A Simple Method for Decreasing the Liquid Junction Potential in a Flow-through-Type Differential pH Sensor Probe Consisting of pH-FETs by Exerting Spatiotemporal Control of the Liquid Junction
Abstract
:1. Introduction
2. Experimental Section
2.1. Sensor Probe and Automated pH Measurement System

2.2. Vibration and Mixing at the Liquid Junction during Measurement and Evaluation of Their Effect
| Solution # | [KCI] (mM) | [Na2HPO4] (mM) | [KH2PO4] (mM) | Buffer Capacity (mM/pH) | pH after Air Bubbling |
|---|---|---|---|---|---|
| #1 | 0 | 0.061 | 0.017 | 0.064 | 6.625 |
| #2 | 12.5 | 0.061 | 0.017 | 0.058 | 6.625 |
| #3 | 50.0 | 0.061 | 0.017 | 0.065 | 6.625 |
| #4 | 200.0 | 0.061 | 0.017 | 0.059 | 6.549 |
2.3. Solutions, Buffer Capacities, and Authentic pH Values
2.4. Verification of the Mechanism Producing the Liquid Junction Potential
3. Results and Discussion
3.1. A newly Developed Method for Decreasing the Liquid Junction Potential
3.2. Effects of Vibration and Mixing on the Liquid Junction
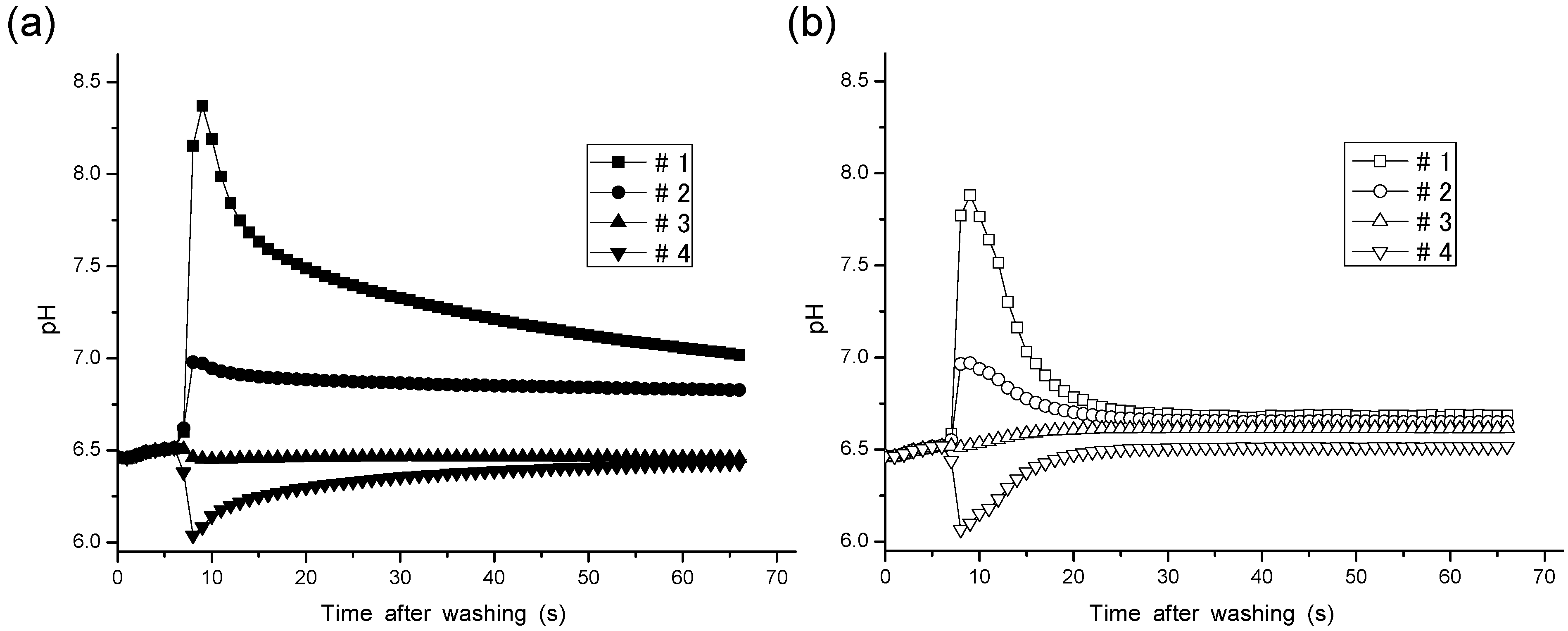
| Method | pH Values/Errors | #1 | #2 | #3 | #4 | Average of Absolute Error |
|---|---|---|---|---|---|---|
| Steady method | pH (Auto-pH) | 7.019 | 6.828 | 6.458 | 6.429 | - |
| Measurement Error | 0.394 | 0.2.3 | −0.617 | −0.120 | 0.221 | |
| Thrbulent method | pH (Auto-pH) | 6.684 | 6.649 | 6.615 | 6.517 | - |
| Measurement Error | 0.060 | 0.025 | −0.032 | −0.032 | 0.0318 |
3.3. Dependency of the Measurement Error on the Ion Concentrations
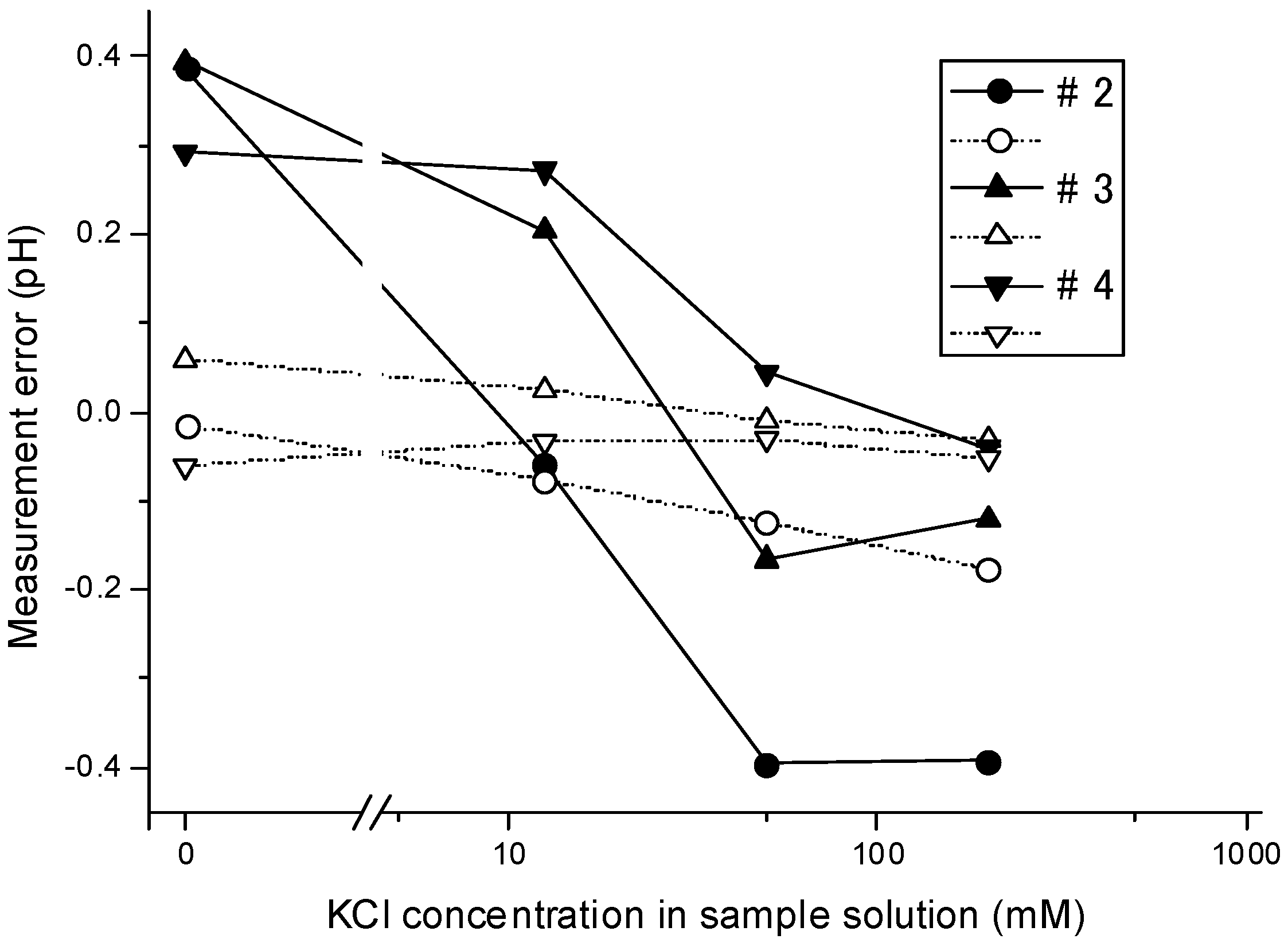
3.4. Dependency of the Overshoot on the Ion Concentration
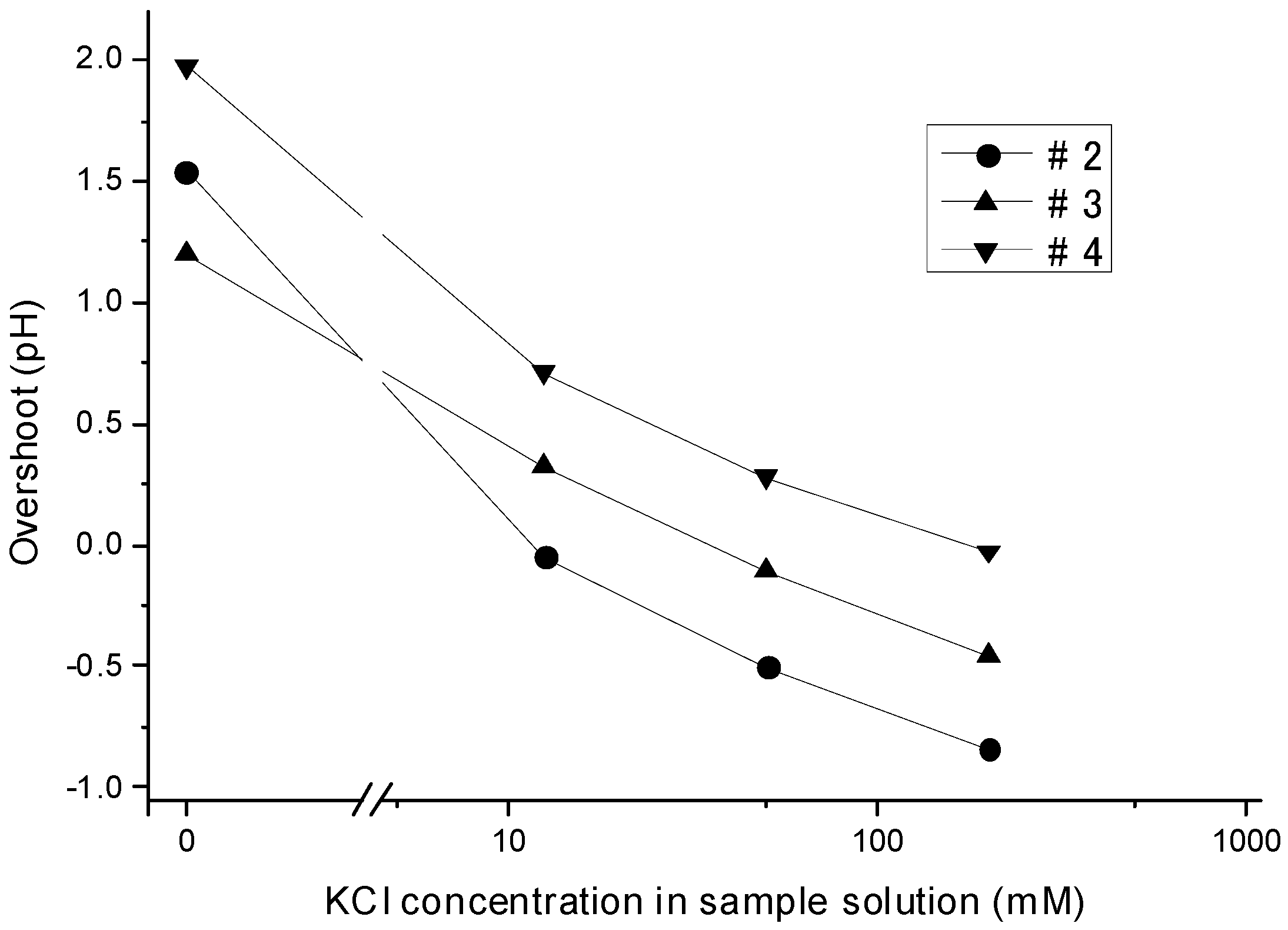
3.5. Mechanism by Which the Liquid Junction Potential Was Produced

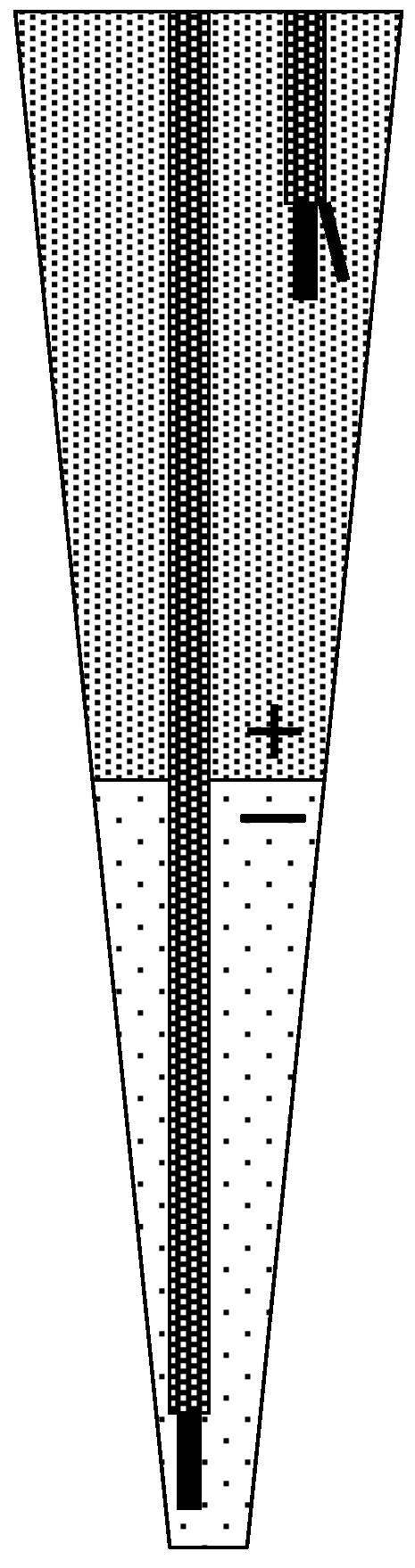
