Recent Advances in Sensing Oropharyngeal Swallowing Function in Japan
Abstract
:1. Introduction
2. Outline of Biomechanical Assessment of Swallowing-Related Organs
3. Sensing of Lip Movement
3.1. Lip Movement in Swallowing
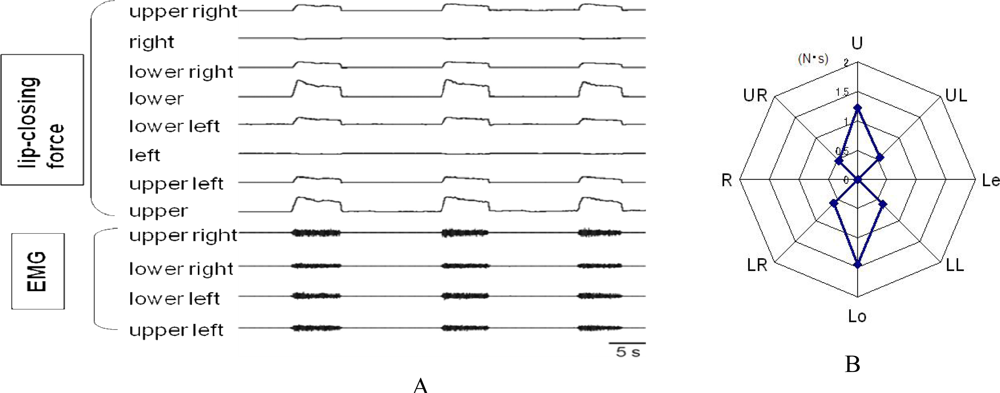
3.2. Assessment of Lip-Closing Force
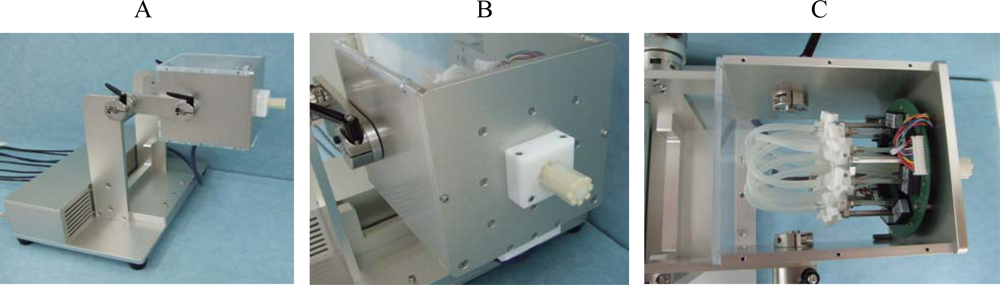
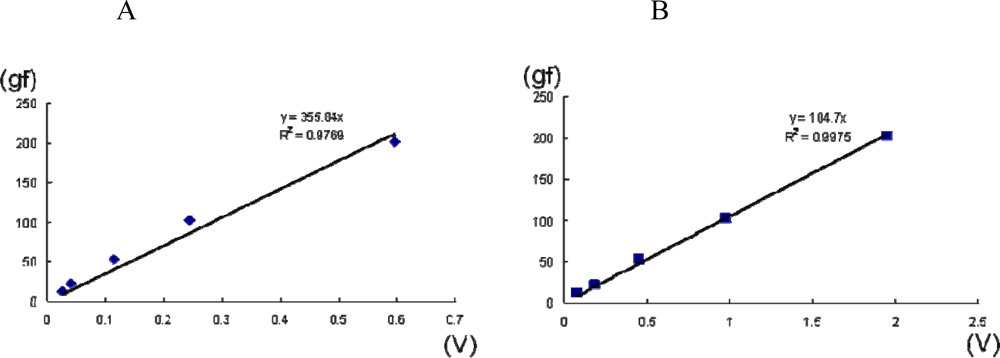
3.3. Novel Equipment: A System for Measuring Multi-Directional Lip-Closing Force
4. Sensing of Tongue Movement
4.1. Tongue Movement in Swallowing
4.2. Sensing Probe
4.3. Artificial Palate with Sensors
4.4. Sensor Sheet
5. Sensing of Laryngeal Movement
5.1. Laryngeal Movement in Swallowing
5.2. Motion Detection from the Skin Surface
5.3. Electric Impedance Measurement
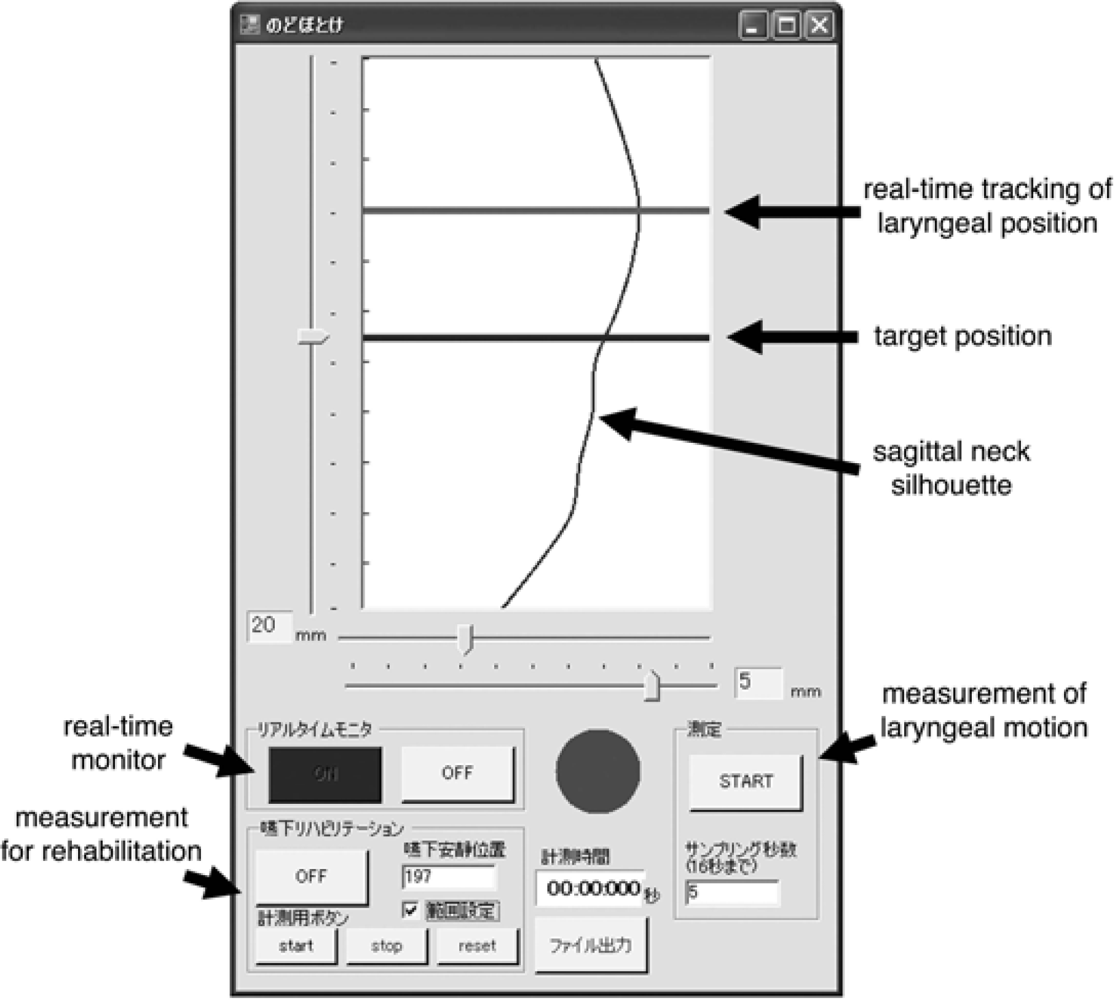
5.4. Sagittal Neck-Silhouette Measurement
6. Conclusions
Acknowledgments
References and Notes
- Robbins, J.; Barezi, S.R. Disorders of Swallowing. In Principles of Geriatric Medicine and Gerontology, 5th ed; Hazzard, W.R., Blass, J.P., Halter, J.B., Eds.; McGraw Hill Inc: New York, NY, USA, 2003; pp. 1193–1212. [Google Scholar]
- Ono, T.; Hori, K.; Tamine, K.; Maeda, Y. Evaluation of Tongue Motor Biomechanics During Swallowing / from Oral Feeding Models to Quantitative Sensing Methods. Jpn. Dent. Sci. Rev 2009. [Google Scholar] [CrossRef]
- Leopold, N.A.; Kagel, M.C. Swallowing, Ingestion and Dysphagia: a Reappraisal. Arch. Phys. Med. Rehabil 1983, 64, 371–373. [Google Scholar]
- Feinberg, M.J. Radiographic Techniques and Interpretation of Abnormal Swallowing in Adult and Elderly Patients. Dysphagia 1993, 8, 356–358. [Google Scholar]
- Palmer, J.B.; Rudin, N.J.; Lara, G.; Crompton, A.W. Coordination of Mastication and Swallowing. Dysphagia 1992, 7, 187–200. [Google Scholar]
- Palmer, J.B.; Hiiemae, K.M. Ingestion of Oral and Pharyngeal Bolus Propulsion—A New Model for the Physiology of Swallowing. Jpn. J. Dysphag. Rehabil 1997, 1, 15–30. [Google Scholar]
- Ono, T.; Hori, K.; Nokubi, T.; Sumida, A.; Furukawa, S. Evaluation of Mastication and Swallowing of Gummy Jelly by using Digital Subtruction Angiography. Dent. Jpn. (Tokyo) 2005, 40, 57–60. [Google Scholar]
