Piezoelectric Bimorph Cantilever for Vibration-Producing-Hydrogen
Abstract
:1. Introduction
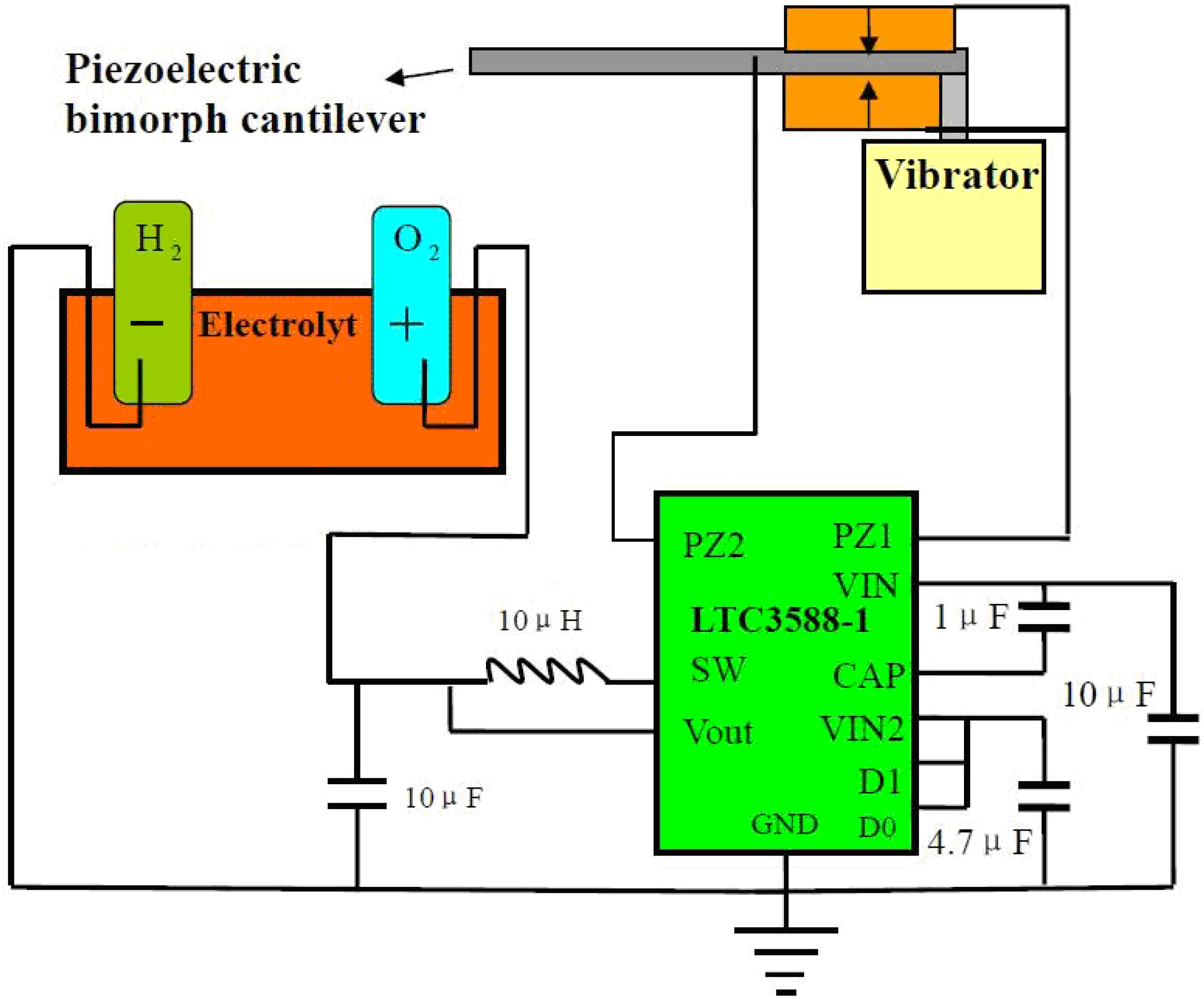
2. Experimental Section
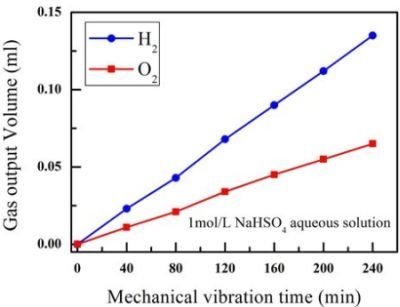
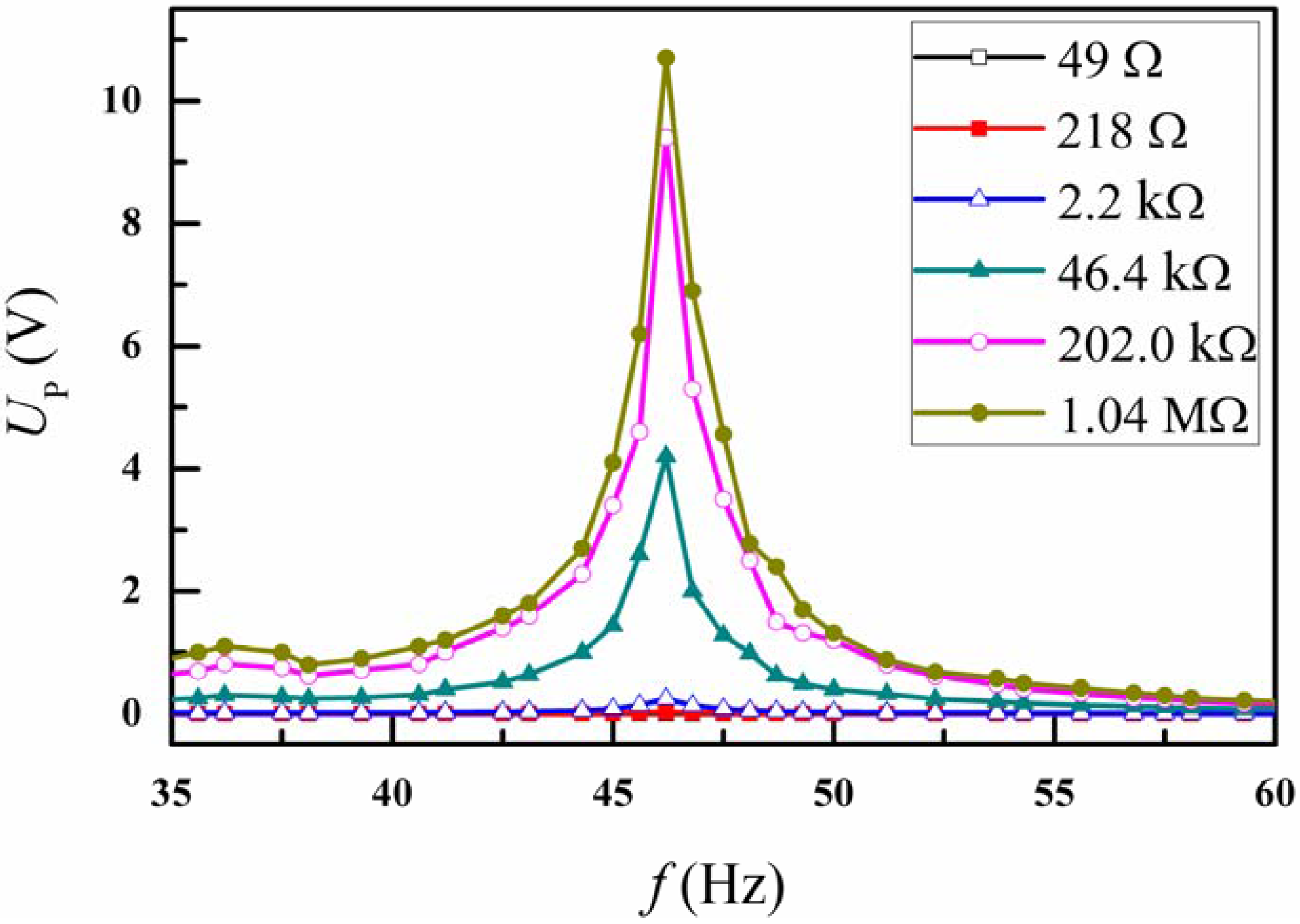
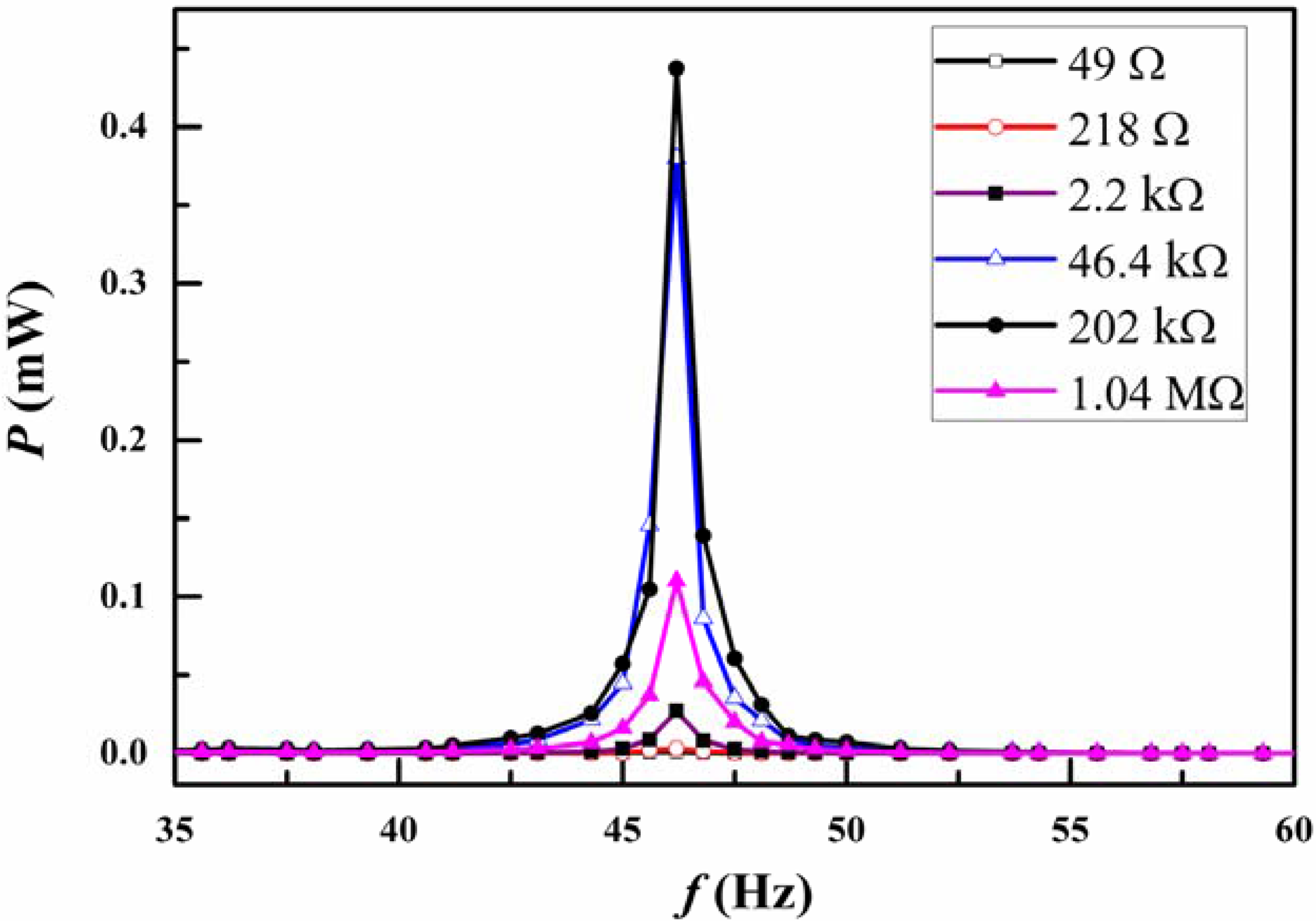
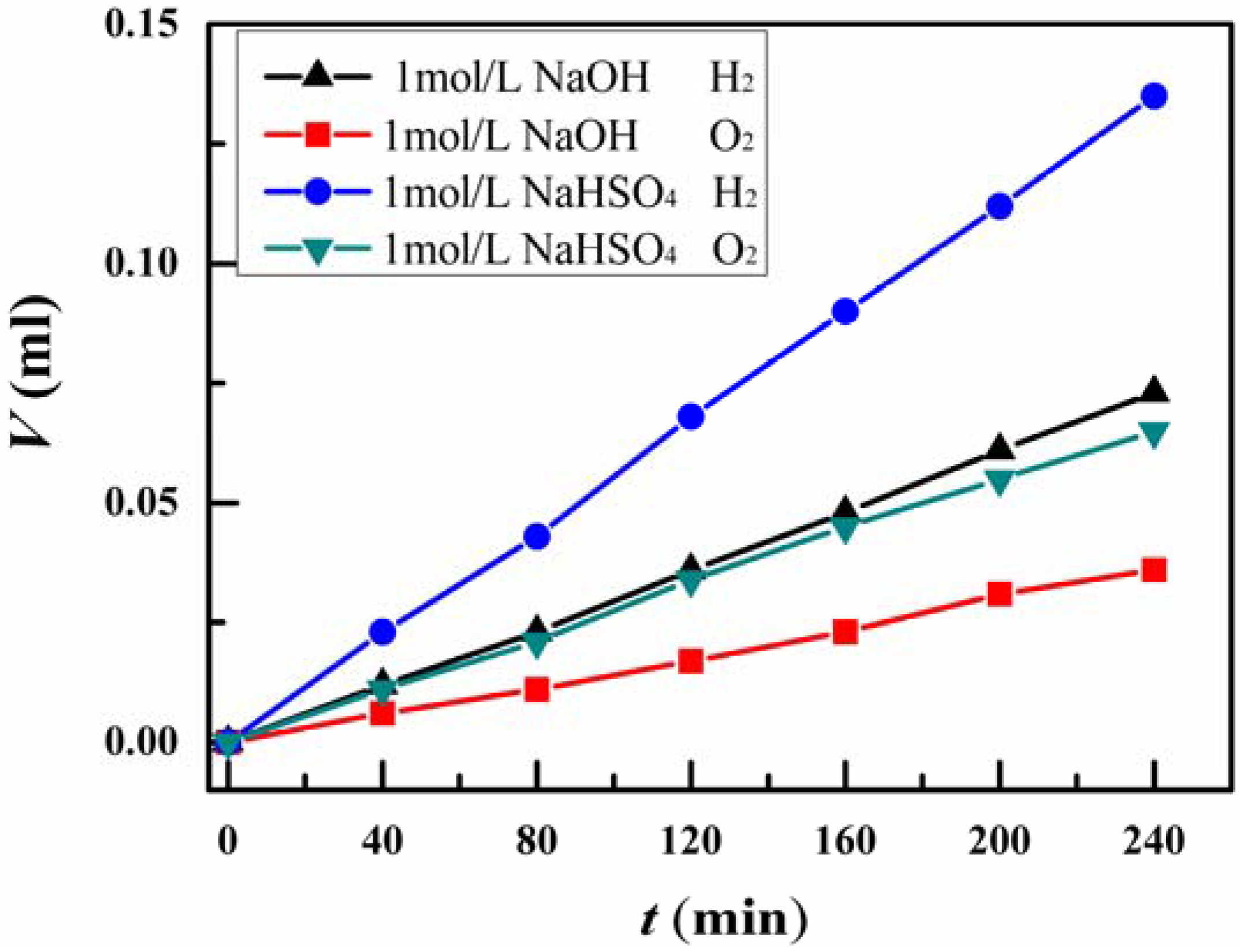
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Hansen, J.; Johnson, D.; Lacis, A.; Lebedeff, S.; Lee, P.; Rind, D.; Russell, G. Climate impact of increasing atmospheric carbon dioxide. Science 1981, 213, 957–966. [Google Scholar]
- Sherif, S.A.; Barbir, F.; Veziroglu, T.N. Wind energy and the hydrogen economy—review of the technology. Solar Energy 2005, 78, 647–660. [Google Scholar]
- Das, D.; Nejat, V.T. Hydrogen production by biological processes: a survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar]
- Kazuhior, S.; Kazuaki, M.; Ryu, A.; Yoshimoto, A.; Hironori, A. A new photocatalytic water splitting system under visiable light irradiation mimicking a Z-scheme mechanism in photosynthesis. J. Photochem. Photobiol. 2002, 148, 71–77. [Google Scholar]