3.6. Prospects for Miniaturizing the Device and Improving the Measurement Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haber, F.; Klemensiewicz, Z. Über elektrische phasengrenzkräfte. Z. Phys. Chem. 1909, 67, 385–431. [Google Scholar]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef]
- Bergveld, P. Thirty years of ISFETOLOGY what happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators B-Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Helmholtz, H. Ueber galvanische ströme, verursacht durch concentrationsunterschiede; Folgerungen avs der mechanischen wärmetheorie. Ann. Phys. Chem. 1878, 3, 201–216. [Google Scholar] [CrossRef]
- Kakiuchi, T. Salt bridge in electroanalytical chemistry: Past, present, and future. J. Solid State Electrochem. 2011, 15, 1661–1671. [Google Scholar] [CrossRef]
- Nernst, W. Zur kinetik der in lösung befindlichen körper. Z. Phys. Chem. 1888, 2, 613–637. [Google Scholar]
- Nernst, W. Die elektromotorische wirksamkeit der jonen. Z. Phys. Chem. 1889, 4, 129–181. [Google Scholar]
- Planck, M. Uber die erregung von electricität und wärme in electrolyten. Ann. Phys. Chem. 1890, 39, 161–186. [Google Scholar] [CrossRef]
- Planck, M. Ueber die potentialdifferenz zwischen zwei verdünnten lösungen binärer electrolyte. Ann. Phys. Chem. 1890, 40, 561–576. [Google Scholar] [CrossRef]
- Henderson, P. Zur thermodynamik der flüssigkeitsketten. Z. phys. Chem. 1907, 59, 118–127. [Google Scholar]
- Henderson, P. Zur thermodynamik der flüssigkeitsketten. Z. phys. Chem. 1908, 63, 325–345. [Google Scholar]
- Negbauer, W. Experimentaluntersuchungen über potentialdifferenzen an den berührungsflächen sehr verdünnter lösungen. Ann. Phys. Chem. 1891, 44, 737–758. [Google Scholar] [CrossRef]
- MacInnes, D.A.; Yeh, Y.L. The potentials at the junctions of monovalent chloride solutions. J. Am. Chem. Soc. 1921, 43, 2563–2573. [Google Scholar] [CrossRef]
