Application of Visible and Near-Infrared Hyperspectral Imaging to Determine Soluble Protein Content in Oilseed Rape Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Chemical Analysis of Soluble Protein Content
2.3. Hyperspectral Imaging System and Image Acquisition

2.4. Spectral Data Extraction
2.5. Image Visualization and Distribution Map
2.6. Multivariate Data Analysis
2.7. Sensitive Wavelengths Selection
2.7.1. Weighted Regression Coefficients
2.7.2. Genetic Algorithm-Partial Least Squares
2.7.3. Successive Projections Algorithm
2.8. Model Evaluation
3. Results and Discussion
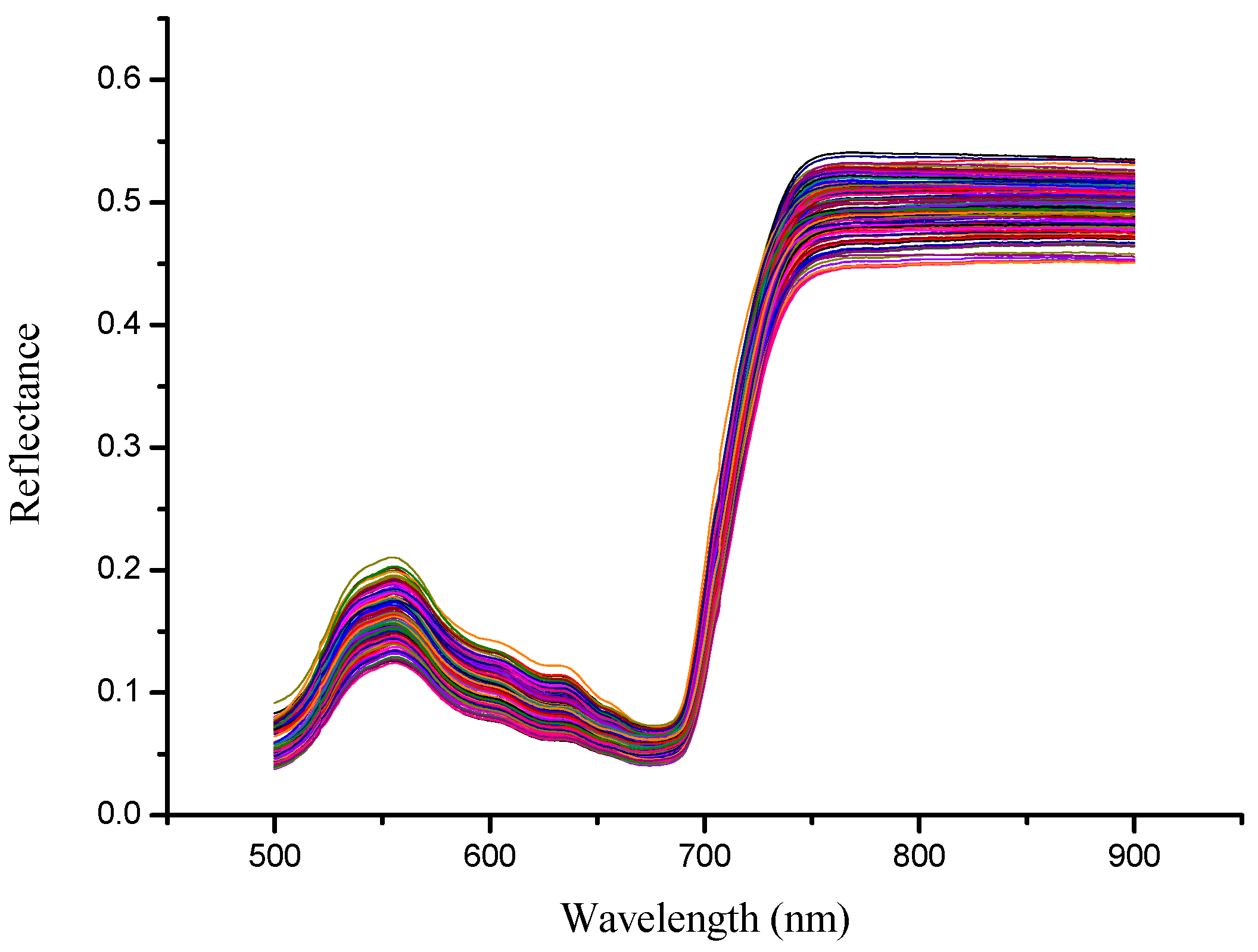
3.1. Spectral Feature of Rape Leaves
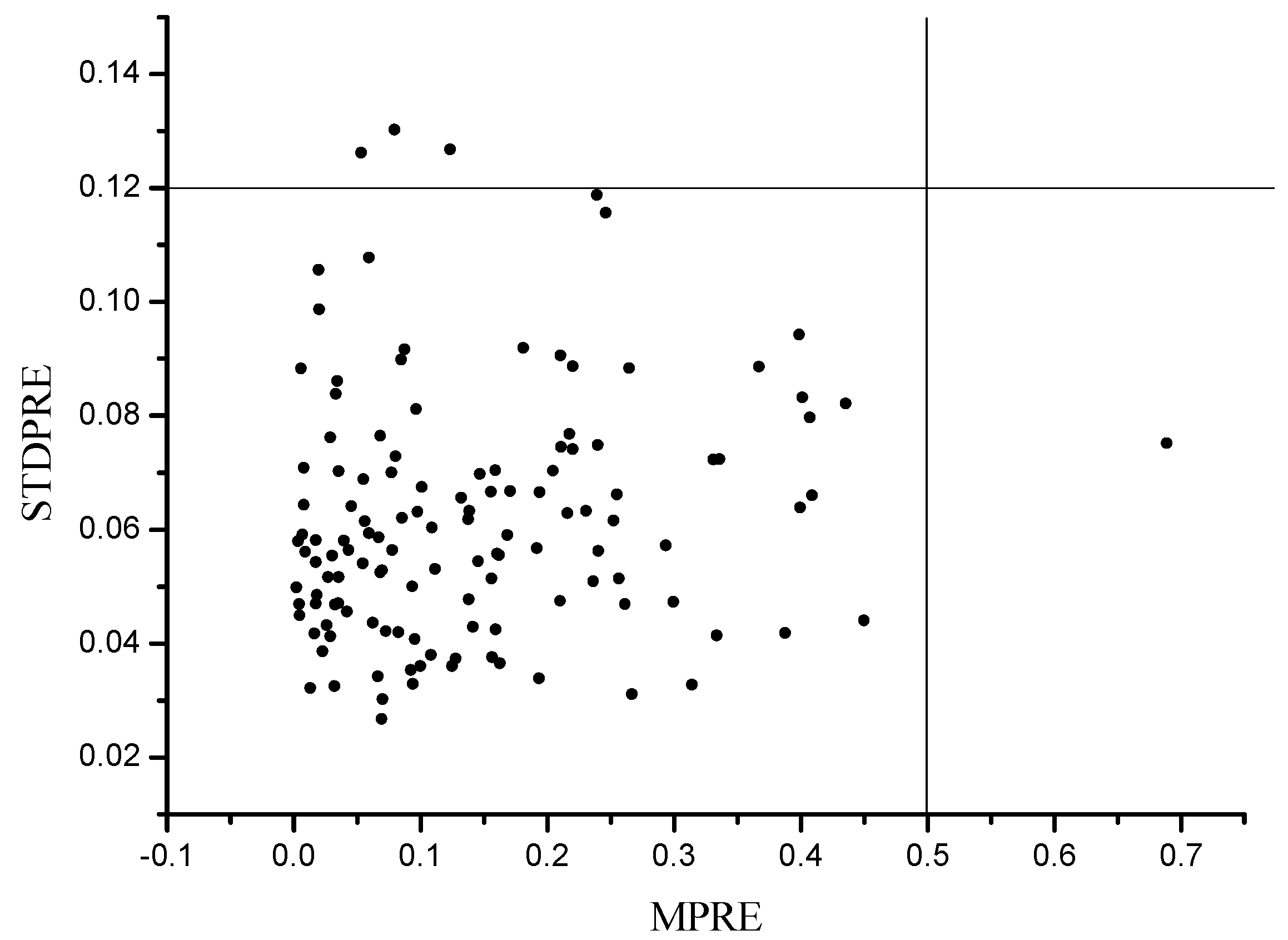
3.2. Outlier Detection
3.3. Statistics of Measured Samples
| Sample Sets | Number | Range (mg/g) | Mean (mg/g) | SD (mg/g) |
|---|---|---|---|---|
| Calibration | 92 | 1.3842–2.9966 | 2.1811 | 0.4944 |
| Prediction | 32 | 1.4555–2.9950 | 2.2262 | 0.4985 |
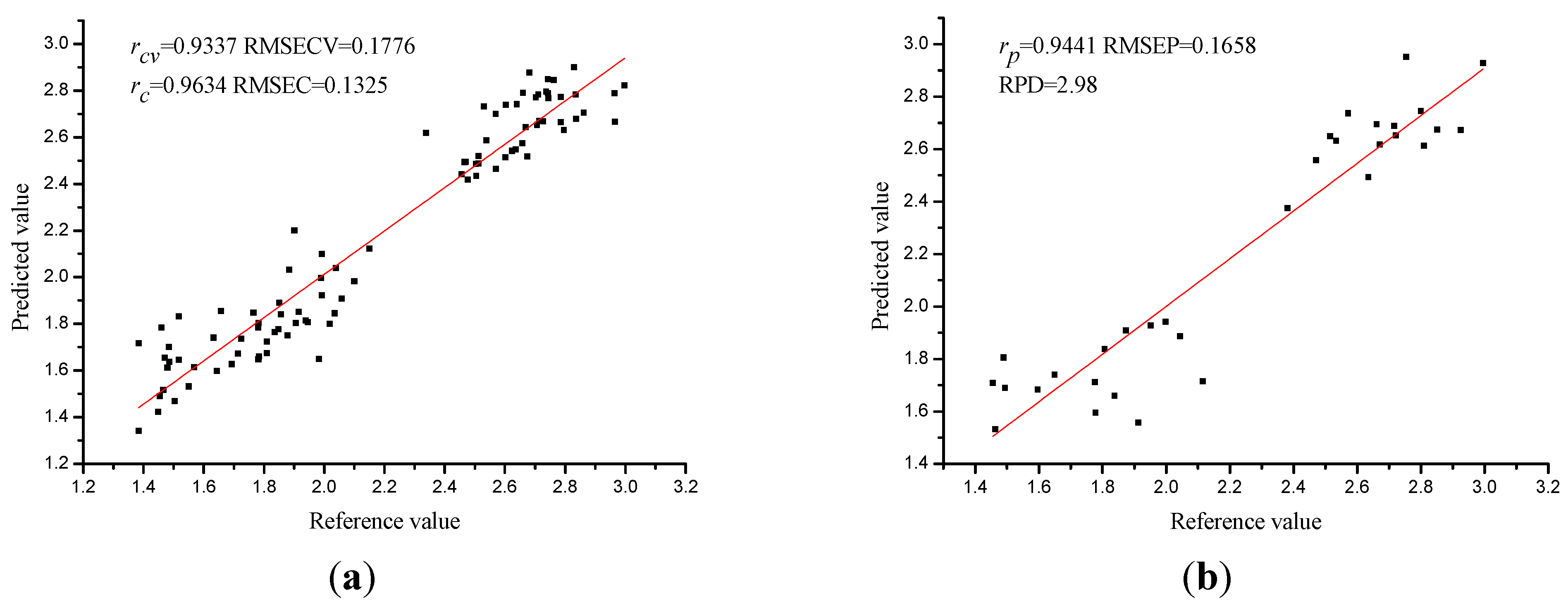
3.4. PLS Model Using Full Spectra
3.5. Sensitive Wavelengths Selection
| Methods | Number | Sensitive Wavelengths (nm) |
|---|---|---|
| Bw | 18 | 501, 508, 542, 707, 720, 739, 761, 769, 789, 809, 852, 859, 865, 871, 880, 892, 897, 899 |
| SPA | 15 | 892, 543, 897, 618, 782, 554, 701, 635, 746, 505, 852, 712, 677, 512, 684 |
