Interstitial Photoacoustic Sensor for the Measurement of Tissue Temperature during Interstitial Laser Phototherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
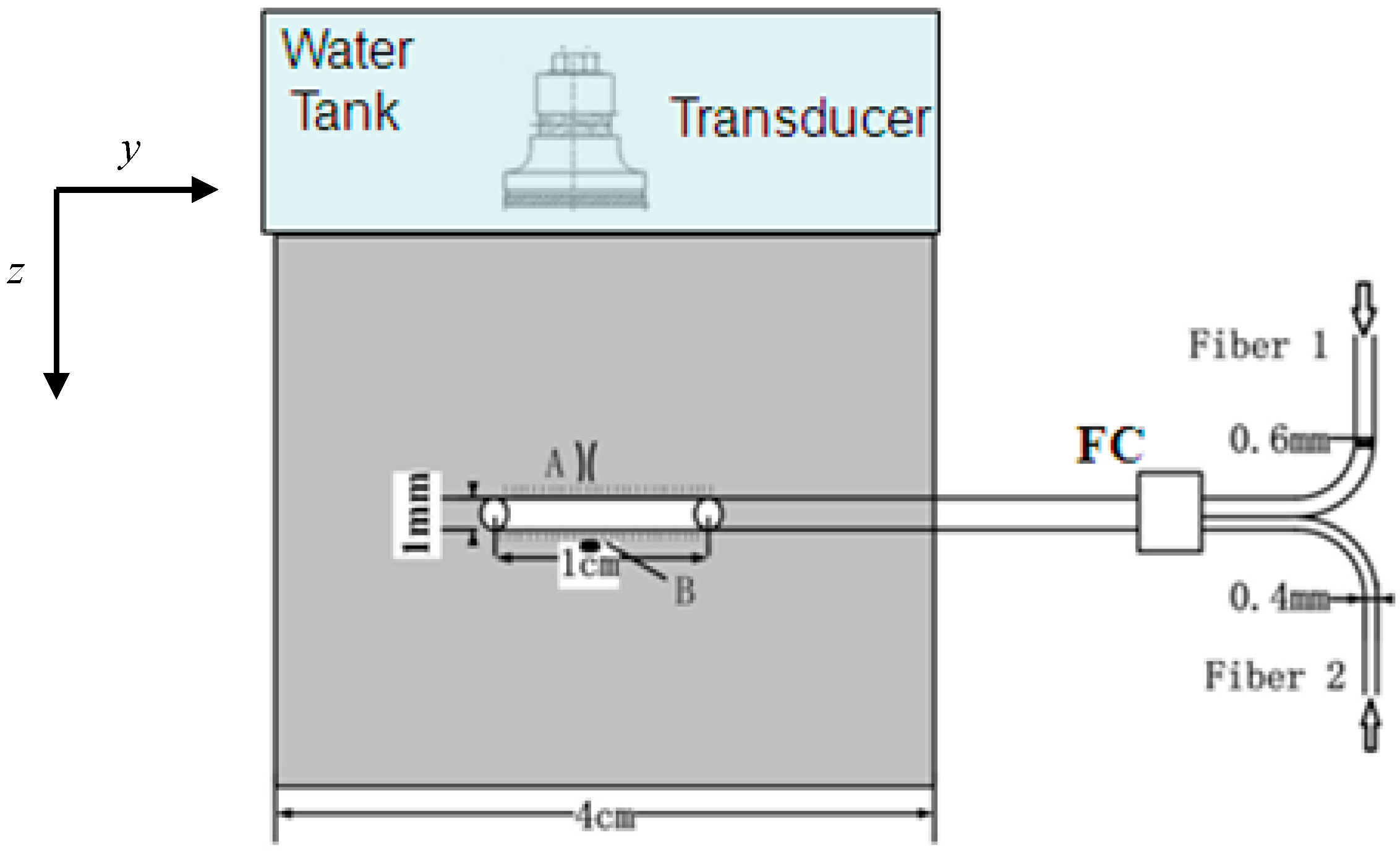
2.2. Theoretical Foundation of PA Measurement of Temperature
| Anisotropic Factor (g) | Scattering Coefficient (µs) | Absorption Coefficient (µa) |
|---|---|---|
| 0.93 | 5.6 mm−1 | 0.73 mm−1 |
| ρ (g/cm3) | c (J·g−1·°C−1) | k (W·cm−1·°C−1) |
|---|---|---|
| 1.05 | 3.59 | 0.00566 |
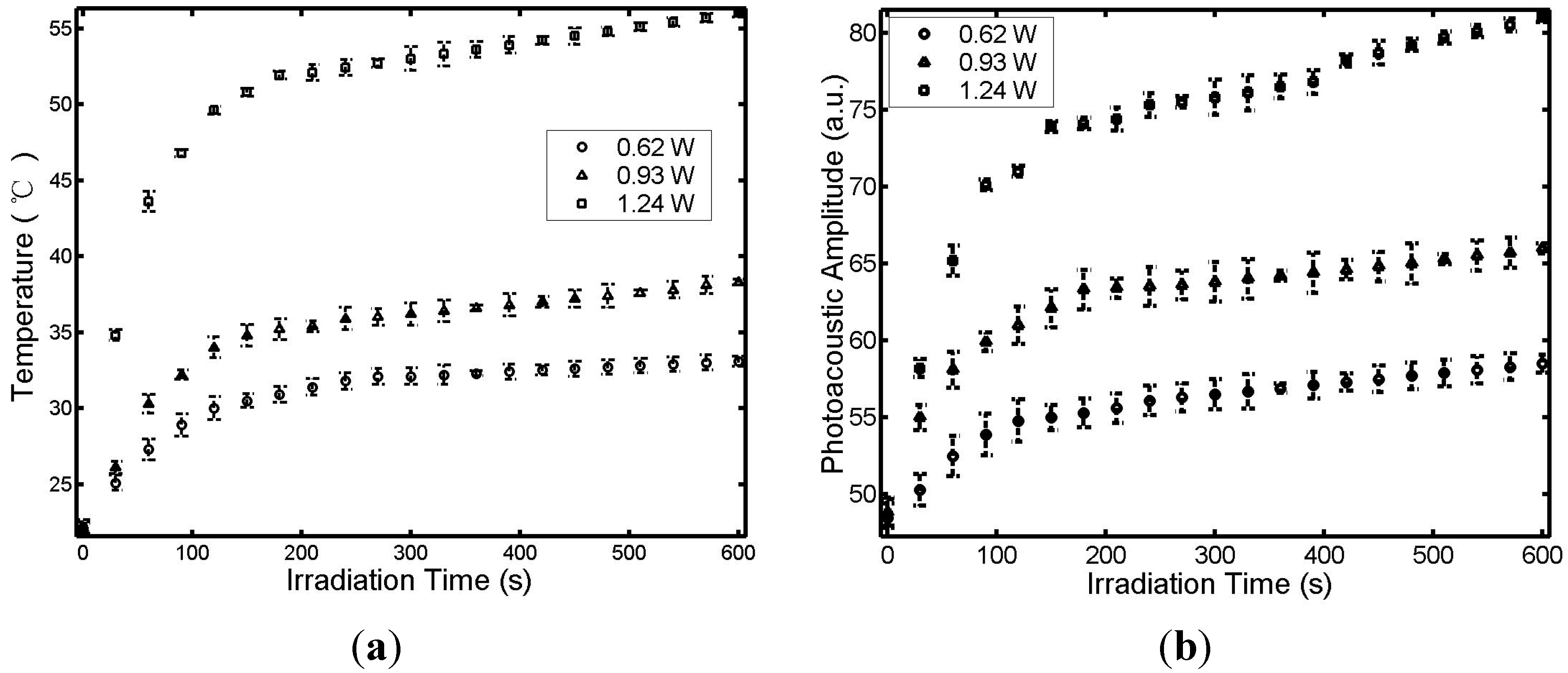
3. Results and Discussion



4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anghileri, L.J.; Robert, J. Hyperthermia in Cancer Treatment; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Chen, W.R.; Adams, R.L.; Heaton, S.; Dickey, D.T.; Bartels, K.E.; Nordquist, R.E. Chromophore-enhanced laser-tumor tissue photothermal interaction using 808 nm diode laser. Cancer Lett. 1995, 88, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Adams, R.L.; Bartels, K.E.; Nordquist, R.E. Chromophore-enhanced in vivo tumor cell destruction using an 808-nm diode laser. Cancer Lett. 1995, 94, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. PNAS 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Lereu, A.L.; Farahi, R.H.; Tetard, L.; Enoch, S.; Thundat, T.; Passian, V. Plasmon assisted thermal modulation in nanoparticles. Opt. Express 2013, 21, 12145–12158. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Adams, R.L.; Higgins, A.K.; Bartels, K.E.; Nordquist, R.E. Photothermal effects on murine mammary tumors using indocyanine green and an 808-nm diode laser: An in vivo efficacy study. Cancer Lett. 1996, 98, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Zhu, W.-G.; Dynlacht, J.R.; Liu, H.; Nordquist, R.E. Long-term tumor resistance induced by laser photo-immunotherapy. Int. J. Cancer 1999, 81, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Liu, H.; Nordquist, J.A.; Nordquist, R.E. Tumor cell damage and leukocyte infiltration after laser immunotherapy treatment. Lasers Med. Sci. 2000, 15, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Singhal, A.K.; Liu, H.; Nordquist, R.E. Laser immunotherapy induced antitumor immunity and its adoptive transfer. Cancer Res. 2001, 61, 459–461. [Google Scholar] [PubMed]