- Scatchard, G. Electromotive-force measurements with a saturated potassium chloride bridge or with concentration cells with a liquid junction. J. Am. Chem. Soc. 1931, 53, 574–578. [Google Scholar]
- Tower, O.F. Studien über superoxyd-elektroden. Z. Phys. Chem. 1895, 18, 17–50. [Google Scholar]
- Tower, O.F. Ueber potentialdifferenzen an den berührungsflächen verdünnter lösungen. Z. Phys. Chem. 1896, 20, 198–206. [Google Scholar]
- Bjerrum, N. Über die elimination des diffusionspotentials zwischen zwei verdünnter wässerigen lösungen durch einschalten einer konzentrierten chlorkaliumlösung. Z. Phys. Chem. 1905, 53, 428–440. [Google Scholar]
- Bjerrum, N. Über die elimination des flüssigkeitspotentials bei messungen von elektrodenpotentialen. Z. Electrochem. Angew. Phys. Chem. 1911, 17, 389–393. [Google Scholar]
- Bjerrum, N. Über die gültigkeit der planckschen formel für das diffusionspotential. Z. Electrochem. Angew. Phys. Chem. 1911, 17, 58–61. [Google Scholar]
- Fales, H.A.; Vosburgh, W.C. Planck’s formula for the potential difference between solutions and the values of certain important cells. J. Am. Chem. Soc. 1918, 40, 1291–1316. [Google Scholar] [CrossRef]
- Covington, A.K.; Rebelo, M.J.F. Reference electrodes and liquid junction effects in ion-selective electrode potentiometry. Ion-Sel. Electrode Rev. 1983, 5, 93–128. [Google Scholar]
- Izutsu, K. Electrochemical approach to ion solvations. Applications of ion-selective electrodes as sensors for ion solvations and the problem of the liquid junction potential between different solvents. Anal. Sci. 1991, 7, 1–8. [Google Scholar] [CrossRef]
- Picknett, R.G. Liquid junction potential between dilute electrolytes and saturated potassium chloride. Trans. Faraday Soc. 1968, 64, 1059–1069. [Google Scholar] [CrossRef]
- Covington, A.K.; Whalley, P.D.; Davison, W. Procedures for the measurement of pH in low ionic strength solutions including freshwater. Analyst 1983, 108, 1528–1532. [Google Scholar] [CrossRef]