- Ono, T.; Hori, K.; Ikebe, K.; Nokubi, T.; Nago, S.; Kumakura, I. Factors Influencing Eating Ability of Old In-patients in a Rehabilitation Hospital in Japan. Gerodontology 2003, 20, 24–31. [Google Scholar]
- Yoshida, Y.; Otsuka, A.; Sakai, S.; Manabe, M.; Kito, Y.; Ono, T.; Kamiya, S.; Tsuchiya, T. The Force of Lip Closure in Children (1) The Relationship Between the Force of Lip Closure and Age. Jpn. J. Ped. Dent 2004, 42, 436–440, (in Japanese, abstract in English).. [Google Scholar]
- Nakao, M. Relevance of Lip Pressure to Post-treatment Stability–Part I: Development of a Lip Pressure Gauge and Measurement of Maximum Lip Pressure in Subjects with Normal Occlusion. Jpn. J. Jaw Deform 2007, 17, 254–258, (in Japanese, abstract in English).. [Google Scholar]
- Kinoshita, M.; Kambara, T.; Kawamoto, T. Maximum Lip Closure Pressure in Growing Children. Shika Igaku 2007, 70, 30–34, (in Japanese, abstract in English).. [Google Scholar]
- Fukui, T.; Kikutani, T.; Tamura, F.; Inaba, S. Relationship Between Vertical Labial-closing Pressure and Age. Jpn. J. Dysphag. Rehabil 2005, 9, 265–271, (in Japanese, abstract in English).. [Google Scholar]
- Miura, H.; Kariyasu, M.; Sumi, Y.; Yamasaki, K. Labial Closure Force, Activities of Daily Living, and Cognitive Function in Frail Elderly Persons. Nippon Ronen Igakkai Zasshi 2008, 45, 520–525, (in Japanese, abstract in English).. [Google Scholar]
- Ono, T.; Yoshida, Y.; Otsuka, A.; Aoyama, T.; Murata, N.; Aizawa, S.; Achiwa, T.; Kamiya, S.; Tsuchiyta, T. The Force Lip Closure in Children (2) The Relationship Between the Force of Lip Closure and Occlusion. Jpn. J. Ped. Dent 2004, 42, 441–446, (in Japanese, abstract in English).. [Google Scholar]
- Jung, M.H.; Yang, W.S.; Nahm, D.S. Effects of Upper Lip Closing Force on Craniofacial Structures. Am. J. Orthod. Dentofacial. Orthop 2003, 123, 58–63. [Google Scholar]
- Ruan, W.H.; Su, J.M.; Ye, X.W. Pressure from the Lips and the Tongue in Children with Class III Malocclusion. J. Zhejiang Univ. Sci. B 2007, 8, 296–301. [Google Scholar]