- Chen, W.R.; Liu, H.; Ritchey, J.W.; Bartels, K.E.; Lucroy, M.D.; Nordquist, R.E. Effect of different components of laser immunotherapy in treatment of metastatic tumors in rats. Cancer Res. 2002, 62, 4295–4299. [Google Scholar] [PubMed]
- Chen, W.R.; Jeong, S.W.; Lucroy, M.D.; Wolf, R.F.; Howard, E.W.; Liu, H.; Nordquist, R.E. Induced anti-tumor immunity against DMBA-4 metastatic mammary tumors in rats using laser immunotherapy. Int. J. Cancer 2003, 107, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ferrel, G.L.; Guerra, M.C.; Hode, T.; Lunn, J.A.; Adalsteinsson, O.; Nordquist, R.E.; Liu, H.; Chen, W.R. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photochem. Photobiol. Sci. 2011, 10, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Naylor, M.F.; Nordquist, R.E.; Teague, T.K.; Howard, C.A.; Murray, C.; Chen, W.R. In situ photoimmunotherapy for late-stage melanoma patients: A preliminary study. Cancer Biol. Ther. 2010, 10, 1077–1214. [Google Scholar]
- Zhang, H.G.; Mehta, K.; Cohen, P.; Guha, C. Hyperthermia on immune regulation: A temperature’s story. Cancer Lett. 2008, 271, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gu, Y.; Du, N.; Hode, T.; Nordquist, R.E.; Wolf, R.F.; Howard, E.; Lunn, J.A.; Adalsteinsson, O.; Chen, W.R. Laser immunotherapy: Concept, possible mechanism, clinical applications, and recent experimental results. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1434–1438. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.; Chen, W.R. Host antitumour immune responses to HIFU ablation. Int. J. Hyperth. 2007, 23, 165–171. [Google Scholar] [CrossRef]
- Welch, A.J.; van Gemert, M.J.C. Optical-Thermal Response of Laser-Irradiated Tissue, 1st ed.; Plenum Publishing Corporation: New York, NY, USA, 1995. [Google Scholar]
