Abstract
Understanding drivers of biodiversity is a long-standing goal of basic and applied ecological research. In riverine systems, there remains a critical need to identify these drivers as efforts to manage and protect rivers grow increasingly desperate in the face of global change. We explored one commonly cited potential driver of riverine biodiversity, stream size (e.g., stream order, watershed area, width), using a systematic literature review paired with an analysis of broad-scale macroinvertebrate and fish communities. Of the 165 papers reviewed, we found mostly positive, but no universal, relationship between biodiversity and stream size despite inconsistent use of over 30 measures of stream size. One-third of studies failed to report explanatory mechanisms driving biodiversity–stream size relationships. Across over 4000 macroinvertebrate and fish samples from 1st–8th order streams in the contiguous USA, our analysis showed biodiversity (Shannon diversity, functional diversity, beta diversity) generally increased with measures of stream size. However, because of inconsistent and generally weak relationships between biodiversity and stream size across organismal groups, we emphasize the need to look beyond simple physical stream size measures to understand and predict riverine biodiversity, and strongly suggest that studies search for more mechanistic explanations of biodiversity patterns in lotic systems.
1. Introduction
Understanding patterns of biodiversity across landscapes is a fundamental goal of both basic and applied ecological research [1,2]. Identifying the drivers of biodiversity patterns is also necessary for effective management of natural systems, and often forms foundational assumptions of many management and restoration programs [3,4]. In freshwater systems, the need to understand drivers of diversity is amplified by human reliance on these systems for drinking water, food and recreation, and by increasing anthropogenic stressors that lead to their degradation. However, biodiversity in freshwater ecosystems is among the most threatened of any system [5]. River (lotic) ecosystems are among the most affected because they are intricately linked with surrounding landscapes and therefore susceptible to alteration of landscapes throughout their watersheds [6]. The current and future estimated declines of aquatic organisms due to climate change and anthropogenic alterations are substantial [7,8,9]. These losses come at a time when lotic ecologists are still scrambling to identify the physical and biological processes that determine and predict patterns in biodiversity. Additionally, the evaluation of water quality and subsequent management decisions are often based on assumed relationships between biodiversity and local environmental drivers [10,11,12].
Stream size is often cited as a major driver of biodiversity in lotic ecosystems [13,14,15,16]. Streams generally grow larger from their origins at headwaters, forming ever wider and deeper channels as tributaries merge and the amount of water carried in a channel increases. One of the foundational works of stream ecology, The River Continuum Concept (RCC) [17], has long provided a framework for studying the continually changing gradient of physical and biological conditions that accompanies this accumulation in discharge from headwaters to large rivers. Other changes along this same gradient of increased discharge include decreasing topographic gradient, increasing depth and width, shifts in substrate type from coarser to finer, increased openness of the riparian canopy, changes in trophic structure as the energy base shifts from the allochtonous inputs of leaf litter to a trophic structure driven more by autotrophic production, and concomitant shifts of stream biota in response to all of these physical factors [17]. The original RCC dealt only tangentially with the relationship between biodiversity and stream size, though it did offer a prediction of maximum total biotic diversity at streams of intermediate size as a result of maximum temperature variability and maximum productivity:respiration [17]. Decades of work have added layers of complexity and understanding to this general RCC concept, solidifying the view that changes in stream size were major drivers of processes in riverine systems (e.g., [18,19,20,21,22]); therefore, it is no surprise that stream size is often invoked as a major predictor or determinant of biodiversity in riverine systems.
Studies that investigate the relationship between some measure of stream size and biodiversity are common, with the general impression that biodiversity increases with stream size [23,24,25,26]. A variety of mechanistic explanations for increased biodiversity with increasing stream size have been proposed. Among these explanations are increased habitat size or area [13,27,28,29], increasing habitat complexity [30,31,32,33], habitat stability [14,34], effects of temperature or temperature variability [35,36,37], effects of flow variability [38] and an increasingly complex trophic base as nutrients and particulates from allochthonous decomposition are transported downstream to mix with autochtonous production [22]. It is also expected that many of these mechanisms may function interactively and that different mechanisms will drive these patterns across different taxonomic and functional groups of organisms, and that mechanism will vary across spatial scale [39,40,41].
However, an examination of ecological literature, such as the review described in this paper, reveals several factors that can potentially lead to a lack of clarity and some general misconceptions regarding the relationship between stream size and biodiversity. Perhaps the most obvious of these factors is that definitions and measures of stream size are disparate and varied, ranging from simple measures of stream width, to complex measures based on hydrologic models. Additionally, studies quantifying biodiversity–stream size relationships may have very different purposes. While some studies seek to mechanistically understand these relationships, other studies may simply use stream size as a convenient predictor, or a sort of master variable because stream size is so widely correlated with myriad other physical and biological variables. However, because of these numerous correlations, relationships between stream size and biodiversity are often difficult to explain mechanistically. Many studies do not attempt to provide these mechanistic links at all, and often, explorations of this relationship may simply be post hoc or the product of stream size being a relatively simple measure against which to predict diversity. Both of these issues—a diverse range of measurements for stream size, and lack of consideration of mechanistic underpinnings—are potentially problematic for ecological studies of riverine systems. Lack of consistent measurement reduces the ability to compare results across studies, and a lack of mechanistic underpinnings limits the utility of stream size–biodiversity relationships to reveal deeper insights regarding the factors that drive differences in biodiversity on landscapes. These issues could have significance for monitoring or management programs that use biodiversity–stream size relationships as benchmarks or indicators of water quality or management success. To date, there has been little synthesis regarding the use of stream size as a predictor or driver of biodiversity patterns in riverine systems. A thorough review and analysis of how stream size influences biodiversity could improve communication regarding drivers of biodiversity in lotic ecosystems, an increasingly important goal considering the global increases in anthropogenic alteration of these systems.