- Otsuka, A. The Activity of Orbicularis Oris Muscle During Lip Closure and Mastication in Male Adults—Comparison of the Muscle Activity Amount Among Different Occlusion Types. Aichi Gakuin Dent. Sci 2006, 44, 187–197, (in Japanese, abstract in English).. [Google Scholar]
- Wakabayashi, K. Relationship between Maxillofacial Morphology and Perioral Muscle Function. Orthod. Waves 2002, 61, 454–465. [Google Scholar]
- Sabashi, K.; Kondo, S. Influence of Frenulum Labii Superioris on Lip Closure in Children. Jpn. J. Ped. Dent 2004, 42, 661–667, (in Japanese, abstract in English).. [Google Scholar]
- Thuer, U.; Ingervall, B. Pressure from the Lips on the Teeth and Malocclusion. Am. J. Orthod. Dentofacial Orthop 1986, 90, 234–242. [Google Scholar]
- Kogue, Y.; Igari, K.; Kudo, A.; Goto, N.; Hozumi, Y.; Mayanagi, H. The Relationship Between the Force of Lip Closure and Masticatory Function in Young Children. Jpn. J. Ped. Dent 2007, 45, 48–64, (in Japanese, abstract in English).. [Google Scholar]
- Kusumoto, O. Changes with Time in Myofunction through Lip Exercise. J. Kyushu Dent. Sci 1992, 46, 355–372, (in Japanese, abstract in English).. [Google Scholar]
- Yawaka, Y.; Shirakawa, T.; Nomura, Y.; Oguchi, H. Measurement of Upper Lip Pressure in the Course of Myofunctional Therapy: The Relationship between Values of Upper Lip Pressures and Electromyogram of m. Orbicuralis Oris in Swallowing Condition. Jpn. J. Ped. Dent 1992, 30, 893–903, (in Japanese, abstract in English).. [Google Scholar]
- Tomita, K.; Okano, T.; Tamura, F.; Mukai, Y. The Relationship between Labial Pressure during Swallowing and Maximum Labial Pressure—A Comparison of Adults and Elderly Subjects. Jpn. J. Dysphag. Rehabil 2002, 6, 16–26, (in Japanese, abstract in English).. [Google Scholar]
- Barlow, S.M.; Burton, M. Ramp-and-hold Force Control in the Upper and Lower Lips: Developing New Neuromotor Assessment Applications in Traumatically Brain Injured Adults. J. Speech Hear. Res 1990, 33, 660–675. [Google Scholar]
- Barlow, S.M.; Rath, E.M. Maximum Voluntary Closing Forces in the Upper and Lower Lips of Humans. J. Speech Hear. Res 1985, 28, 373–376. [Google Scholar]