| GAPLS | 16 | 788, 789, 809, 636, 638, 778, 807, 639, 635, 738, 791, 810, 866, 679, 741, 777 |
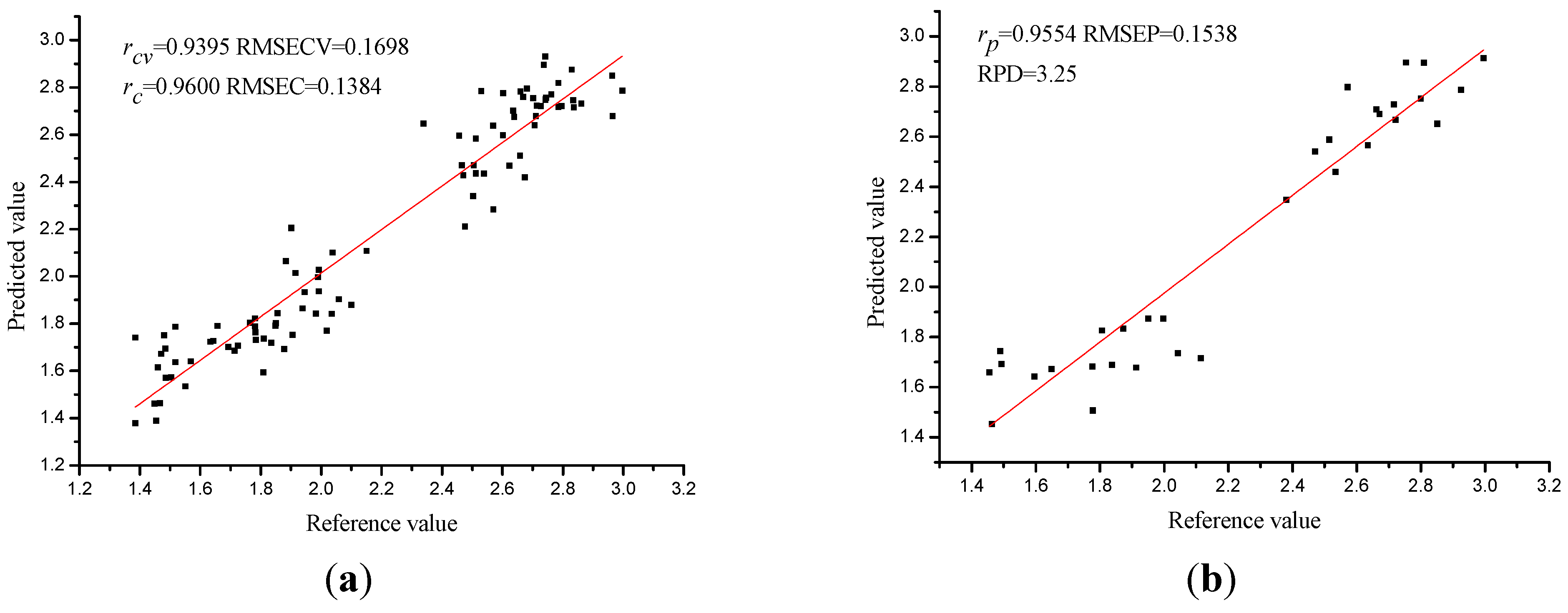
3.6. PLS Models on Selected Wavelengths
| Models | LVs | rcv | RMSECV | rc | RMSEC | rp | RMSEP | RPD |
|---|---|---|---|---|---|---|---|---|
| Bw-PLS | 8 | 0.9095 | 0.2058 | 0.9303 | 0.1813 | 0.9058 | 0.2142 | 2.30 |
| SPA-PLS | 12 | 0.9395 | 0.1698 | 0.9600 | 0.1384 | 0.9554 | 0.1538 | 3.25 |
| GAPLS-PLS | 8 | 0.9288 | 0.1837 | 0.9494 | 0.1553 | 0.9223 | 0.1927 | 2.55 |
3.7. Visualization of Soluble Protein Content Distribution
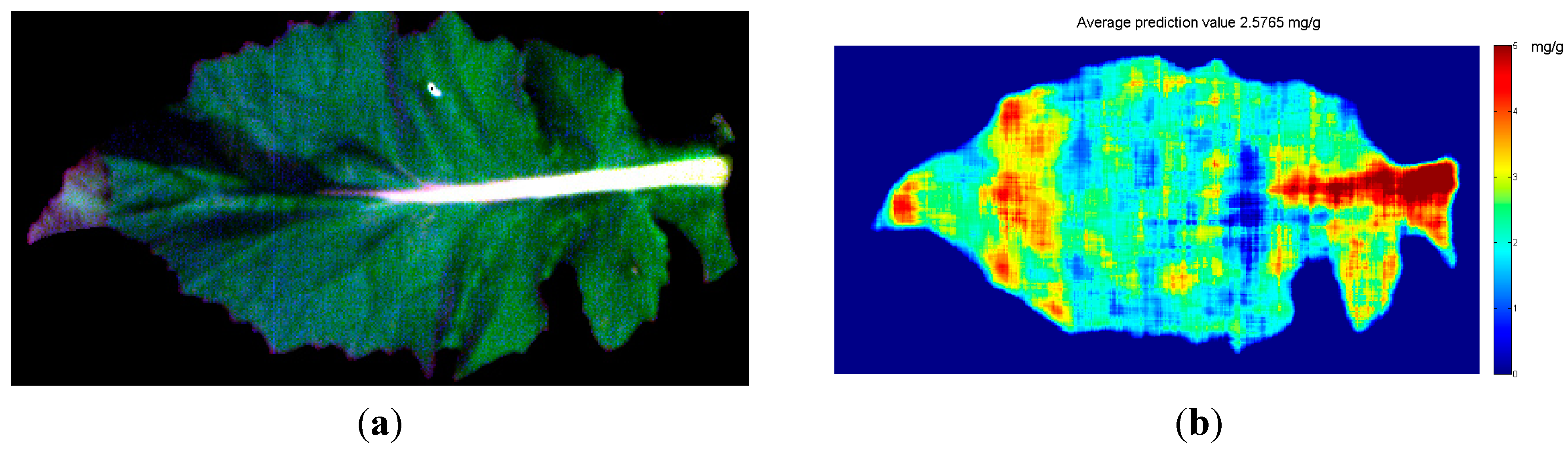
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, N.Q.; Wang, M.H.; Wang, N. Precision agriculture—A worldwide overview. Comput. Electron. Agric. 2002, 36, 113–132. [Google Scholar] [CrossRef]
- Taulavuori, E.; Tahkokorpi, M.; Laine, K.; Taulavuori, K. Drought tolerance of juvenile and mature leaves of a deciduous dwarf shrub Vaccinium myrtillus L. in a boreal environment. Protoplasma 2010, 241, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhatla, S.C. Polypeptide markers for low temperature stress during seed germination in sunflower. Plantarum 2006, 50, 81–86. [Google Scholar] [CrossRef]
- Patel, S.J.; Subramanian, R.B.; Jha, Y.S. Biochemical and molecular studies of early blight disease in tomato. Phytoparasitica 2011, 39, 269–283. [Google Scholar] [CrossRef]