In this paper, we examined biodiversity–stream size relationships in lotic systems. This examination had two congruent parts. First, we performed a systematic review of existing literature with the goal of identifying commonalities and inconsistencies across the range of studies that include biodiversity–stream size relationships. Our literature review had several specific goals:
- Quantify how many published articles determined stream size is a predictor of biodiversity.
- Identify the different measures of stream size used in biodiversity studies.
- Identify the types of biodiversity that are being compared across stream size gradients.
- Determine if biodiversity–stream size relationships may consistently differ among studies, systems, and organismal groups, and whether there is consensus regarding the relationships in each of these categories.
- Catalog the mechanistic explanations proposed for biodiversity–stream size relationships, and how often these explanations are invoked.
Second, we performed an analysis of three existing regional stream datasets spanning the contiguous USA to empirically investigate several questions related to our literature review.
- Were patterns in our data analysis consistent with those identified in our literature review?
- Did biodiversity–stream size relationships change appreciably with different measures of stream size?
- Did biodiversity–stream size relationships differ among organismal groups?
The two sections of this paper were designed to complement each other by combining the broad, generalizable patterns of a literature review with a focused analysis of three large datasets. The datasets themselves are not intended to represent any sort of universally applicable patterns, but serve as case studies of biodiversity–stream size relationships.
2. Materials and Methods
2.1. A Systematic Review of Literature on Stream Size and Biodiversity
First, we systematically searched the Web of Science ([42], date of search 18 November 2016) using search terms and/or word strings related to biodiversity and stream size (Table 1) to gather relevant articles. Second, we further screened these articles by reading each article to determine how stream size was used as a potential determinant of biodiversity. We excluded articles if they did not directly mention a relationship between biodiversity and stream size or if specific relationships could not be determined from the paper. We did not exclude articles based on geographic location, sampling methods, or experimental design. We retained 165 out of 353 (46%) of articles for the literature review based on these criteria (Supplementary S1).

Table 1.
Search terms and/or word strings used in literature search for papers on biodiversity and stream size.
Third, we extracted information from the articles pertaining to two categories relevant to our literature review objectives. The first category included how biodiversity and stream size were measured, as well as what relationship, if any, was found among these terms in the study. We also recorded what mechanisms the authors proposed that explained relationships between stream size and biodiversity. The second category included information related to what type of organisms (e.g., macroinvertebrates, fish) were used in studies and scales of inference at which stream size effects on biodiversity were made. In this category, we also recorded the type of biodiversity measured referring to alpha diversity as local diversity, beta diversity as turnover between local communities, and gamma diversity as regional diversity.
2.2. An Analysis of Stream Size Measures That Influence Biodiversity
2.2.1. Datasets
We explored biodiversity–stream size relationships using datasets from the Maryland Biological Stream Survey (MBSS) and North Carolina Basinwide Monitoring Program (NCBMP) and US Environmental Protection Agency (USEPA) collected during monitoring efforts. These datasets included assessments of community structure for benthic macroinvertebrates and fish from 955, 1222, and 2123 stream/river sites in Maryland and North Carolina and the contiguous USA, respectively (Figure 1). In most cases, macroinvertebrates and fish were collected from the same sites. For the MBSS dataset, macroinvertebrate and fish collection sites were nested within 53 and 104 watersheds (hydrologic unit code (HUC) 10), [43]), respectively. For the NCBMP dataset, macroinvertebrate and fish sites were nested within 206 and 173 watersheds (HUC 10), For the USEPA dataset, macroinvertebrate and fish sites were nested within 1066 and 1126 watersheds (HUC 8), respectively. Stream size across all sites ranged from small headwater streams (1st Strahler order; minimum width: 0.35 m) to large-order rivers (8th Strahler order; maximum width: 2484 m). Stream size was based on Strahler stream order, watershed area, and stream width. Macroinvertebrates from the MBSS and USEPA dataset were identified to mostly the genus-level, whereas those in the NCBMP dataset were identified to either the species-level or genus-level. Fish were identified to the species-level in all datasets.
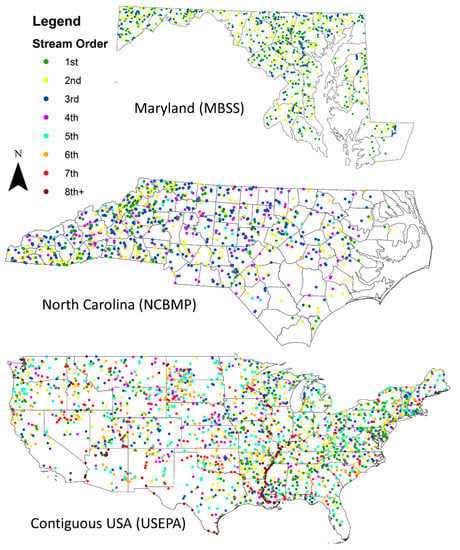
Figure 1.
Map of macroinvertebrate and fish community collection sites from Maryland Biological Stream Survey (MBSS, upper right, n = 952 sites) and North Carolina Basinwide Monitoring Program (NCBMP, lower right, n = 1222 sites) and the contiguous USA (USEPA, center, n = 2123 sites). Colors indicate Strahler stream order from 1st order to 8th order. Map not to scale.