- Barlow, S.M.; Netsell, R. Differential Fine Force Control of the Upper and Lower Lips. J. Speech Hear. Res 1986, 29, 163–169. [Google Scholar]
- Barlow, S.M.; Bradford, P.T. Comparison of Perioral Reflex Modulation in the Upper and Lower Lip. J. Speech Hear. Res 1996, 39, 55–75. [Google Scholar]
- Masuda, Y.; Kato, T.; Sabashi, K.; Nakatsuka, K.; Kanayama, H.; Fujimoto, M.; Morimoto, T. Characteristic of Multidirectional Lip-closing Force Measured by a New Apparatus. J. Dent. Res 2006, 85. Special Issue B, #0392.. [Google Scholar]
- Shaker, R.; Cook, I.J.; Dodds, W.J.; Hogan, W.J. Pressure-flow Dynamics of the Oral Phase of Swallowing. Dysphagia 1988, 3, 79–84. [Google Scholar]
- Ku, D.N.; Ma, P.P.; McConnel, F.M.; Cerenko, D. A Kinematic Study of the Oropharyngeal Swallowing of a Liquid. Ann. Biomed. Eng 1998, 18, 655–669. [Google Scholar]
- Kahrilas, P.J.; Lin, S.; Logemann, J.A.; Ergun, G.A.; Facchini, F. Deglutitive Tongue Action—Volume Accommodation and Bolus Propulsion. Gastroenterology 1993, 104, 152–162. [Google Scholar]
- Dantas, R.O.; Kern, M.K.; Massey, B.T.; Dodds, W.J.; Kahrilas, P.J.; Brasseur, J.G.; Cook, I.J.; Lang, I.M. Effect of Swallowed Bolus Variables on Oral and Pharyngeal Phases of Swallowing. Am. J. Physio 1990, 258, G675–G681. [Google Scholar]
- Pouderoux, P.; Kahrilas, P.J. Deglutitive Tongue Force Modulation by Volition, Volume, and Viscosity in Humans. Gastroenterology 1995, 108, 1418–1426. [Google Scholar]
- Nicosia, M.A.; Hind, J.A.; Roecker, E.B.; Carnes, M.; Doyle, J.; Dengel, G.A.; Robbins, J. Age Effects on the Temporal Evolution of Isometric and Swallowing Pressure. J. Gerontol. A. Biol. Sci. Med. Sci 2000, 55, M634–M640. [Google Scholar]
- Hayashi, R.; Tsuga, K.; Hosokawa, R.; Yoshida, M.; Sato, Y.; Akagawa, Y. A Novel Handy Probe for Tongue Pressure Measurement. Int. J. Prosthodont 2002, 15, 385–388. [Google Scholar]
- Utanohara, Y.; Hayashi, R.; Yoshikawa, M.; Yoshida, M.; Tsuga, K.; Akagawa, Y. Standard Values of Maximum Tongue Pressure Taken Using Newly Developed Disposable Tongue Pressure Measurement Device. Dysphagia 2008, 23, 286–290. [Google Scholar]