- Lamb, A.B.; Larson, A.T. Reproducible liquid junction potentials: The flowing junction. J. Am. Chem. Soc. 1920, 42, 229–237. [Google Scholar] [CrossRef]
- Shibata, M.; Yamanuki, M.; Iwamoto, Y.; Nomura, S.; Kakiuchi, T. Stability of a Ag/AgCl reference electrode equipped with an ionic liquid salt bridge composed of 1-methyl-3-octylimidazolium bis(trifluoromethanesulfonyl)-amide in potentiometry of pH standard buffers. Anal. Sci. 2010, 26, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Sakaida, H.; Kakiuchi, T. Determination of the activity of hydrogen ions in dilute sulfuric acids by use of an ionic liquid salt bridge sandwiched by two hydrogen electrodes. Anal. Chem. 2011, 83, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Rius-Ruiz, F.X.; Kisiel, A.; Michalska, A.; Maksymiuk, K.; Riu, J.; Rius, F.X. Solid-state reference electrodes based on carbon nanotubes and polyacrylate membranes. Anal. Bioanal. Chem. 2011, 399, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lai, C.Z.; Fierke, M.A.; Stein, A.; Bühlmann, P. Advantages and limitations of reference electrodes with an ionic liquid junction and three-dimensionally ordered macroporous carbon as solid contact. Anal. Chem. 2012, 84, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, E.A. A study of cells with liquid-liquid junctions. J. Am. Chem. Soc. 1930, 52, 1315–1337. [Google Scholar] [CrossRef]
- Scatchard, G. The activities of strong electrolytes. III. The use of the flowing junction to study the liquid junction potential between dilute hydrochloric acid and saturated potassium chloride solutions; and the revision of some single-electrode potentials. J. Am. Chem. Soc. 1925, 47, 696–709. [Google Scholar] [CrossRef]