2.2.2. Trait Data and Measures of Taxonomic, Functional and Beta Diversity
We incorporated functional trait information related to macroinvertebrate and fish taxa using two common trait databases to evaluate functional diversity [44,45]. For macroinvertebrates, we incorporated a total of 59 traits related to: life history, mobility, morphology, ecology, and trophic habitat [44]. For fish, we incorporated a total of 61 traits related to: trophic ecology, body size, reproductive ecology, life history traits, habitat preference, and salinity tolerance [45]. Trait information was not available for all taxa within the datasets and only taxa with sufficient trait data were used to calculate functional diversity. This analysis included 239/243 macroinvertebrates and 61/79 fish in the MBSS dataset and 417/1048 of macroinvertebrates and 133/151 fish in the NCBMP dataset and 209/974 of macroinvertebrates and 402/623 of fish in the USPEA dataset. We have included a list of taxa missing trait information in Supplementary S3.
We calculated several measurements of macroinvertebrate and fish biodiversity including Shannon (or Shannon-Wiener) diversity, functional diversity and beta diversity. Shannon diversity accounts for both abundance and evenness of species present in a local community, rather than simply species richness, and is commonly used to quantify biodiversity. We used Rao’s quadratic entropy (Rao Q; [46,47]) as a measure of functional diversity. Rao Q measures the breadth of functional traits present within a community. We calculated beta diversity at the watershed level using species richness as diversity measures [48]. Beta diversity is the number of distinct compositional units within each watershed calculated by dividing gamma richness (watershed-level richness) by alpha richness (local) [46]. We calculated Rao Q and beta diversity using the packages FD [49] and vegetarian [50] in R (version 3.2.2; R Project for Statistical Computing, Vienna, Austria), respectively.
2.2.3. Data Analysis
We assessed relationships between stream size metrics (Strahler stream order, watershed area, stream width) and Shannon diversity, Rao Q and beta diversity using analysis of variance (ANOVA). We used each biodiversity and stream size metric as a response variable and fixed explanatory variables, respectively (e.g., model 1 = Shannon diversity ~ Strahler stream order). We tested each biodiversity and stream size metric combination separately for organismal groups (macroinvertebrates, fish) and data sources (MBSS, NCBMP, USEPA). We assessed the relationship between beta diversity with only watershed area because we calculated one value per watershed. For each model, we visually assessed residual variances for homogeneity of variance and normality and log transformed response and explanatory variables when needed to meet these assumptions. We tested for differences in mean levels of biodiversity metrics among Strahler stream orders using post hoc Tukey’s honest significant difference multiple comparisons (Tukey HSD). We performed ANOVA using the R base package and Tukey HSD using agricolae [51] in R and determined statistical significance based on a criterion of p < 0.05.
3. Results
3.1. Measures of Biodiversity and Stream Size in Reviewed Literature
Diversity measurements ranged in scale including local (i.e., alpha, 81%), regional (i.e., gamma diversity, 4%), beta (37%), or combinations of alpha, gamma and beta (Figure 2a). Among the measurements used, taxonomic richness (77% of papers) and Shannon diversity index (21%) were the most common, whereas abundance (e.g., total abundance or density, 12%), evenness (e.g., Pielou’s, 10%), beta diversity (9%), community composition (4%) and functional diversity (3%) were used less commonly (Figure 2b). Various other metrics were used less frequently including: biomass (1%), genetic diversity (<1%), nestedness (1%), and occurrence (<2%).
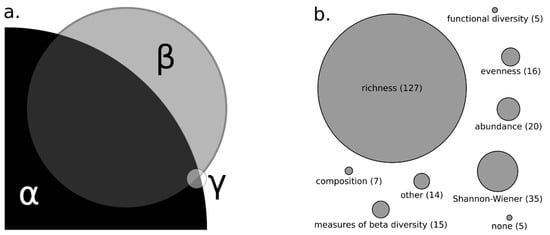
Figure 2.
Proportion of reviewed publications utilizing alpha-diversity (81%), beta-diversity (37%), and gamma-diversity (4%) as a scale for biodiversity comparison (a). Overlap of circles representing diversity types indicates the number of studies that used multiple metrics. Alpha-diversity refers to local diversity, beta-diversity as turnover between local communities, and gamma-diversity as regional diversity. Measures of biodiversity used to infer relationships between stream size and biodiversity (b). Measures of beta-diversity include dissimilarity and turnover. Other includes measures such as biomass, genetic diversity, nestedness, and occurrence.
We found a variety of stream size measurements used across studies inferring biodiversity–stream size relationships (Figure 3a). Stream order (e.g., Strahler) and stream width were used in 42% and 29% of reviewed studies, respectively, whereas water depth (16%), network measures (11%), watershed area (10%) and water discharge (9%) were used less commonly (Figure 3a). Network measures included measures such as link distance from source or mouth, links magnitude, and several other network-related measures. Even though Strahler order can be interpreted as a network measure, we separated it into its own category because of its prevalence.
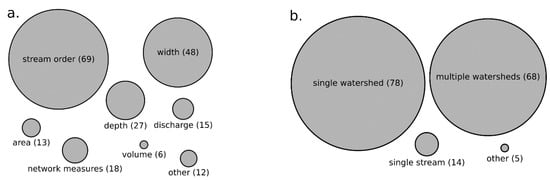
Figure 3.
Measures of stream size used to infer biodiversity–stream size relationships (a) Network measures includes measures such as link distance from source, downstream, links magnitude, and several other measures. Geographic scale of studies inferring relationships between stream size and biodiversity (b) The category “other” includes continental, global, and single reach.
Most papers reviewed assessed biodiversity across single (47%) or multiple (41%) watersheds (Figure 3b). We found that assessments in single streams (8%) or across other scales including single stream reaches, continental or global scales were less common (3%).
3.2. Organisms Studied and Their Relationship with Stream Size in Reviewed Literature
We found a wide range of organismal groups assessed across reviewed papers (Figure 4). Macroinvertebrates (not including mussels; 46%) and fish (39%) were the most commonly assessed organisms. We found that algae (7%), microbes (4%) and riparian plants (4%), mussels (3%), amphibians (3%), and macrophytes (2%) were less commonly assessed (Figure 4).
