The Synthesis and Anion Recognition Property of Symmetrical Chemosensors Involving Thiourea Groups: Theory and Experiments
Abstract
:1. Introduction
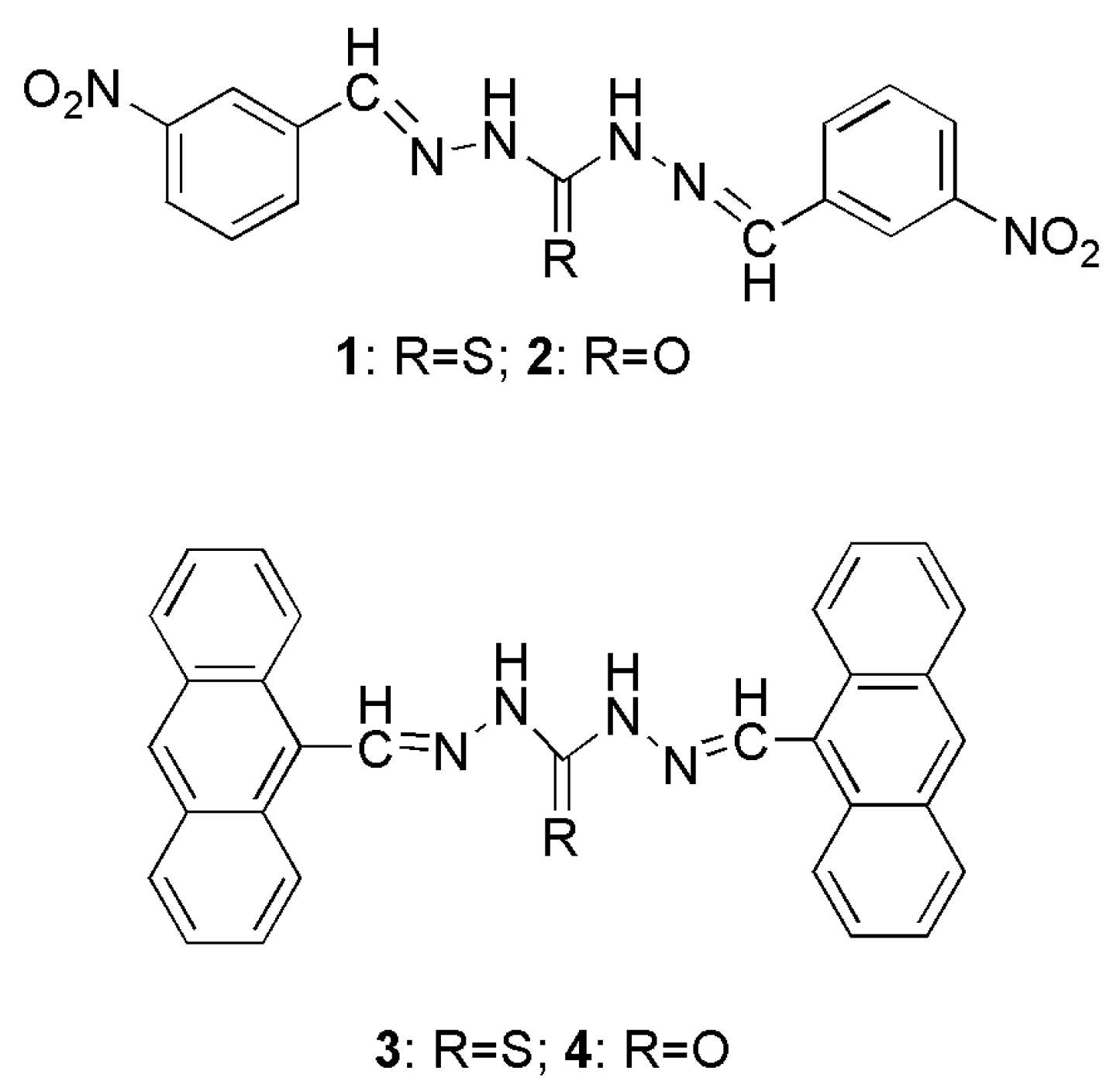
2. Experimental Section
2.1. General Information
2.2. Chemistry
3. Results and Discussion
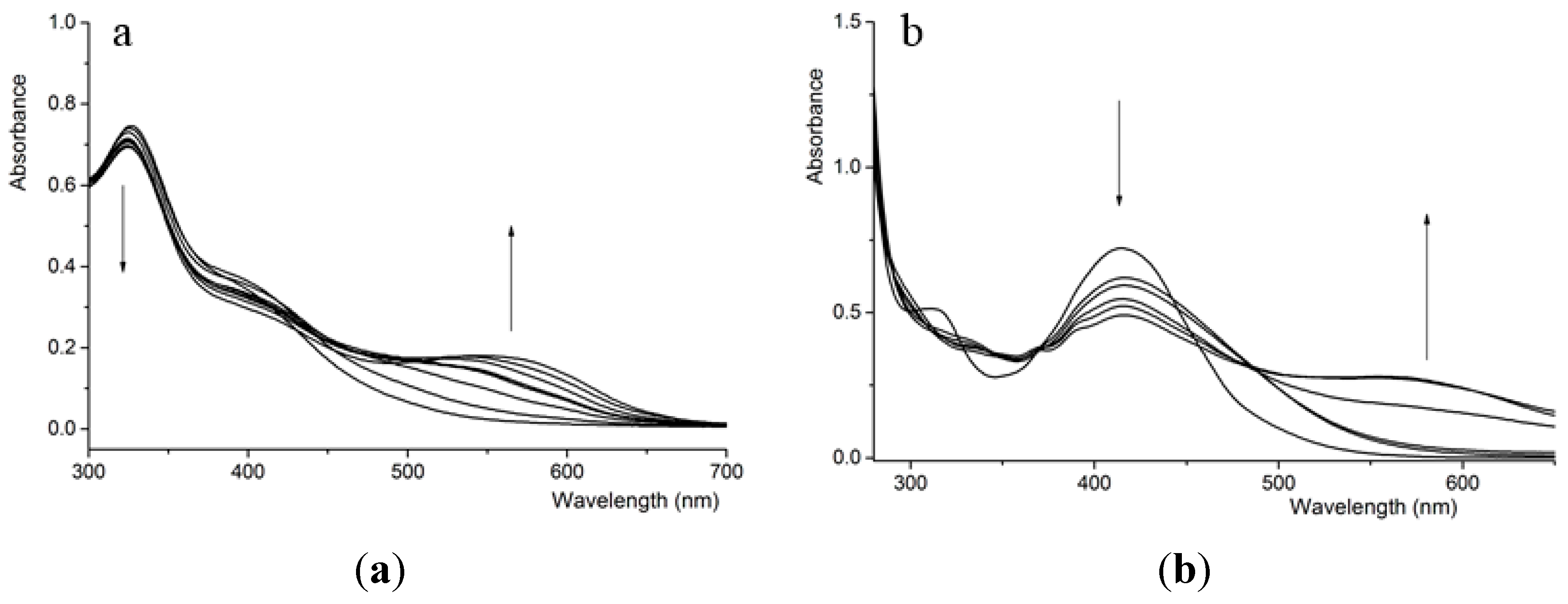
3.1. UV-Vis Titration
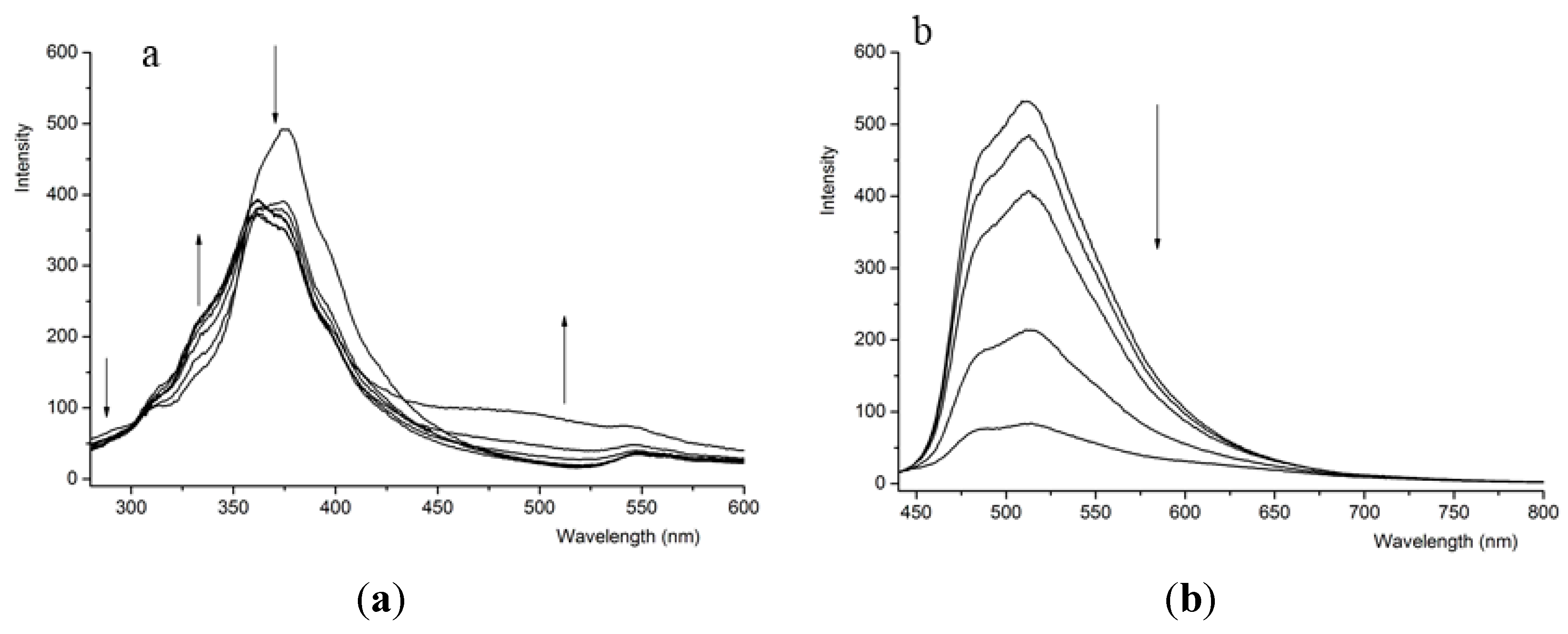
3.2. Fluorescence Response
3.3. The Calculation of Binding Constant
| Anion a | Compound | |
|---|---|---|
| 1 | 3 | |
| AcO− | (1.33 ± 0.08) × 104 | (3.93 ± 0.06) × 104 |
| F− | (2.06 ± 0.14) × 103 | (2.64 ± 0.02) × 104 |
| H2PO4− | (9.25 ± 0.27) × 102 | (4.45 ± 0.24) × 103 |
| Cl−, Br− or I− | ND b | ND |
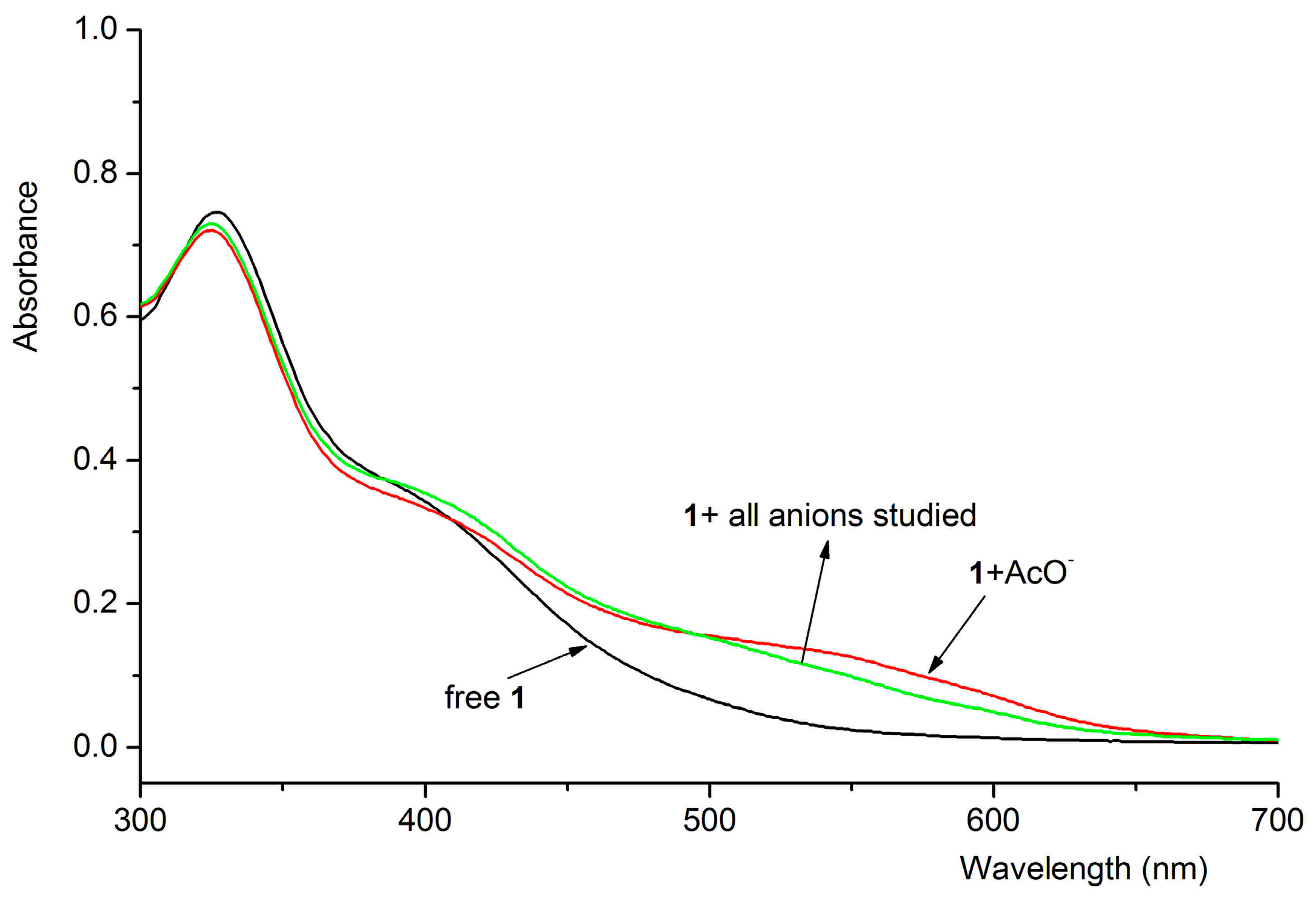
3.4. Interference Experiment
3.5. Theoretical Investigation
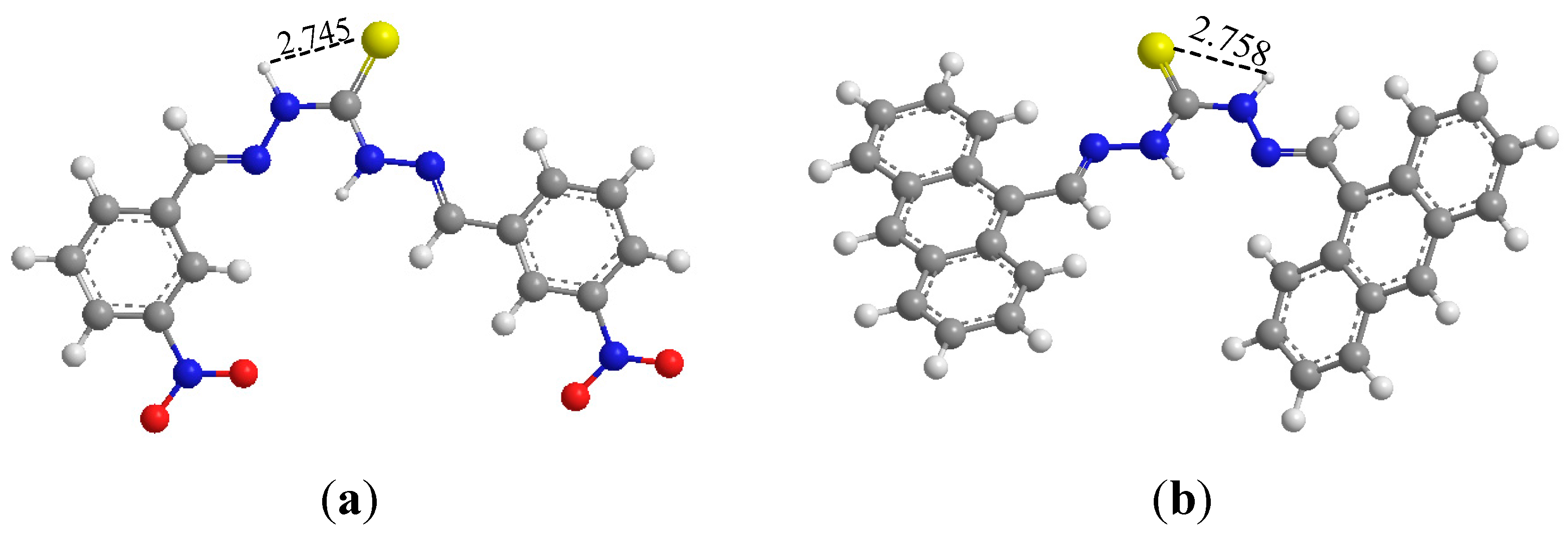
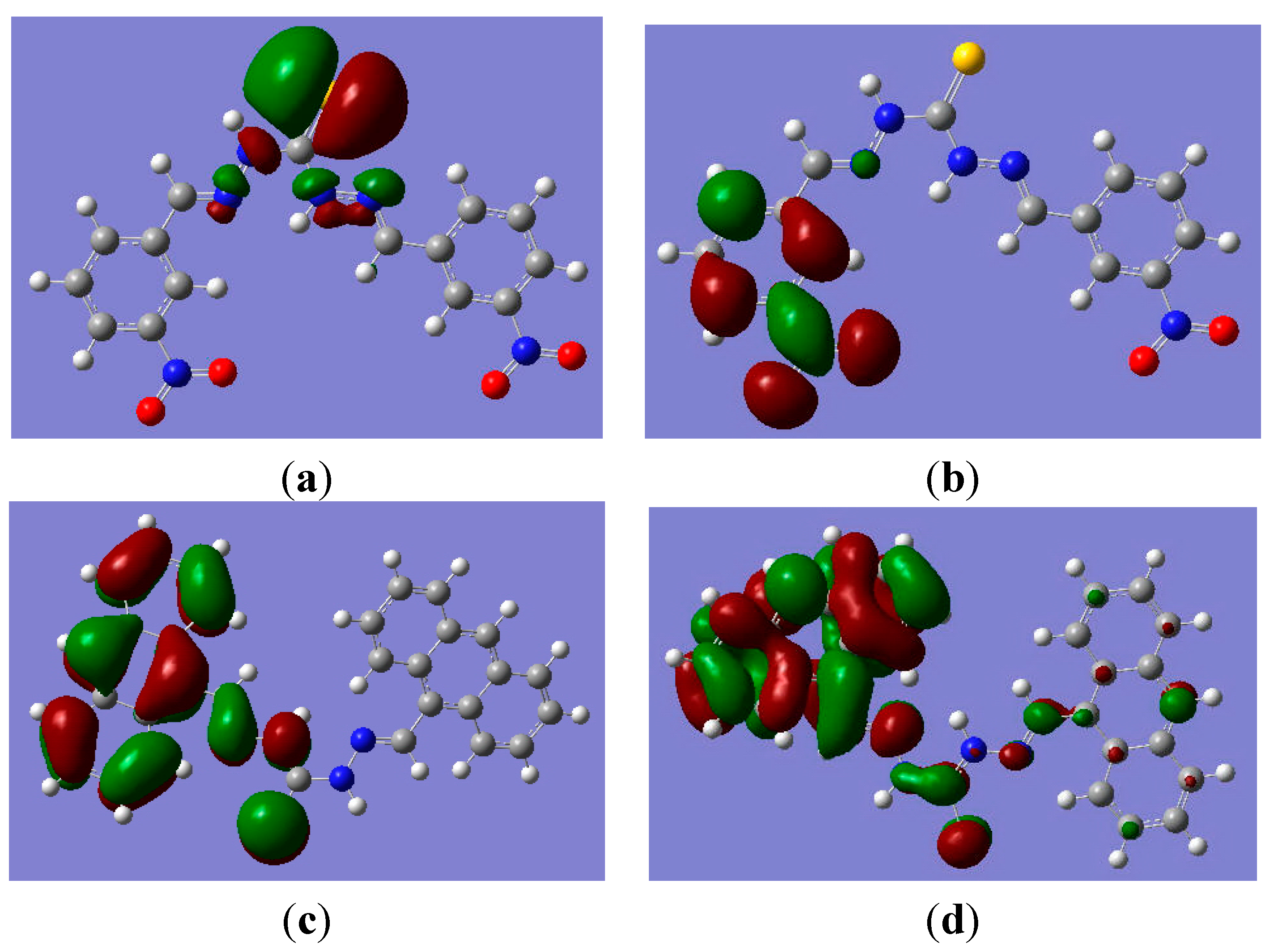
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Busschaert, N.; Caltagirone, C.; van Rossom, W.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef] [PubMed]
- Santos-Figueroa, L.E.; Moragues, M.E.; Climent, E.; Agostini, A.; Martínez-Máñez, R.; Sancenón, F. Chromogenic and fluorogenic chemosensors and reagents for anions: A comprehensive review of the years 2010–2011. Chem. Soc. Rev. 2013, 42, 3489–3613. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Du, J.G.; Yang, W.C.; Liu, Y.; Fu, Z.Y.; Wei, X.F.; Yan, R.F.; Yao, N.C.; Guo, Y.P.; Zhang, J.L.; Xu, X.F. The development and amino acid binding ability of nano-materials based on azo derivatives: Theory and experiment. Mat. Sci. Eng. C 2014, 38, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.W.; Mustoe, C.L.; White, N.G.; Brown, A.; Thompson, A.L.; Kennepohl, P.; Beer, P.D. Evidence for halogen bond covalency in acyclic and interlocked halogen-bonding receptor anion recognition. J. Am. Chem. Soc. 2015, 137, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Schubert, U.S. Beyond click chemistry—Supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, S.; Ni, X.L. Anion recognition triggered nanoribbon-like self-assembly: A fluorescent chemosensor for nitrate in acidic aqueous solution and living cells. Anal. Chem. 2015, 87, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Assaf, K.I.; Ural, M.S.; Pan, F.; Georgiev, T.; Simova, S.; Rissanen, K.; Gabel, D.; Nau, W.M. Water structure recovery in chaotropic anion recognition: high-affinity binding of dodecaborate clusters to γ-cyclodextrin. Angew. Chem. Int. Ed. 2015, 54, 6852–6856. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Cai, Y.; Li, Q.; Shi, B.B.; Yao, H.; Zhang, Y.M.; Wei, T.B. Fluorescent “turn-on” detecting CN− by nucleophilic addition induced schiff-base hydrolysis. Spectrochim. Acta. A 2015, 141, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Cornes, S.P.; Davies, C.H.; Blyghton, D.; Sambrook, M.R.; Beer, P.D. Contrasting anion recognition behaviour exhibited by halogen and hydrogen bonding rotaxane hosts. Org. Biomol. Chem. 2015, 13, 2582–2587. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.F.; Ho, P.Y.; Wong, W.L.; Gong, C.B. A multifunctional bimetallic molecular device for ultrasensitive detection, naked-eye recognition, and elimination of cyanide ions. Chem. A Eur. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Lim, C.S.; Bhuniya, S.; Cho, B.R.; Kim, J.S. A highly selective colorimetric and ratiometric two-photon fluorescent probe for fluoride ion detection. Org. Lett. 2011, 13, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bhuniya, S.; Mahajan, R.K.; Puri, R.; Liu, H.; Ko, K.C.; Lee, J.Y.; Kim, J.S. Fluorescence turn-on sensors for HSO4−. Chem. Commun. 2009, 46, 7128–7130. [Google Scholar] [CrossRef] [PubMed]
- Joo, T.Y.; Singh, N.; Lee, G.W.; Jang, D.O. Benzimidazole-based ratiometric fluorescent receptor for selective recognition of acetate. Tetrahedron Lett. 2007, 48, 8846–8850. [Google Scholar] [CrossRef]