- Gulen, H.; Turhan, E.; Eris, A. Changes in peroxidase activities and soluble proteins in strawberry varieties under salt-stress. Acta Physiol. Plant. 2006, 28, 109–116. [Google Scholar] [CrossRef]
- Zheng, W.S.; Fei, Y.H.; Huang, Y. Soluble protein and acid phosphatase exuded by ectomycorrhizal fungi and seedlings in response to excessive Cu and Cd. J. Environ. Sci. 2009, 21, 1667–1672. [Google Scholar] [CrossRef]
- Zeng, J.M.; Zhang, L.; Li, Y.Q.; Wang, Y.; Wang, M.C.; Duan, X.; He, Z.G. Over-producing soluble protein complex and validating protein-protein interaction through a new bacterial co-expression system. Protein Expres. Purif. 2010, 69, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Overton, T.R.; Clark, J.H. Effects of Yucca shidigera Extract and Soluble Protein on Performance of Cows and Concentrations of Urea Nitrogen in Plasma and Milk. J. Dairy Sci. 1998, 81, 1022–1027. [Google Scholar] [CrossRef]
- Ledoux, M.; Lamy, F. Determination of proteins and sulfobetaine with the Folin-phenol reagent. Anal. Biochem. 1986, 157, 28–31. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hoff, J.E. A simple method for the approximate determination of soluble protein in potato tubers. Potato Res. 1975, 18, 428–432. [Google Scholar] [CrossRef]
- González-Lara, R.; González, L.M. Analysis of the soluble proteins in grape musts by reversed-phase HPLC. Chromatographia 1991, 32, 463–465. [Google Scholar] [CrossRef]