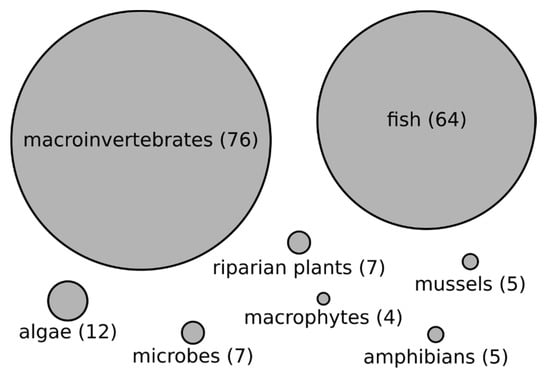
Figure 4.
Types of organisms assessed in studies inferring biodiversity–stream size relationships.
We found 53% of the reviewed papers found positive relationships between biodiversity and stream size. Only 7% of papers found negative biodiversity–stream size relationships, whereas 28% found relationships that were more complicated. Few papers found no relationships (15%) or did not report any relationships (5%). Biodiversity–stream size relationships were labeled more complicated if a positive or negative relationship was not maintained across all stream sizes. Relationships were not consistent across all organismal groups. For macroinvertebrates, 43% of papers found positive relationships between measures of biodiversity and stream size, whereas 22% of papers listed negative or no relationship. For fish, a higher percentage of papers (56%) found positive biodiversity–stream size relationships compared to macroinvertebrates and only 16% found negative or no relationship. We found that 50% of the papers studying algae found positive biodiversity–stream size relationship with 16% finding no relationship.
We found ~35% of papers did not provide explanatory mechanisms behind biodiversity and stream size relationships. When mechanisms were provided, a diverse range were used to explain relationships between biodiversity and stream size. Complexity, size and stability of habitats and water temperature were most often used as explanatory mechanisms behind biodiversity–stream size relationships.
3.3. Relationships between Biodiversity and Strahler Stream Order across Sites in Maryland, North Carolina, and the Contiguous USA
Our data analysis revealed biodiversity–stream size relationships were mostly positive or null across macroinvertebrates and fish (ANOVA, p < 0.05, Figure 5a–f, see Supplementary S4 for F-statistic, degrees of freedom and exact p-values); however, positive relationships tended to be stronger for fish communities than macroinvertebrates. Across Strahler stream orders, we found macroinvertebrate Shannon diversity did not differ between 2nd and 3rd order streams, but macroinvertebrate Shannon diversity was lower in 1st order streams compared to 2–3rd orders in the MBSS dataset (Tukey’s HSD, p < 0.05, Figure 5a). In the NCBMP dataset, we found macroinvertebrate Shannon diversity did not increase with stream order, although it was higher in 4–6th order than in 3rd order streams but not higher than in 1–2nd order streams (Tukey’s HSD, p < 0.05, Figure 5c). In the USEPA dataset, we found macroinvertebrate Shannon diversity in 7–8th orders was lower compared to 1–6th order streams, and was lowest in 8th order streams (Tukey’s HSD, p < 0.05, Figure 5e). Macroinvertebrate Rao Q increased with increasing stream order (ANOVA, p < 0.05, Figure 6a) in the MBSS dataset but was only higher in 5th order compared to 1–6th order streams in the NCBMP dataset (Tukey’s HSD, p < 0.05, Figure 6c). In the USEPA dataset, we found macroinvertebrate Rao Q were not different among 2–6th order streams, although it was lowest in 8th order streams (Tukey’s HSD, p < 0.05, Figure 5e).
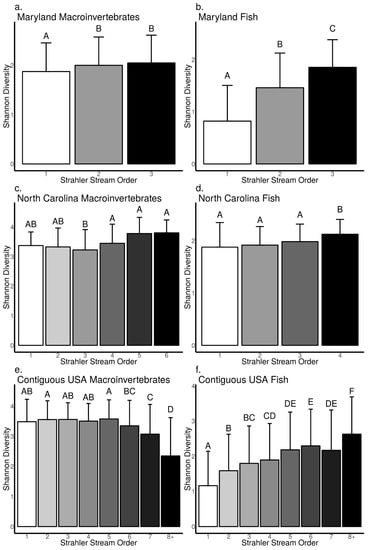
Figure 5.
Relationship between Shannon diversity and Strahler stream order for macroinvertebrate (a,c,e) and fish (b,d,f) communities collected from Maryland (MBSS), North Carolina (NCBMP) and contiguous USA (USEPA). Error bars represent standard deviation and different letters indicate significant differences between orders determined using Tukey’s HSD multiple comparisons.
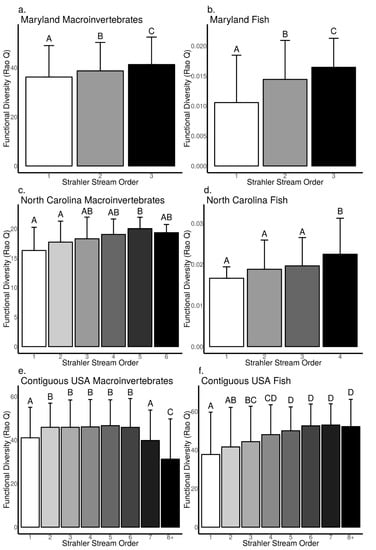
Figure 6.
Relationship between functional diversity (Rao Q) and Strahler stream order for macroinvertebrate (a,c,e) and fish (b,d,f) communities collected from Maryland (MBSS), North Carolina (NCBMP) and contiguous USA (USEPA). Error bars represent standard deviation and different letters indicate significant differences between orders determined using Tukey’s HSD multiple comparisons.