- Scatchard, G.; Buehrer, T.F. An effect of the breadth of junction of the electromotive force of a simple concentration cell. J. Am. Chem. Soc. 1931, 53, 574–578. [Google Scholar] [CrossRef]
- Dohner, R.E.; Wegmann, D.; Morf, W.E.; Simon, W. Reference electrode with free-flowing free-diffusion liquid junction. Anal. Chem. 1986, 58, 2585–2589. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Mohri, S.; Nakamura, M.; Naruse, K. Automation of pH measurement using a flow-through type differential pH sensor system based on pH-FET. IEEJ Trans. SM 2007, 127, 367–370. [Google Scholar] [CrossRef]
- Yamada, A.; Mohri, S.; Nakamura, M.; Naruse, K. A fully automated pH measurement system for 96-well microplates using a semiconductor-based pH sensor. Sen. Actuat. B-Chem. 2010, 143, 464–469. [Google Scholar] [CrossRef]
- Yamada, A.; Mohri, S.; Nakamura, M.; Naruse, K. Optimizing the conditions for pH measurement with an automated pH measurement system using a flow-through-type differential sensor probe consisting of pH-FETs. J. Robot. Mechatron. 2010, 22, 197–203. [Google Scholar]
- Ogata, K. Modern Control Engineering, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009; pp. 159–268. [Google Scholar]
- Nakatsuka, T.; Nakamura, M.; Sano, T. Applications of hydrogen ion sensitive FET to the kinetic study of fast reaction in solution. Bull. Chem. Soc. Jpn. 1988, 61, 799–802. [Google Scholar] [CrossRef]
- Mohri, S.; Shimizu, J.; Goda, N.; Miyasaka, T.; Fujita, A.; Nakamura, M.; Kajiya, F. Measurements of CO2, lactic acid and sodium bicarbonate secreted by cultured cells using a flow-through type pH/CO2 sensor system based on ISFET. Sens. Actuators B-Chem. 2006, 115, 519–525. [Google Scholar] [CrossRef]
- Mohri, S.; Yamada, A.; Goda, N.; Nakamura, M.; Naruse, K.; Kajiya, F. Application of a flow-through type pH/CO2 sensor system based on ISFET for evaluation of the glucose dependency of the metabolic pathways in cultured cells. Sens. Actuators B-Chem. 2008, 134, 447–450. [Google Scholar] [CrossRef]
