.; Omatu, S. Measurement system for metal-oxide gas sensors. Artif. Life Robot. 2013, 17, 357–361. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Gruber, V.; Bahn, M. The ‘Gas-Snake’: Design and validation of a versatile membrane-based gas flux measurement system in a grassland soil respiration study. Agric. For. Meteorol. 2012, 154, 166–173. [Google Scholar] [CrossRef]
- Pignanelli, E.; Kuhn, K.; Schutze, A. Versatile gas measurement system based on combined NDIR transmission and photoacoustic spectroscopy. In Proceedings of the IEEE Sensors Conference, University of Limerick, Limerick, Ireland, 28–31 October 2011.
- Shibata, H.; Ito, M.; Asakursa, M.; Watanabe, K. A digital hygrometer using a polyimide film relative humidity sensor. Trans. Instrum. Meas. 1996, 45, 564–569. [Google Scholar] [CrossRef]
- Shekhah, O.; Roques, N.; Mugnaini, V.; Munuera, C.; Ocal, C.; Veciana, J.; Woll, C. Grafting of monocarboxylic substituted polychlorotriphenylmethyl radicals onto a COOH-functionalized self-assembled monolayer through copper (II) metal ions. Langmuir 2008, 24, 6640–6648. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.E.; Franzreb, M.; Weidler, P.G.; Shekhah, O.; Wöll, C. Magnetic Cores with Porous Coatings: Growth of Metal-Organic Frameworks on Particles Using Liquid Phase Epitaxy. Adv. Funct. Mater. 2013, 23, 1210–1213. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; Bashir, A.; Shekhah, O.; Alawady, A.R.E.; Birkner, A.; Grunwald, C.; Wöll, C. Fabrication of a surface plasmon resonance biosensor based on gold nanoparticles chemisorbed onto a 1, 10-decanedithiol self-assembled monolayer. Thin Solid Films 2009, 518, 387–391. [Google Scholar] [CrossRef]
- Hersee, S.D.; Ballingall, J.M. The operation of metalorganic bubblers at reduced pressure. J. Vac. Sci. Technol. A 1990, 8, 800–804. [Google Scholar] [CrossRef]
- De Voorde, B.V.; Bueken, B.; Denayer, J.; de Vos, D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5778. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapsanis, C.; Omran, H.; Chernikova, V.; Shekhah, O.; Belmabkhout, Y.; Buttner, U.; Eddaoudi, M.; Salama, K.N. Insights on Capacitive Interdigitated Electrodes Coated with MOF Thin Films: Humidity and VOCs Sensing as a Case Study. Sensors 2015, 15, 18153-18166. https://doi.org/10.3390/s150818153
Sapsanis C, Omran H, Chernikova V, Shekhah O, Belmabkhout Y, Buttner U, Eddaoudi M, Salama KN. Insights on Capacitive Interdigitated Electrodes Coated with MOF Thin Films: Humidity and VOCs Sensing as a Case Study. Sensors. 2015; 15(8):18153-18166. https://doi.org/10.3390/s150818153
Chicago/Turabian StyleSapsanis, Christos, Hesham Omran, Valeriya Chernikova, Osama Shekhah, Youssef Belmabkhout, Ulrich Buttner, Mohamed Eddaoudi, and Khaled N. Salama. 2015. "Insights on Capacitive Interdigitated Electrodes Coated with MOF Thin Films: Humidity and VOCs Sensing as a Case Study" Sensors 15, no. 8: 18153-18166. https://doi.org/10.3390/s150818153
APA StyleSapsanis, C., Omran, H., Chernikova, V., Shekhah, O., Belmabkhout, Y., Buttner, U., Eddaoudi, M., & Salama, K. N. (2015). Insights on Capacitive Interdigitated Electrodes Coated with MOF Thin Films: Humidity and VOCs Sensing as a Case Study. Sensors, 15(8), 18153-18166. https://doi.org/10.3390/s150818153