- Grätzel, M. Artificial photosynthesis: water cleavage into hydrogen and oxygen by visible light. Acc. Chem. Res. 1981, 14, 376–384. [Google Scholar]
- Frank, E.O. Inoranic materials as Catalysts for photochemical splitting of water. Chem. Mater. 2008, 20, 35–54. [Google Scholar]
- Agrafiotis, C.; Roeb, M.; Konstandopoulos, A.G.; Nalbandian, L.; Zaspalis, V.T.; Sattler, C.; Stobbe, P.; Steele, A.M. Solar water splitting for hydrogen production with monolithic reactors. Solar Energy 2005, 79, 409–421. [Google Scholar]
- Bard, A.J.; Fox, M.A. Artificial photosynthesis: Solar splitting of water to hydrogen and oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar]
- Kamen, M.D.; Gest, H. Evidence for a nitrogenase system in the photosynthetic bacterium rbodospirillum rubrum. Science 1949, 109, 560–560. [Google Scholar]
- Debabrata, D.; Veziroglu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar]
- Khanal, S.K.; Chen, W.-H.; Li, L.; Sung, S.H. Biological hydrogen production: effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar]
- Yu, H.Q.; Zhu, Z.H.; Hu, W.R.; Zhang, H.S. Hydrogen production from rice winery wastewater in an upflow anaerobic reactor by using mixed anaerobic cultures. Int. J. Hydrogen Energy 2002, 27, 1359–1365. [Google Scholar]
- Wakayama, T.; Nakada, E.; Asada, Y.; Miyake, J. Effect of light/dark cycle on bacterial hydrogen production by Rhodobacter sphaeroides RV. From hour to second range. Appl. Biochem. Biotechnol. 2000, 84, 431–440. [Google Scholar]
- Yoshiyuki, U.; Tatsushi, K.; Susumu, S.; Otsuka, S.; Morimoto, M. Biological production of hydrogen from cellulose by natural anaerobic microflora. J. Ferment. Bioeng. 1995, 97, 395–397. [Google Scholar]
- Lallart, M.; Inman, D.J.; Guyomar, D. Transient performance of energy harvesting strategies under constant force magnitude excitation. J. Intell. Mater. Syst. Struct. 2010, 21, 1279–1291. [Google Scholar]
- Yang, R.; Qin, R.; Li, C.; Zhu, G.; Wang, Z.L. Converting biomechanical energy into electricity by a muscle-movement-driven nanogenerator. Nano Lett. 2009, 9, 1201–1205. [Google Scholar]