For fish communities, Shannon diversity increased with increasing stream order in the MBSS and USEPA datasets (ANOVA, p < 0.001, Figure 5b,f), but was only higher in 4th order compared to 1–3rd order streams in the NCBMP dataset (Tukey’s HSD, p < 0.05, Figure 5d). Rao Q for fish communities increased with increasing stream order in all datasets (ANOVA, p < 0.001, Figure 6b,d,f).
3.4. Relationships between Biodiversity and Watershed Area across Sites in Maryland, North Carolina, and the Contiguous USA
As watershed area increased, we found macroinvertebrate Shannon diversity did not increase in the MBSS dataset (ANOVA, p = 0.83, Figure 7a), but increased in the NCBMP dataset (ANOVA, p = 0.02, Figure 7c) and decreased in the USEPA dataset (ANOVA, p < 0.001, Figure 7e). Similarly, macroinvertebrate Rao Q did not increase with increasing watershed area in the MBSS dataset (ANOVA, p = 0.88, Figure 8a) but did increase with increasing watershed area in the NCBMP dataset and decreased in the USEPA datasets (ANOVA, p < 0.001 for both, Figure 8c,e). Macroinvertebrate beta diversity increased with increasing watershed area in all datasets (ANOVA, p < 0.001 for both, Figure 9a,c,e).
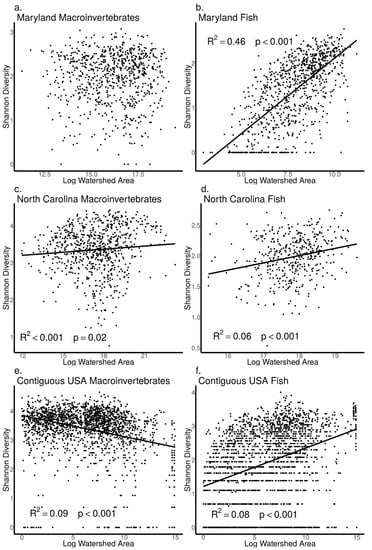
Figure 7.
Relationship between Shannon diversity and watershed area (log transformed) for macroinvertebrate (a,c,e) and fish (b,d,f) communities collected from Maryland (MBSS), North Carolina (NCBMP) and contiguous USA (USEPA). Lines, R2 and p-values indicate significant positive relationships determined by linear models.
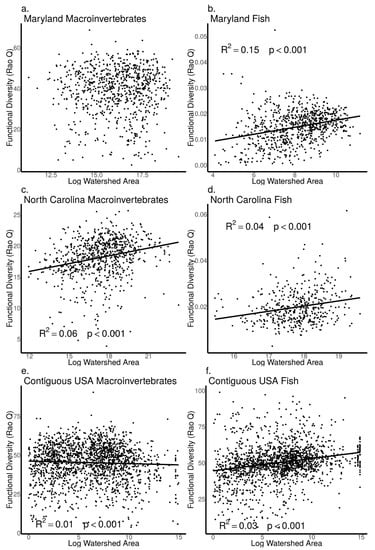
Figure 8.
Relationship between functional diversity (Rao Q) and watershed area (log transformed) for macroinvertebrate (a,c,e) and fish (b,d,f) communities collected from Maryland (MBSS), North Carolina (NCBMP) and contiguous USA (USEPA). Lines, R2 and p-values indicate significant positive relationships determined by linear models.
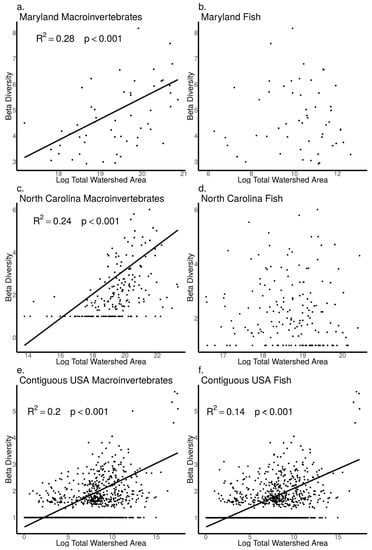
Figure 9.
Relationship between beta diversity and watershed area (log transformed) for macroinvertebrate (a,c,e) and fish (b,d,f) communities collected from Maryland (MBSS), North Carolina (NCBMP) and contiguous USA (USEPA). Lines, R2 and p-values indicate significant positive relationships determined by linear models.
For fish communities, Shannon diversity increased as watershed area increased in all datasets (ANOVA, p < 0.001 for all, Figure 7b,d,f). Fish community Rao Q also increased as watershed area increased in all datasets (ANOVA, p < 0.001 for all, Figure 8b,d,f). Fish beta diversity did not change with increasing watershed area in the MBSS and NCBMP dataset (ANOVA, p = 0.89, 0.41, respectively; Figure 9b,c), but increased in the USEPA dataset (ANOVA, p < 0.001, Figure 9f).
3.5. Relationships between Biodiversity and Stream Width across Sites in Maryland, North Carolina, and the Contiguous USA
As stream width increased, we found macroinvertebrate Shannon diversity did not increase in the MBSS dataset (ANOVA, p = 0.91, Figure 10a), but increased in the NCBMP dataset (ANOVA, p < 0.001, Figure 10c) and decreased in the USEPA dataset (ANOVA, p < 0.001, Figure 10e). Similarly, macroinvertebrate Rao Q did not increase with increasing stream width in the MBSS dataset (ANOVA, p = 0.90, Figure 11a) but did increase with increasing stream width in the NCBMP and USEPA datasets (ANOVA, p < 0.001 for both, Figure 11c, e).