- Yamada, A.; Mohri, S.; Nakamura, M.; Naruse, K. Glucose dependency of the metabolic pathway of HEK 293 cells measured by a flow-through type pH/CO2 sensor system using ISFETs. IEEJ Trans. EIS 2011, 131, 1535–1539. [Google Scholar] [CrossRef]
- Matsuura, K.; Asano, Y.; Yamada, A.; Naruse, K. Detection of Micrococcus Luteus biofilm formation in microfluidic environments by pH measurement using an ion-sensitive field-effect transistor. Sensors 2013, 13, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, A.; Mohri, S.; Nakamura, M.; Naruse, K. A Simple Method for Decreasing the Liquid Junction Potential in a Flow-through-Type Differential pH Sensor Probe Consisting of pH-FETs by Exerting Spatiotemporal Control of the Liquid Junction. Sensors 2015, 15, 7898-7912. https://doi.org/10.3390/s150407898
Yamada A, Mohri S, Nakamura M, Naruse K. A Simple Method for Decreasing the Liquid Junction Potential in a Flow-through-Type Differential pH Sensor Probe Consisting of pH-FETs by Exerting Spatiotemporal Control of the Liquid Junction. Sensors. 2015; 15(4):7898-7912. https://doi.org/10.3390/s150407898
Chicago/Turabian StyleYamada, Akira, Satoshi Mohri, Michihiro Nakamura, and Keiji Naruse. 2015. "A Simple Method for Decreasing the Liquid Junction Potential in a Flow-through-Type Differential pH Sensor Probe Consisting of pH-FETs by Exerting Spatiotemporal Control of the Liquid Junction" Sensors 15, no. 4: 7898-7912. https://doi.org/10.3390/s150407898
APA StyleYamada, A., Mohri, S., Nakamura, M., & Naruse, K. (2015). A Simple Method for Decreasing the Liquid Junction Potential in a Flow-through-Type Differential pH Sensor Probe Consisting of pH-FETs by Exerting Spatiotemporal Control of the Liquid Junction. Sensors, 15(4), 7898-7912. https://doi.org/10.3390/s150407898