- Hong, K.S.; Xu, H.-F.; Konishi, H.; Li, X.C. Direct water splitting through vibrating piezoelectric microfiber in water. J. Phys. Chem. Lett. 2010, 1, 997–1002. [Google Scholar]
- Roundy, S.; Write, P.K.; Rabaey, J. A study of low level vibrations as a power source for wireless sensor nodes. Comput. Commun. 2003, 26, 1131–1141. [Google Scholar]
- Fang, H.B.; Liu, J.Q.; Xu, Z.Y. Fabrication and performance of MEMS-based piezoelectric power generator for vibration energy harvesting. Microelectron. J. 2006, 37, 1280–1284. [Google Scholar]
- Shen, D.; Park, J.H. Micromachined PZT cantilever based on SOI structure for low frequency vibration energy harvesting. Sens. Actuat. A Phys. 2009, 154, 103–108. [Google Scholar]
- Ren, B.; Or, S.W.; Zhang, Y.-Y. Piezoelectric energy harvesting using shear mode 0.71Pb(Mg1/3Nb2/3)O3–0.29PbTiO3 single crystal cantilever. Appl. Phys. Lett. 2010, 96, 083502. [Google Scholar]
- Levie, R. The electrolysis of water. J. Electroanal. Chem. 1999, 476, 92–93. [Google Scholar]