- Beierlein, F.R.; Clark, T.; Braunschweig, B.; Engelhardt, K.; Glas, L.; Peukert, W. Carboxylate ion pairing with alkali-metal ions for β-Lactoglobulin and its role on aggregation and interfacial adsorption. J. Phys. Chem. B 2015, 119, 5505–5517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, S.; Iglesias-Cans, M.; Krah, A.; Yildiz, O.; Leone, V.; Matthies, D.; Cook, G.M.; Faraldo-Gómez, J.D.; Meier, T. A new type of Na(+)-driven ATP synthase membrane rotor with a two-carboxylate ion-coupling motif. PLoS Biol. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V.; Peru, K.M. Electrospray ionization mass spectro-metry studies of cyclodextrin-carboxylate ion inclusion complexes. Rapid Commun. Mass Spectrom. 2009, 23, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- White, N.G.; Colaço, A.R.; Marques, I.; Félix, V.; Beer, P.D. Halide selective anion recognition by an amide-triazolium axle containing [2]rotaxane. Org. Biomol. Chem. 2014, 12, 4924–4931. [Google Scholar] [CrossRef] [PubMed]
- Carreira-Barral, I.; Rodríguez-Blas, T.; Platas-Iglesias, C.; de Blas, A.; Esteban-Gómez, D. Cooperative anion recognition in copper(II) and zinc(II) complexes with a ditopic tripodal ligand containing a urea group. Inorg. Chem. 2014, 53, 2554–2568. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Nagamine, M.; Karasawa, S.; Ishihara, M.; Unno, M.; Yano, Y. Anion recognition by 2,2′-binaphthalene derivatives bearing urea and thiourea groups at 8- and 8′-positions by UV-Vis and fluorescence spectroscopies. Tetrahedron 2011, 67, 943–950. [Google Scholar] [CrossRef]
- Bao, X.P.; Zhou, Y.H. Synthesis and recognition properties of a class of simple colorimetric anion chemosensors containing OH and CONH groups. Sens. Actuators B Chem. 2010, 147, 434–441. [Google Scholar] [CrossRef]
- Yoon, D.W.; Gross, D.E.; Lynch, V.M.; Sessler, J.L.; Hay, B.P.; Lee, C.H. Benzene-, pyrrole-, and furan-containing diametrically strapped calix[4] pyrroles—An experimental and theoretical study of hydrogen-bonding effects in chloride anion recognition. Angew Chem. Int. Ed. 2008, 47, 5038–5042. [Google Scholar] [CrossRef] [PubMed]
- Saikia, E.; Borpuzari, M.P.; Chetia, B.; Kar, R. Experimental and theoretical study of urea and thiourea based new colorimetric chemosensor for fluoride and acetate ions. Spectrochim. Acta A 2015, 152, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Basaran, I.; Khansari, M.E.; Pramanik, A.; Wong, B.M.; Hossain, A. An exclusive fluoride receptor: Fluoride-induced proton transfer to a quinoline-based thiourea. Tetrahedron Lett. 2014, 55, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.J.; Busschaert, N.; Kirby, I.L.; Herniman, J.; Light, M.E.; Wells, N.J.; Marques, I.; Félix, V.; Gale, P.A. Acylthioureas