- Ratcliffe, M.; Panozzo, J.F. The Application of Near Infrared Spectroscopy to Evaluate Malting Quality. J. I. Brewing. 1999, 105, 85–88. [Google Scholar] [CrossRef]
- Lu, C.; Han, D. The component analysis of bottled red sufu products using near infrared spectroscopy. J. Near Infrared Spec. 2005, 13, 139–145. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, F.; Jin, Z.; He, Y.; Fang, H.; Ye, Q.; Zhou, W. Determination of acetolactate synthase activity and protein content of oilseed rape (Brassica napus L.) leaves using visible/near-infrared spectroscopy. Anal. Chim. Acta 2008, 629, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Nie, P.C.; Huang, M.; Kong, W.W.; He, Y. Nondestructive determination of nutritional information in oilseed rape leaves using visible/near infrared spectroscopy and multivariate calibrations. Sci. China Inform. Sci. 2011, 54, 598–608. [Google Scholar] [CrossRef]
- Guo, W.L.; Du, Y.P.; Zhou, Y.C.; Yang, S.; Lu, J.H. At-line monitoring of key parameters of nisin fermentation by near infrared spectroscopy, chemometric modeling and model improvement. World J. Microbiol. Biotechnol. 2012, 28, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; EImasry, G.; Sun, D.W.; Allen, P. Prediction of some quality attributes of lamb meat using near-infrared hyperspectral imaging and multivariate analysis. Anal. Chim. Acta 2012, 714, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, Y. Use of visible and near infrared spectroscopy and least squares-support vector machine to determine soluble solids content and pH of cola beverage. J. Agric. Food Chem. 2007, 55, 8883–8888. [Google Scholar] [CrossRef] [PubMed]
- Osborne, S.D.; Kunnemeyer, R.; Jordan, R.B. Methods of wavelength selection for partial least squares. Analyst 1997, 122, 1531–1537. [Google Scholar] [CrossRef]
- EIMasry, G.; Sun, D.W.; Allen, P. Near-infrared hyperspectral imaging for predicting colour, pH and tenderness of fresh beef. J. Food Eng. 2012, 110, 127–140. [Google Scholar] [CrossRef]
- Leardi, R. Application of genetic algorithm–PLS for feature selection in spectral data sets. J. Chemometr. 2000, 14, 643–655. [Google Scholar] [CrossRef]
- Leardi, R.; Gonzalez, A.L. Genetic algorithms applied to feature selection in PLS regression: How and when to use them. Chemometr. Intell. Lab. 1998, 41, 195–207. [Google Scholar] [CrossRef]
- Liu, F.; He, Y. Application of successive projections algorithm for variable selection to determine organic acids of plum vinegar. Food Chem. 2009, 115, 1430–1436. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, Y.; He, Y. Variable selection in visible/near infrared spectra for linear and nonlinear calibrations: A case study to determine soluble solids content of beer. Anal. Chim. Acta 2009, 635, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gaston, E.; Frias, J.M.; Cullen, P.J.; O’Donnell, C.P.; Gowen, A.A. Prediction of polyphenol oxidase activity using visible near-infrared hyperspectral imaging on mushroom (Agaricus bisporus) caps. J. Agric. Food Chem. 2010, 58, 6226–6233. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liu, F.; Kong, W.; He, Y. Application of Visible and Near-Infrared Hyperspectral Imaging to Determine Soluble Protein Content in Oilseed Rape Leaves. Sensors 2015, 15, 16576-16588. https://doi.org/10.3390/s150716576
Zhang C, Liu F, Kong W, He Y. Application of Visible and Near-Infrared Hyperspectral Imaging to Determine Soluble Protein Content in Oilseed Rape Leaves. Sensors. 2015; 15(7):16576-16588. https://doi.org/10.3390/s150716576
Chicago/Turabian StyleZhang, Chu, Fei Liu, Wenwen Kong, and Yong He. 2015. "Application of Visible and Near-Infrared Hyperspectral Imaging to Determine Soluble Protein Content in Oilseed Rape Leaves" Sensors 15, no. 7: 16576-16588. https://doi.org/10.3390/s150716576
APA StyleZhang, C., Liu, F., Kong, W., & He, Y. (2015). Application of Visible and Near-Infrared Hyperspectral Imaging to Determine Soluble Protein Content in Oilseed Rape Leaves. Sensors, 15(7), 16576-16588. https://doi.org/10.3390/s150716576