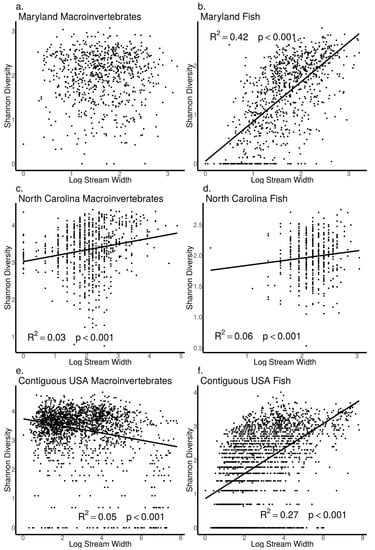
Figure 10.
Relationship between Shannon diversity and stream width (log+1 transformed) for macroinvertebrate (a,c,e) and fish (b,d,f) communities collected from Maryland (MBSS), North Carolina (NCBMP) and contiguous USA (USEPA). Lines, R2 and p-values indicate significant positive relationships determined by linear models.
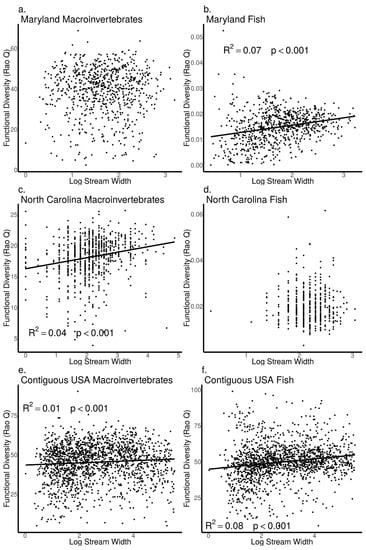
Figure 11.
Relationship between functional diversity (Rao Q) and stream width (log+1 transformed) for macroinvertebrate (a,c,e) and fish (b,d,f) communities collected from Maryland (MBSS), North Carolina (NCBMP) and contiguous USA (USEPA). Lines, R2 and p-values indicate significant positive relationships determined by linear models.
For fish communities, Shannon diversity increased as stream width increased in all datasets (ANOVA, p < 0.001 for all, Figure 10b,d,e). Fish community Rao Q also increased as stream width increased in both the MBSS and USEPA datasets (ANOVA, p < 0.001, Figure 11b,f) but not in the NCBMP dataset (ANOVA, p = 0.56, Figure 11d).
4. Discussion
Stream size is arguably the most common variable used to explain patterns of biodiversity in lotic ecosystems [23,24,25,26]. To identify general patterns across the range of ecological studies that include biodiversity–stream size relationships, we used a systematic literature review of 165 relevant articles. We found studies mainly assessed biodiversity at local sites and most commonly used species richness and Strahler stream order as measures of biodiversity and stream size, respectively. However, we found inconsistencies across biodiversity–stream size studies in that over 20 different measures of biodiversity and over 30 measures of stream size were used. Furthermore, there was no universal relationship between biodiversity and stream size across organismal groups but positive relationships were most common, especially when fish communities were assessed. Most surprisingly, over 1/3 of reviewed papers did not provide mechanisms to explain biodiversity–stream size relationships leading to a breakdown in communicating drivers of biodiversity in lotic systems.
Our novel data analysis of over 4000 macroinvertebrate and fish samples collected throughout Maryland, North Carolina, and the contiguous USA aimed to evaluate the consistency of biodiversity–stream size patterns from the literature review and explore how these relationships may change with different measures of stream size and organismal groups. We found biodiversity typically increased as stream size increased, confirming general patterns from the literature review. Positive and null relationships did not change dramatically when using different stream size measures (i.e., Strahler stream order, watershed area, stream width). However, positive relationships were more frequently encountered and stronger for fish compared to macroinvertebrate communities. Differences in the strength of biodiversity–stream size relationships between macroinvertebrates and fish suggest life histories, habitat preferences, and dispersal abilities also influence patterns of biodiversity. These differences could also be due to differences in taxonomic resolution (genus-level in the MBSS and USEPA vs. mostly species-level in the NCBMP dataset) and spatial distribution of sites across the contiguous USA.
However, despite statistically significant positive relationships between stream size and α-diversity measured as both taxonomic and functional diversity, in many cases those relationships were quite weak, calling into question whether some positive relationships were biologically meaningful. Such was the case for Shannon diversity of both macroinvertebrates and fish in the North Carolina (NCBMP) and the contiguous USA (USEPA) dataset, as well as for functional diversity for Maryland and contiguous USA fish and North Carolina and contiguous USA invertebrates. In all these cases, despite a highly statistically significant slope for stream size-biodiversity relationship, r2 was ≤0.08. The low variance explained by stream size for all other relationships suggests that, while stream size commonly has some detectable positive influence on biodiversity, it is far from a major driver of biodiversity patterns. Therefore, we posit that positive α-diversity–stream size relationships are common, but not the rule, in lotic systems, and urge significant caution in generalizing these relationships.
4.1. Patterns from Our Literature Review and Data Analysis Support Key Concepts in Riverine Ecology
The generally positive biodiversity–stream size relationships we found provide empirical support for several important concepts in riverine ecology [17,20]. Riverine ecosystems are strongly influenced by the unidirectional flow of water, accumulating from small-order headwater streams to larger-order mainstem rivers. Along this longitudinal gradient, the RCC [17] proposed total biotic diversity would be maximized at intermediate stream sizes because of suitable water temperatures and ratio of productivity:respiration. Our results from both the literature review and data analysis support the idea that biodiversity increases along the longitudinal gradient, especially for fish communities, although our analysis did not find a peak at intermediate streams sizes. The lack of hump-shaped curve in biodiversity as stream size increases could be because most streams investigated ranged from 1–4th order (maximum 8th order), whereas predictions by the RCC were conceptualized using a 1–12th order stream gradient. Future work could aim to examine biodiversity–stream size gradients with more samples collected from large-order rivers than analyzed here to further explore hump-shaped curves in biodiversity across stream size gradients.