- Appleby, A.J.; Crepy, G.; Jacquelin, J. High efficiency water electrolysis in alkaline solution. Int. J. Hydrogen Energy 1978, 3, 21–37. [Google Scholar]
- Jia, Y.; Luo, H.; Zhao, X.; Wang, F. Giant magnetoelectric response from a piezoelectric/magnetostrictive laminated composite combined with a piezoelectric transformer. Adv. Mater. 2008, 20, 4776–4779. [Google Scholar]
- Connelly, K.A.; Hicham, I. The photoreaction of TiO2 and Au/TiO2 single crystal and power surfaces with organic adsorbates. Emphasis on hydrogen production from renewables. Green Chem. 2012, 14, 260–280. [Google Scholar]
- Bowker, M. Sustainable hydrogen production by the application of ambient temperature photocatalysis. Green Chem. 2011, 13, 2235–2246. [Google Scholar]
- Kurihara, T.; Hiroaki, O.; Miseki, Y.; Kato, H.; Kudo, A. Highly efficient water splitting over K3Ta3B2O12 photocatalyst without loading cocatalyst. Chem. Lett. 2006, 35, 274–275. [Google Scholar]
- Mall, S.; Hsu, T.L. Electromechanical fatigue behavior of graphite/epoxy laminate embedded with piezoelectric actuator. Smart Mater. Struct. 2000, 9, 78–84. [Google Scholar]