- Yoshida, M.; Kikutani, T.; Tsuga, K.; Utanohara, Y.; Hayashi, R.; Akagawa, Y. Decreased Tongue Pressure Reflects Symptom of Dysphagia. Dysphagia 2006, 21, 61–65. [Google Scholar]
- Ono, T.; Kumakura, I.; Arimoto, M.; Hori, K.; Dong, J.; Iwata, H.; Nokubi, T.; Tsuga, K.; Akagawa, Y. Influence of Bite Force and Tongue Pressure on Oro-Pharyngeal Residue in the Elderly. Gerodontology 2007, 24, 143–150. [Google Scholar]
- Murayama, M.; Mukai, Y.; Kaneko, Y. Effect of Trunk and Head Posture on Lingual Pressure up to the Palate During Swallowing. JJSDH 1996, 17, 134–148, (in Japanese, abstract in English).. [Google Scholar]
- Furuya, J. Effects of Wearing Complete Dentures on Swallowing in Elderly. J. Jpn. Stomatol. Soc 1999, 66, 361–369, (in Japanese, abstract in English).. [Google Scholar]
- Saito, Y.; Motoyoshi, Y.; Arimoto, M.; Itoi, K.; Shimazaki, T.; Namura, S. Tongue Pressure on the Palate During Deglutition. Orthod. Waves 1999, 58, 303–308, (in Japanese, abstract in English).. [Google Scholar]
- Kitaoka, N.; Komoda, J.; Ichikawa, T.; Ishikawa, M.; Nagao, K.; Kawano, F.; Hada, M. Observation of Tongue Pressure to Palate at Swallowing: Comparison Between Young Dentition and Elderly Complete Denture Wearers. J. Jpn. Prosthodont. Soc 2000, 44, 379–385, (in Japanese, abstract in English).. [Google Scholar]
- Yokoyama, M.; Michiwaki, Y.; Ozawa, M.; Kinumatsu, Y.; Michi, K. A Basic Study on Tongue Pressure during Normal Swallowing. Assessment of the Reproducibility and Individual Differences. J. Jpn. Stomatol. Soc 2000, 49, 171–176, (in Japanese, abstract in English).. [Google Scholar]
- Nagao, K.; Kitaoka, N.; Kawano, F.; Komoda, J.; Ichikawa, T. Influence of Changes in Occlusal Vertical Dimension on Tongue Pressure to Palate during Swallowing. Prosthodont. Res. Pract 2002, 1, 16–23. [Google Scholar]
- Yorozuya, A.; Tamura, F.; Mukai, Y. Evaluation of Shifting of Linguopalatal Pressure—Effect of Palatal Plate Thickness on Lingual Movement During Swallowing. Jpn. J. Dysphag. Rehabil 2002, 6, 93–103, (in Japanese, abstract in English).. [Google Scholar]
- Ono, T.; Hori, K.; Nokubi, T. Pattern of Tongue Pressure on Hard Palate during Swallowing. Dysphagia 2004, 19, 259–264. [Google Scholar]