Our results showing that macroinvertebrate communities responded infrequently or weakly to the stream size gradient may suggest greater support for other key concepts in riverine ecology, such as habitat patch dynamics [52] or functional process zones [20], rather than simple longitudinal gradients. These concepts bring together the importance of physical habitat attributes, such as stream width, and hydrogeomorphic (e.g., flow dynamics), temporal (e.g., disturbance regimes) and biotic (e.g., competition) controls on biodiversity. Indeed, authors have refuted claims of positive biodiversity–stream size relationships when assessing macroinvertebrates [52,53]. Although increases in habitat size [54], complexity [32], stability [55] and temperature variability [56] often correlate with increasing stream size and influence macroinvertebrate diversity, other variables must also be at play. For example, metacommunity dynamics are becoming increasingly recognized as key drivers of macroinvertebrate biodiversity patterns [57,58].
4.2. Difference between Macroinvertebrates and Fish in the Frequency and Strength of Biodiversity–Stream Size Relationships
Differing patterns between macroinvertebrates and fish in biodiversity–stream size relationships highlight the potential importance of functional traits and dispersal as important drivers of lotic biodiversity patterns. On the one hand, macroinvertebrate dispersal can be dominated by the downstream movement of organisms via drift [59,60]. When macroinvertebrate dispersal is directed upstream by adult insects, the process of colonization is often highly seasonal according to life histories, and stochastic due to weak flight abilities [59,60]. Thus, the ability of macroinvertebrates to colonize local habitats is often determined by the proximity of colonization sources [61,62]. On the other hand, fish have strong swimming ability, allowing them to move more freely throughout the river network in the absence of man-made barriers. Longer life cycles allow individuals to continuously disperse throughout the year rather than during certain periods. Thus, increased dispersal ability in fish compared to macroinvertebrates may allow stronger habitat preferences and organization in a more consistent manner than macroinvertebrates along the river gradient. It is true that metacommunity dynamics play a role in determining fish communities along river gradients e.g., [18,63], but it is likely that macroinvertebrates are more constrained by dispersal [57], and their stochastic colonization across the river network could explain the differing strength of biodiversity–stream size relationships we found in this study. Additionally, many fish are orders of magnitude larger than benthic invertebrates, and these differences in scale may also result in very different mechanistic explanations for stream size–biodiversity relationships. For both fish and macroinvertebrates, habitat size has been identified as an important correlate of biodiversity [64,65,66], and the often-cited explanation is a greater diversity of habitat types with increasing habitat size [16,66,67]. However, the drastic differences in size between these organismal groups suggest that these mechanisms will be operating at very different spatial scales. Differences in the biodiversity–stream size relationship across organismal groups, and differences in the likely mechanistic basis behind them, suggests caution to river ecologists and managers who might seek to identify general relationships for purposes of prediction or management. Therefore, studies examining patterns across organismal groups, as performed here, should be continued to further investigate the importance of functional traits and dispersal.
4.3. Important Lack of Explanatory Mechanisms across Behind Biodiversity–Stream Size Relationships
The abundance of studies in our literature review (over 1/3rd) that did not suggest a mechanism responsible for the relationship between biodiversity and stream size indicates an important communication breakdown among river ecologists and managers. This finding raises the question, Is a mechanistic explanation necessary for biodiversity–stream size relationships to be of use? We contend that the answer to this question completely depends on the purpose of the study, and that there are two general categories of purpose: (1) studies that seek to describe biodiversity across the landscape [68]; (2) studies that seek to understand the drivers of biodiversity on a landscape [32]. In the case of category 1, the mechanisms that drive biodiversity–stream size relationships will often be inconsequential to the goals of a study. These goals often include simple descriptions of how biodiversity is distributed on a landscape, establishing baselines for biomonitoring, and establishing the presence or abundance of a focal set of species in a set of locales of interest. In studies of this sort, mechanistic explanations are generally not necessary to advance the goals set by investigators because these studies are purely descriptive.
However, studies seeking to understand the drivers of biodiversity patterns across landscapes (category 2) require mechanistic explanations for the biodiversity–stream size relationships they describe because these studies are explicitly seeking the mechanisms that control biodiversity, and stream size itself is usually not a mechanistic explanation. Stream size is a predictor that is correlated with an extremely large number of other variables and, in many ways, can be considered a master variable similar to the way that pH is used as an effective predictor of microbial distributions in environmental microbiology [69]. Most measures of stream size cannot, in themselves, provide a mechanistic explanation because there are no logical mechanistic links between measures of stream size and biodiversity patterns. For example, by far the two most commonly used measures of stream size are width and Strahler order, but neither of these measures have any direct mechanistic links to controls on biodiversity. Stream size measures are correlated with many other variables that can influence biodiversity—discharge, substrate distributions, topographic gradient, dispersal potential, and trophic state to name a few—but most stream size measures themselves do not provide logical mechanisms.
We do not suggest that all measures of stream size have no mechanistic explanatory power. For example, discharge, flow accumulation, and stream area are all direct measures of physical properties that can have an immediate influence on populations and communities, and can potentially provide a true mechanistic explanation for biodiversity patterns. However, even in the case of these variables, investigators should exercise caution because of the large numbers of correlations between potential drivers of biodiversity patterns. A variable like discharge or area can provide an explanation itself, but that variable will also be heavily correlated with many others, so blithely assuming that the measured variables are the true drivers of biodiversity patterns without additional investigation is not logically sound science. One additional consideration when considering mechanism is that investigators should not expect that one single explanation for stream size–biodiversity relationships would apply broadly across systems, taxa, or geographic regions [40]. From our literature review, the studies that did attempt to provide mechanistic explanations produced a wide range, even within similar taxonomic groups.