- Mital, M.; Scott, E.P. Thermal detection of embedded tumors using infrared imaging. J. Biomech. Eng. 2007, 129, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gescheit, I.M.; Dayan, A.; Ben-David, M.; Gannot, I. Minimal-invasive thermal imaging of a malignant tumor: A simple model and algorithm. Med. Phys. 2010, 37, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Zagzebski, J.A.; Chen, Q.; Techavipoo, U.; Frank, G.; Johnson, C.; Wright, A.; Lee, F.T. Ultrasound monitoring of temperature change during radofrequency ablation: Preliminary in vivo results. Ultrasound Med. Biol. 2002, 28, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Li, X.; Figueroa, D.; Towner, R.A.; Garteiser, P.; Saunders, D.; Smith, N.; Liu, H.; Hode, T.; Nordquist, R.E.; et al. Assessment of thermal effects of interstitial laser phototherapy on mammary tumors using proton resonance frequency method. J. Biomed. Opt. 2011, 16, 128001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gnyawali, S.C.; Wu, F.; Liu, H.; Tesiram, Y.A.; Abbott, A.; Towner, R.A.; Chen, W.R. Magnetic resonance imaging guidance for laser photothermal therapy. J. Biomed. Opt. 2008, 13, 044033. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Kim, H.J.; Jeong, W.C.; Chauhan, M.; Kwon, O.I.; Woo, E.J. Detection of temperature distribution via recovering electrical conductivity in MREIT. Phys. Med. Biol. 2013, 58, 2697–2711. [Google Scholar] [CrossRef] [PubMed]
- Larina, I.V.; Larin, K.V.; Esenaliev, R.O. Real-time optoacoustic monitoring of temperature in tissues. J. Phys. D Appl. Phys. 2005, 38, 2633–2639. [Google Scholar] [CrossRef]
- Pramanik, M.; Wang, L.V. Thermoacoustic and photoacoustic sensing of temperature. J. Biomed. Opt. 2009, 14, 054024. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Chen, H.; Xie, W. In vivo determination of acute myocardial ischemia based on photoacoustic imaging with a focused transducer. J. Biomed. Opt. 2011, 16, 076011. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Cox, B.; Zemp, R.J. Estimating optical absorption, scattering, and Grueneisen distributions with multiple-illumination. Appl. Opt. 2011, 50, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, C.; Razansky, D. Optoacoustic imaging and tomography: Reconstruction approaches and outstanding challenges in image performance and quantification. Sensors 2013, 13, 7345–7384. [Google Scholar] [CrossRef] [PubMed]