as anion transporters: the effect of intramolecular hydrogen bonding. Org. Biomol. Chem. 2014, 12, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D.P.; De Costa, M.D. Fluorescence characteristics of [(benzoyloxy)methyl]anth-racene donor-acceptor systems. J. Phys. Chem. A. 2015, 119, 7973–7979. [Google Scholar] [CrossRef] [PubMed]
- Jaividhya, P.; Ganeshpandian, M.; Dhivya, R.; Akbarsha, M.A.; Palaniandavar, M. Fluorescent mixed ligand copper(II) complexes of anthracene-appended Schiff bases: studies on DNA binding, nuclease activity and cytotoxicity. Dalton Trans. 2015, 44, 1997–2010. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, P.G.; Modi, N.R.; Pandya, A.; Joshi, B.K.; Joshi, K.V.; Menon, S.K. An ICT based “turn on/off” quinoline armed calix[4]arene fluoroionophore: Its sensing efficiency towards fluoride from waste water and Zn2+ from blood serum. Analyst 2012, 137, 5491–5494. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.K.; Mishra, R.K.; Kumar, V.; Chowdhury, P.K. A coumarin based ICT probe for fluoride in aqueous medium with its real application. Talanta 2010, 82, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Toraskar, M.P.; Kadam, V.J.; Kulkarni, V.M. Synthesis and antifungal activity of some azetidin-ones. Int. J. ChemTech Res. 2009, 1, 1194–1199. [Google Scholar]