- Ono, T.; Iwata, H.; Hori, K.; Tamine, K.; Kondoh, J.; Hamanaka, S.; Maeda, Y. Evaluation of Tongue-jaw-swallow Related Muscle Coordination during Voluntarily Triggered Swallow. Int. J. Prosthodont 2009, 22, 151–156. [Google Scholar]
- Hori, K.; Ono, T.; Nokubi, T. Coordination of Tongue Pressure and Jaw Movement in Mastication. J. Dent. Res 2006, 85, 187–191. [Google Scholar]
- Hori, K.; Ono, T.; Iwata, H.; Nokubi, T.; Kumakura, I. Tongue Pressure against Hard Palate during Swallowing in Post-Stroke Patients. Gerodontology 2005, 22, 227–233. [Google Scholar]
- Ono, T.; Hori, K.; Tamine, K.; Shiroshita, N.; Kondoh, J.; Maeda, Y. Application of Tongue Pressure Measurement to Rehabilitation of Dysphagic Patients with Prosthesis. Prosthodont. Res. Pract 2008, 7, 240–242. [Google Scholar]
- Hori, K.; Ono, T.; Tamine, K.; Kondo, J.; Hamanaka, S.; Maeda, Y.; Dong, J.; Hatsuda, M. A Newly Developed Sensor Sheet for Measuring Tongue Pressure in Swallowing. J. Prosthodont. Res 2009, 53, 28–32. [Google Scholar]
- Malacaria, C. A Thin, Flexible, Matrix Based Sensor. Sensors Mag 1998, 102–104. [Google Scholar]
- Pehlivan, M.; Yüceyar, N.; Ertekin, C.; Celebi, G.; Ertaş, M.; Kalayci, T.; Aydoĝdu, I. An Electronic Device Measuring the Frequency of Spontaneous Swallowing: Digital Phagometer. Dysphagia 1996, 11, 259–264. [Google Scholar]
- Takagi, T.; Miyaoka, Y.; Hashima, K.; Matsunaga, K.; Yamada, Y. Analysis of Swallowing Movement using a Simple and Safe Device. J. Jpn. Soc. Stomatognath. Funct 2001, 8, 25–30. [Google Scholar]
- Reddy, N.P.; Katakam, A.; Gupta, V.; Unnikrishnan, R.; Narayanan, J.; Canilang, E.P. Measurements of Acceleration during Videofluorographic Evaluation of Dysphagic Patients. Med. Eng. Phys 2000, 22, 405–412. [Google Scholar]
- Toyosato, A.; Nomura, S. The Analysis of the Swallow Dynamic Phase using Piezo-Electric Pulse Transducer. J. Jpn. Soc. Stomatognath. Funct 2004, 10, 162–163. (in Japanese).. [Google Scholar]
- Toyosato, A.; Nomura, S.; Igarashi, A.; Ii, N.; Nomura, A. A Relation between the Piezo-Electric Pulse Transducer Waveforms and Food Bolus Passage during Pharyngeal Phase. Prosthodont. Res. Pract 2007, 6, 272–275. [Google Scholar]