- Ritz, J.P.; Roggan, A.; Isbert, C.; Muller, G.; Buhr, H.J.; Germer, C.T. Optical properties of native and coagulated porcine liver tissue between 400 and 2400 nm. Lasers Surg. Med. 2001, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.; Verhey, J.F. A finite element method model to simulate laser interstitial thermotherapy in anatomical inhomogeneous regions. Biomed. Eng. Online 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.N.; Vitkin, I.A.; Kolios, M.C.; Sherar, M.D. The effect of dynamic optical properties during interstitial laser photocoagulation. Phys. Med. Biol. 2000, 45, 1335–1357. [Google Scholar] [CrossRef] [PubMed]
- Farahi, R.H.; Passian, A.; Tetard, L.; Thundat, T. Pump-probe photothermal spectroscopy using quantum cascade lasers. J. Phys. D Appl. Phys. 2012, 45, 125101. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, H.; Zhou, F.; Li, H.; Chen, W.R. Interstitial Photoacoustic Sensor for the Measurement of Tissue Temperature during Interstitial Laser Phototherapy. Sensors 2015, 15, 5583-5593. https://doi.org/10.3390/s150305583
Li Z, Chen H, Zhou F, Li H, Chen WR. Interstitial Photoacoustic Sensor for the Measurement of Tissue Temperature during Interstitial Laser Phototherapy. Sensors. 2015; 15(3):5583-5593. https://doi.org/10.3390/s150305583
Chicago/Turabian StyleLi, Zhifang, Haiyu Chen, Feifan Zhou, Hui Li, and Wei R. Chen. 2015. "Interstitial Photoacoustic Sensor for the Measurement of Tissue Temperature during Interstitial Laser Phototherapy" Sensors 15, no. 3: 5583-5593. https://doi.org/10.3390/s150305583
APA StyleLi, Z., Chen, H., Zhou, F., Li, H., & Chen, W. R. (2015). Interstitial Photoacoustic Sensor for the Measurement of Tissue Temperature during Interstitial Laser Phototherapy. Sensors, 15(3), 5583-5593. https://doi.org/10.3390/s150305583