- Khan, K.M.; Rasheed, M.; Ullah, Z.; Hayat, S.; Kaukab, F.; Choudhary, M. I.; ur-Rahman, A.; Perveen, S. Synthesis and in vitro leishmanicidal activity of some hydrazides and their analogues. Bioorg. Med. Chem. 2003, 11, 1381–1387. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Bera, R.K.; Baral, M.; Kanungo, B.K. Excited state intramolecular proton transfer (ESIPT) in a dioxotetraamine derived schiff base and its complexation with Fe(III) and Cr(III). J. Photochem. Photobiol. A 2007, 188, 298–310. [Google Scholar] [CrossRef]
- Liu, Y.; Han, B.H.; Zhang, H.Y. Spectroscopic studies on molecular recognition of modified cyclodextrins. Curr. Org. Chem. 2004, 8, 35–46. [Google Scholar] [CrossRef]
- Liu, Y.; You, C.C.; Zhang, H.Y. Supramolecular Chemistry; Nankai University Publication: Tianjin, China, 2001. [Google Scholar]
- Bourson, J.; Pouget, J.; Valeur, B. Ion-responsive fluorescent compounds 4. Effect of cation binding on the photophysical properties of a coumarin linked to monoaza-and diaza-crown ethers. J. Phys. Chem. 1993, 97, 4552–4557. [Google Scholar] [CrossRef]