- Toyosato, A.; Ueda, K.; Nomura, S. Examination of Swallowing Evaluation Method at the Bedside. JADS 2007, 26, 42–46, (in Japanese, abstract in English).. [Google Scholar]
- Yamamoto, Y.; Nakamura, T.; Seki, Y.; Utsuyama, K.; Akashi, K.; Jikuya, K. Neck Electrical Impedance for Measurement of Swallowing. Trans. of IEE 1998, 118-A(3), 210–216, (in Japanese, abstract in English).. [Google Scholar]
- Kusuhara, T.; Nakamura, T.; Yamamoto, Y.; Seki, Y.; Akashi, K.; Jikuya, K. Swallowing Evaluation using Parameter Analysis of IPG. Technical Report of IEICE 1998, MBE98-44,. 15–21, (in Japanese, abstract in English).. [Google Scholar]
- Hayashi, T.; Kaneko, H.; Nakamura, Y.; Ishida, T.; Takahashi, H.; Yamada, Y.; Michimi, N.; Nomura, S. Relationship between Rice-Gruel Properties and Swallowing Motion—Evaluation by Simultaneous Measurement of Larynx Movement, Electromyogram and Swallowing Sound. Jpn. J. Dysphag. Rehabil 2002, 6, 73–81, (in Japanese, abstract in English).. [Google Scholar]
- Murayama, A.; Hayashi, T.; Nakamura, Y.; Michimi, N. Improvement of Larynx-Motion Measurement System SFN-2. A. Technical Report of IEICE,. 2005, MBE2004-111,. 61–64, (in Japanese, abstract in English).. [Google Scholar]
- Fujita, S.; Hayashi, T.; Nakamura, Y.; Michimi, N. Analysis of Laryngeal Elevation during Swallowing Using Skin-contour Change in Anterior Region of the Neck. Proceedings of Program and Abstracts of the 13th Annual Meeting of the Japanese Society of Dysphagia Rehabilitation, Tokyo, Japan, 2007; p. 226. (in Japanese)..
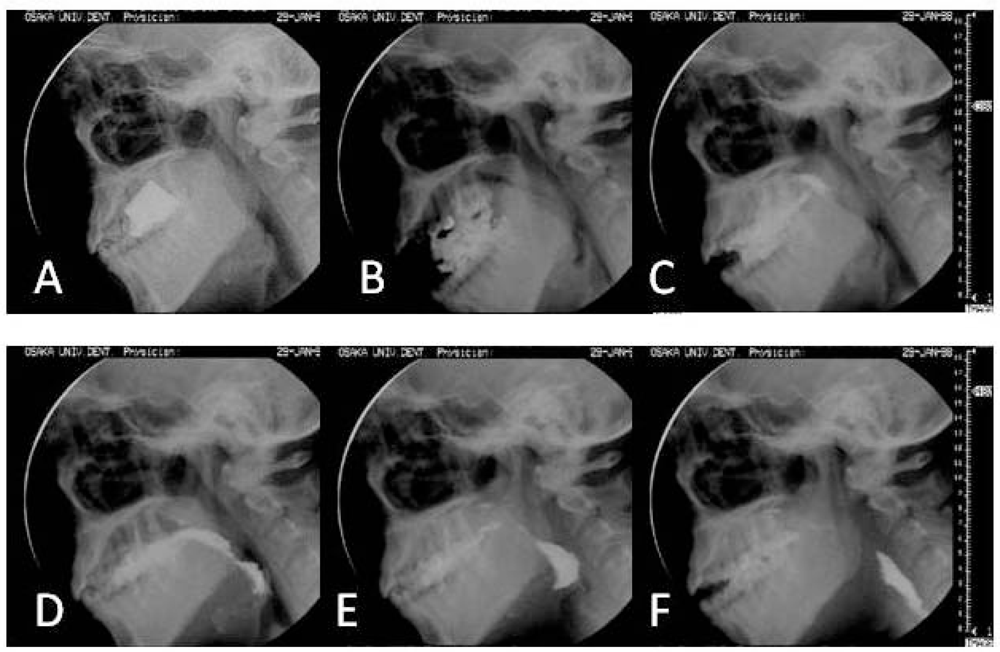




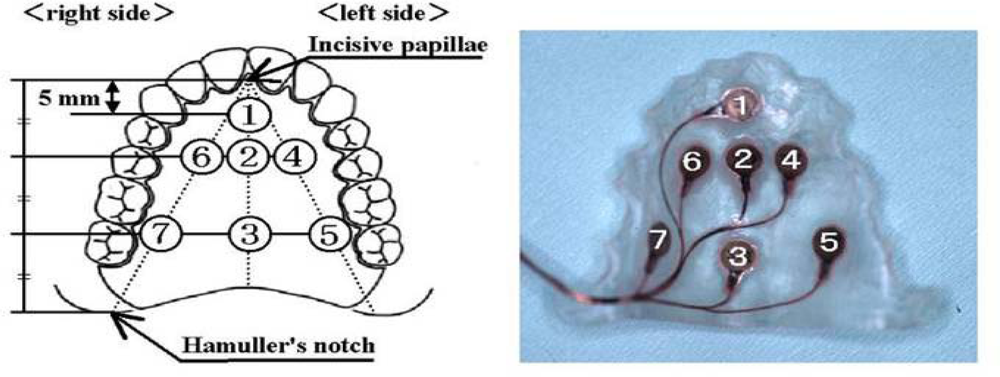

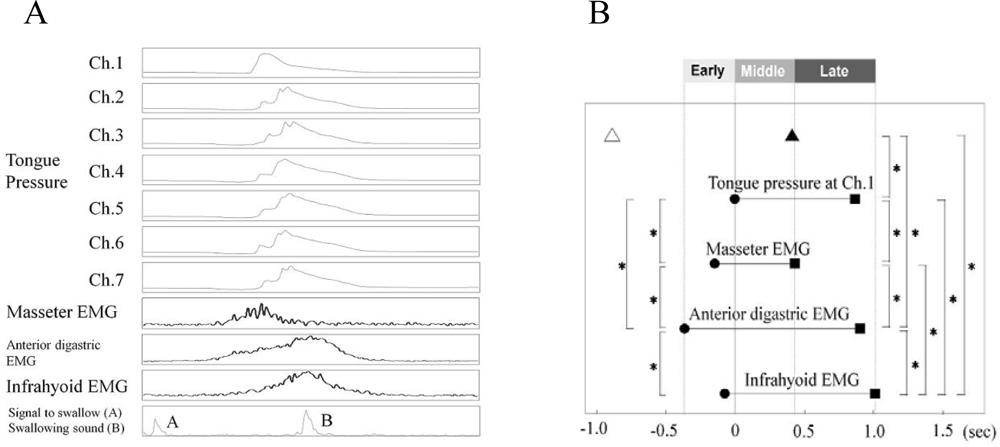

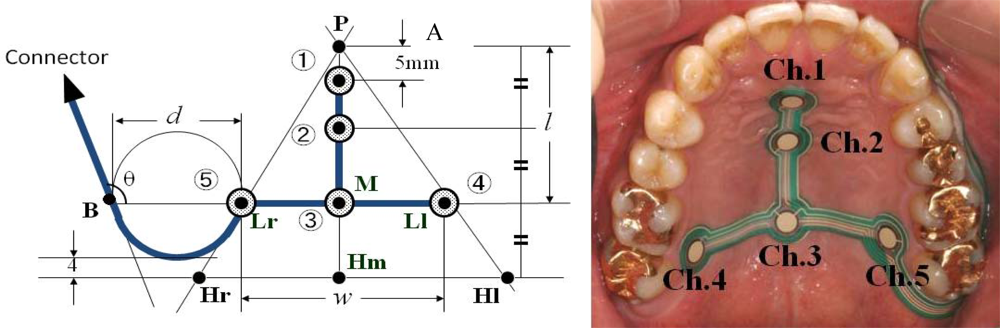
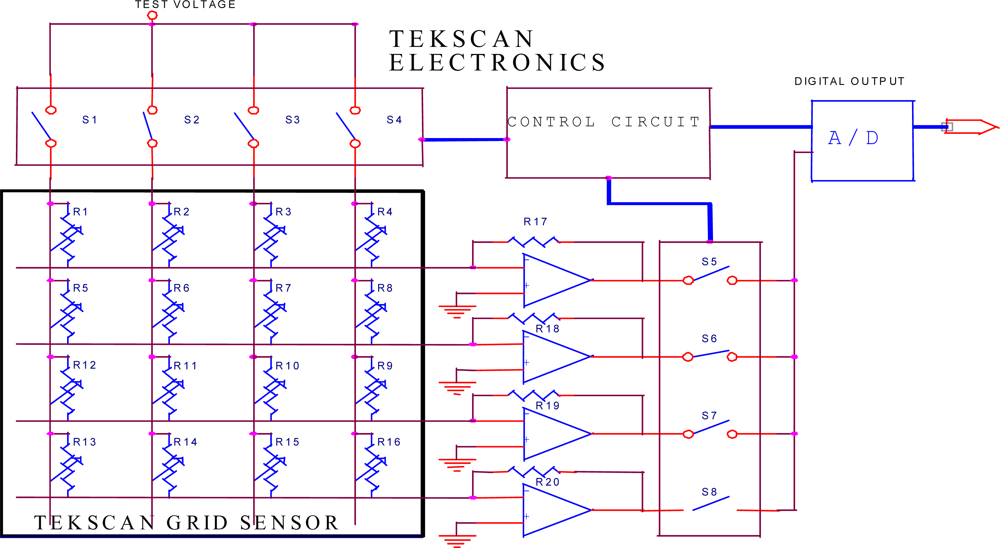


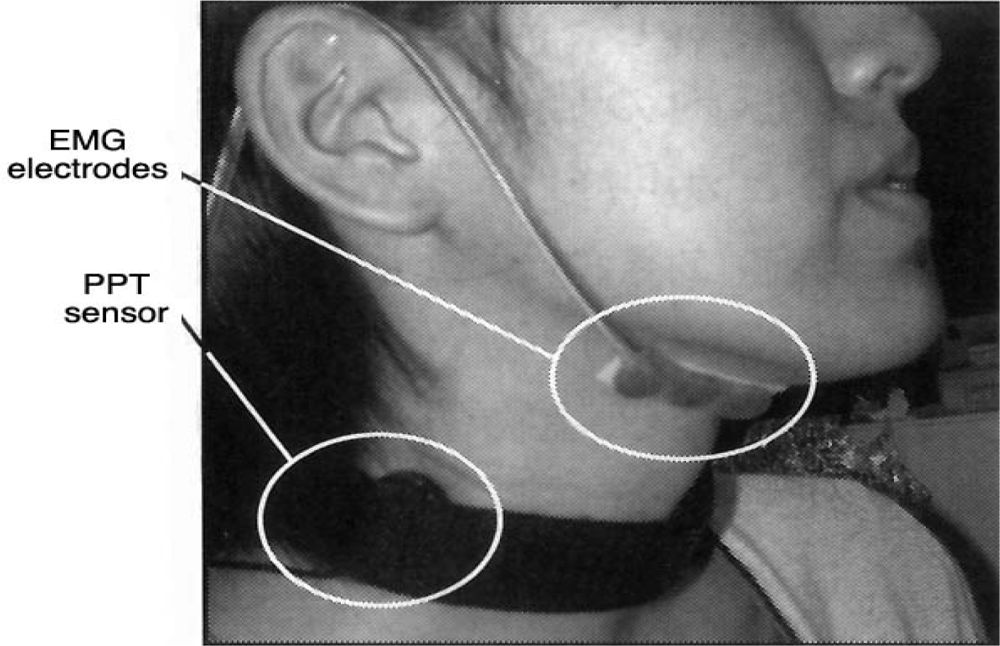
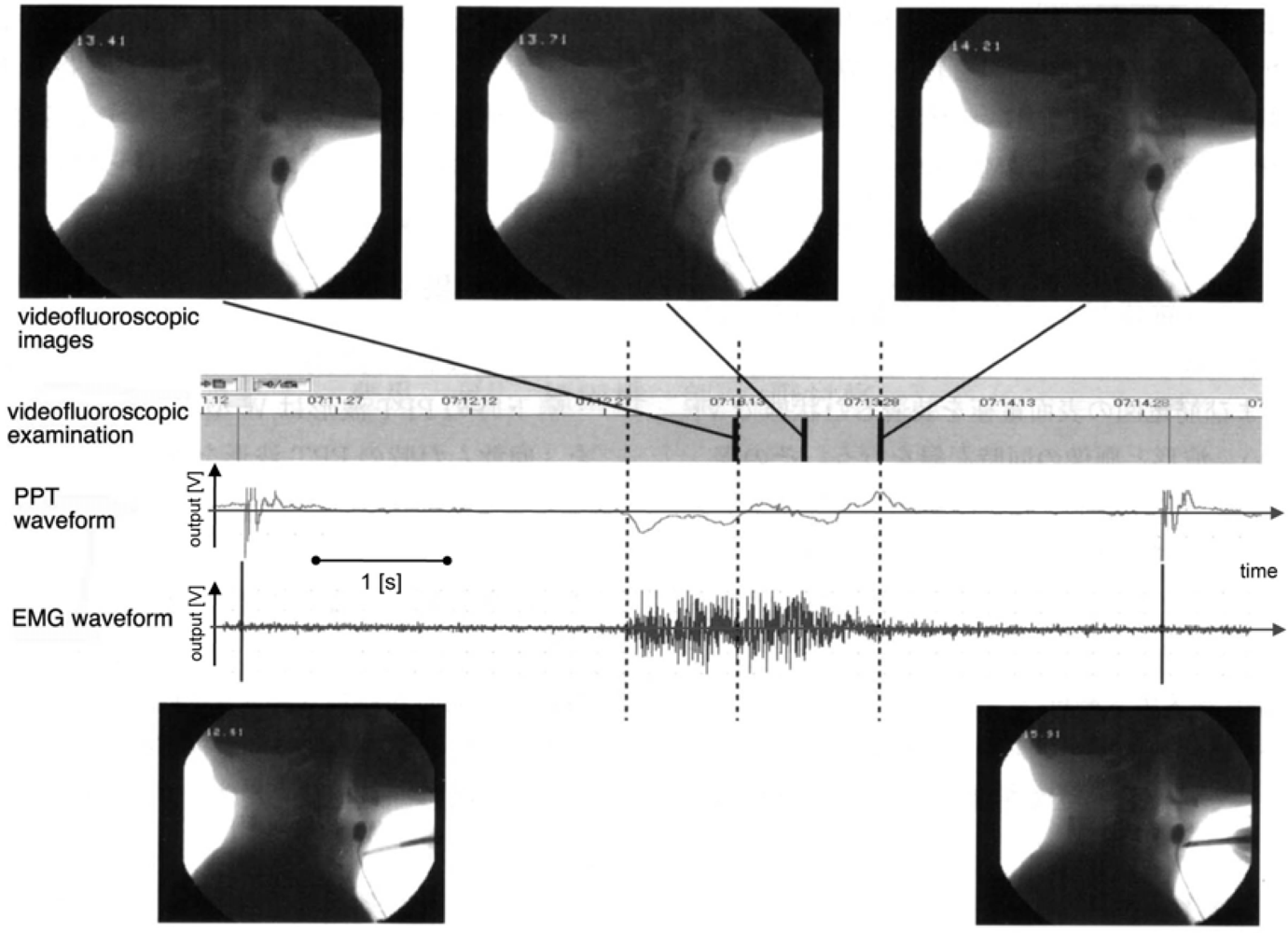

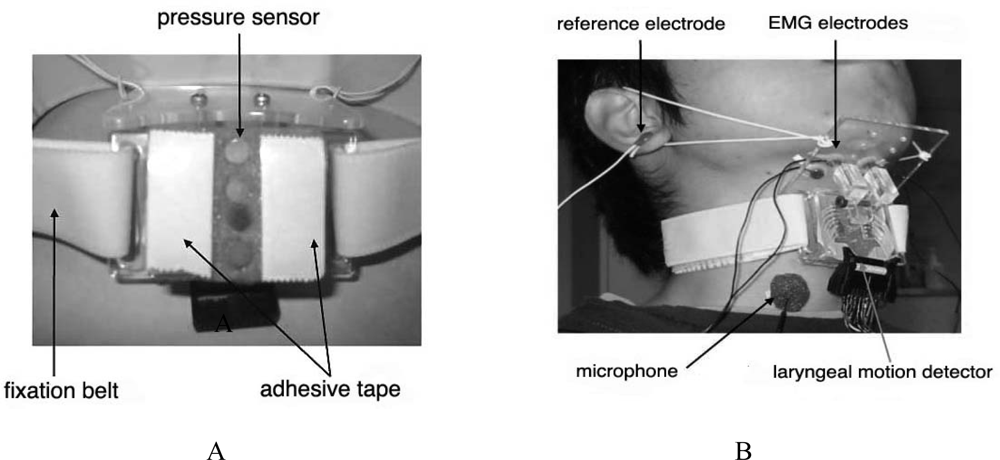


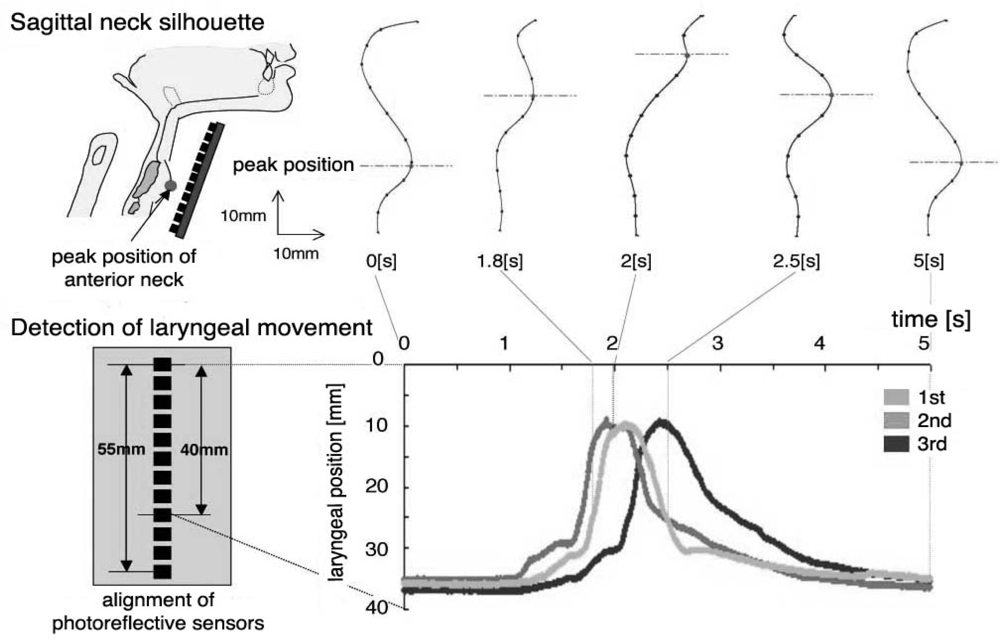
| Ingestion |
| The mouth opens widely as the tongue drops down to make room in the mouth for the bite of food to enter. |
| Stage I transport |