- Hong, K.-S.; Xu, H.; Konishi, H.; Li, X. Piezoelectrochemical effect: A new mechanism for azo dye decolorization in aqueous solution through vibrating piezoelectric microfibers. J. Phys. Chem. C. 2012, 116, 13045–13051. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, J.; Wu, Z.; Jia, Y.; Kan, J.; Cheng, G. Piezoelectric Bimorph Cantilever for Vibration-Producing-Hydrogen. Sensors 2013, 13, 367-374. https://doi.org/10.3390/s130100367
Zhang J, Wu Z, Jia Y, Kan J, Cheng G. Piezoelectric Bimorph Cantilever for Vibration-Producing-Hydrogen. Sensors. 2013; 13(1):367-374. https://doi.org/10.3390/s130100367
Chicago/Turabian StyleZhang, Jun, Zheng Wu, Yanmin Jia, Junwu Kan, and Guangming Cheng. 2013. "Piezoelectric Bimorph Cantilever for Vibration-Producing-Hydrogen" Sensors 13, no. 1: 367-374. https://doi.org/10.3390/s130100367
APA StyleZhang, J., Wu, Z., Jia, Y., Kan, J., & Cheng, G. (2013). Piezoelectric Bimorph Cantilever for Vibration-Producing-Hydrogen. Sensors, 13(1), 367-374. https://doi.org/10.3390/s130100367