- Wong, M.W.; Xie, H.; Kwa, S.T. Anion recognition by azophenol thiourea-based chromogenic sensors: A combined DFT and molecular dynamics investigation. J. Mol. Model. 2013, 19, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Milani, M. The squaramide versus urea contest for anion recognition. Chem. A Eur. J. 2010, 16, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C; et al. Gaussian03, Software for Computational Chemistry; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Yang, Z.; Fu, J.; Zhao, P.; Xu, X. The Synthesis and Anion Recognition Property of Symmetrical Chemosensors Involving Thiourea Groups: Theory and Experiments. Sensors 2015, 15, 28166-28176. https://doi.org/10.3390/s151128166
Shang X, Yang Z, Fu J, Zhao P, Xu X. The Synthesis and Anion Recognition Property of Symmetrical Chemosensors Involving Thiourea Groups: Theory and Experiments. Sensors. 2015; 15(11):28166-28176. https://doi.org/10.3390/s151128166
Chicago/Turabian StyleShang, Xuefang, Zhenhua Yang, Jiajia Fu, Peipei Zhao, and Xiufang Xu. 2015. "The Synthesis and Anion Recognition Property of Symmetrical Chemosensors Involving Thiourea Groups: Theory and Experiments" Sensors 15, no. 11: 28166-28176. https://doi.org/10.3390/s151128166
APA StyleShang, X., Yang, Z., Fu, J., Zhao, P., & Xu, X. (2015). The Synthesis and Anion Recognition Property of Symmetrical Chemosensors Involving Thiourea Groups: Theory and Experiments. Sensors, 15(11), 28166-28176. https://doi.org/10.3390/s151128166