| The tongue moves posteriorly in the oral cavity, carrying the bite of food on its surface, and rotates to deliver the food to the chewing surface of the molars. |
| Food processing |
| Once food reaches the molar region, it is softened and reduced in size as it is chewed, manipulated, and mixed with saliva. During chewing, jaw movement is linked to cyclic motions of the tongue and hyoid bone. The tongue moves upward and downward in association with jaw closing and opening, and also forward and backward. These horizontal motions of the tongue are out of phase with its vertical motions. |
| Stage II transport |
| The portion of food bolus is propelled through the faucial pillars into the oropharynx where it is stored in anticipation of swallowing. Some food remains in the oral cavity, where processing continues. During Stage II transport, cyclic motions of the jaw and tongue continue in a linked pattern. In some cycles, food is pulled backward on the surface of the tongue, while in other cycles the tongue pushes it against the palate and squeezes it back along the palate and into the oropharynx. |
| Hypopharyngeal transit |
| As the pharyngeal swallow begins, the food bolus in the oropharynx is propelled through the hypopharynx into the esophagus. Bolus propulsion is produced primarily by backward thrust of the tongue onto the oropharynx. |
©2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/)
Share and Cite
Ono, T.; Hori, K.; Masuda, Y.; Hayashi, T. Recent Advances in Sensing Oropharyngeal Swallowing Function in Japan. Sensors 2010, 10, 176-202. https://doi.org/10.3390/s100100176
Ono T, Hori K, Masuda Y, Hayashi T. Recent Advances in Sensing Oropharyngeal Swallowing Function in Japan. Sensors. 2010; 10(1):176-202. https://doi.org/10.3390/s100100176
Chicago/Turabian StyleOno, Takahiro, Kazuhiro Hori, Yuji Masuda, and Toyohiko Hayashi. 2010. "Recent Advances in Sensing Oropharyngeal Swallowing Function in Japan" Sensors 10, no. 1: 176-202. https://doi.org/10.3390/s100100176
APA StyleOno, T., Hori, K., Masuda, Y., & Hayashi, T. (2010). Recent Advances in Sensing Oropharyngeal Swallowing Function in Japan. Sensors, 10(1), 176-202. https://doi.org/10.3390/s100100176