4.4. Management Implications
A major goal of river restoration and protection is to increase biodiversity [4,70]. By analyzing the relationship among various biodiversity and stream size measurements at sites across the contiguous USA, our results improve the understanding of how stream size affects biodiversity and indicate that altering stream size alone will not greatly influence biodiversity, especially for macroinvertebrates. Considering stream size is highly correlated with many other physical attributes of streams as previously mentioned, this suggests that focusing on the physical restoration of the stream alone will not lead to positive outcomes. This claim is supported by evidence from previous studies showing little success of restoration in terms of biodiversity when physical attributes such as habitat heterogeneity are manipulated (e.g., [4]). Instead, the success of river restoration in terms of biodiversity likely depends on the combined effects of improving physical habitats, water quality and the potential for colonization from nearby unimpacted streams [4,71]. Likewise, our results suggest some cautions for biomonitoring studies that assess water quality using the distributions of macroinvertebrates across landscapes because we found their relationship with stream size was weak or non-existent. Many factors likely dictate macroinvertebrate distributions making their patterns complex and highly variable [36,49,54]. Stream size may be one of those factors, but the mechanistic explanations for biodiversity–stream size relationships can also be considerably less straightforward than simple congruence between local habitat and organism occurrence [57,72].
5. Conclusions
As river ecologists and managers search for universal drivers of biodiversity, the lack of broad-scale general patterns related to stream size shown here emphasizes the need to consider theories that tie together physical habitat, taxonomic traits (e.g., life history, size, habitat preference) and metacommunity concepts. Multiple drivers may be simultaneously influencing biodiversity–stream size relationships (e.g., [39,41]) or the dominant drivers may change through time [41]. In such situations, identifying mechanistic drivers of biodiversity–stream size relationships may be empirically challenging, and while it may be tempting to assume simple explanations for biodiversity–stream size relationships, the reality may be quite complicated. Our results clearly show the power of using large datasets featuring both macroinvertebrate and fish collections across environmental gradients to facilitate future work in identifying general drivers of biodiversity patterns.
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1424-2818/9/3/26/s1.
Acknowledgments
We thank the Maryland Department of Natural Resources and North Carolina Department of Environmental Quality (NCDEQ), Biological Assessment Branch for allowing to use their comprehensive datasets. We thank Michael Walters (NCDEQ) for his work organizing and sharing the NCBMP dataset with us. We sincerely thank Thilina Surasinghe for inviting us to submit this paper and two anonymous reviewers for their excellent feedback.
Author Contributions
R.V.V., P.M., S.B., K.M.E., and B.L.B. conceived and designed the literature review and data analysis portions of this paper. P.M., S.B., K.M.E. synthesized results from the literature review. R.V.V. and B.L.B. analyzed the fish and macroinvertebrate data. All authors contributed to data interpretation and manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Prevedello, J.A.; Gotelli, N.J.; Metzger, J.P. A stochastic model for landscape patterns of biodiversity. Ecol. Monogr. 2016, 86, 462–479. [Google Scholar] [CrossRef]
- Cabello, J.; Fernández, N.; Alcaraz-Segura, D.; Oyonarte, C.; Piñeiro, G.; Altesor, A.; Delibes, M.; Paruelo, J. The ecosystem functioning dimension in conservation: Insights from remote sensing. Biodivers. Conserv. 2012, 21, 3287–3305. [Google Scholar] [CrossRef]
- Palmer, M.A.; Menninger, H.L.; Bernhardt, E. River restoration, habitat heterogeneity and biodiversity: A failure of theory or practice? Freshwater Biol. 2010, 55, 205–222. [Google Scholar] [CrossRef]
- Vorosmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Gomi, T.; Sidle, R.C.; Richardson, J.S. Understanding processes and downstream linkages of headwater systems. BioScience 2002, 52, 905–916. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Xenopoulos, M.A.; Lodge, D.M.; Alcamo, J.; Marker, M.; Schulze, K.; Van Vuuren, D.P. Scenarios of freshwater fish extinctions from climate change and water withdrawal. Glob. Chang. Biol. 2005, 11, 1557–1564. [Google Scholar] [CrossRef]
- Heino, J. The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biol. Rev. 2013, 88, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Hilsenhoff, W.L. Rapid field assessment of organic pollution with a family-level biotic index. J. N. Am. Benthol. Soc. 1988, 7, 65–68. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Biology of Polluted Waters; Liverpool University Press: Liverpool, UK, 1960. [Google Scholar]
- De Jalon, G.; Mayo, D.; Molles, M.C. Characterization of spanish pyrenean stream habitat: Relationships between fish communities and their habitat. Regul. Rivers Resour. Manag. 1996, 12, 305–316. [Google Scholar] [CrossRef]
- Heino, J.; Paavola, R.; Virtanen, R.; Muotka, T. Searching for biodiversity indicators in running waters: Do bryophytes, macroinvertebrates, and fish show congruent diversity patterns? Biodivers. Conserv. 2005, 14, 415–428. [Google Scholar] [CrossRef]
- Pease, A.A.; GonzÁLez-DÍAz, A.A.; Rodiles-HernÁNdez, R.; Winemiller, K.O. Functional diversity and trait-environment relationships of stream fish assemblages in a large tropical catchment. Freshwater Biol. 2012, 57, 1060–1075. [Google Scholar] [CrossRef]
- Pease, A.A.; Taylor, J.M.; Winemiller, K.O.; King, R.S. Ecoregional, catchment, and reach-scale environmental factors shape functional-trait structure of stream fish assemblages. Hydrobiologia 2015, 753, 265–283. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, D.H. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Hitt, N.P.; Angermeier, P.L. Fish community and bioassessment responses to stream network position. J. N. Am. Benthol. Soc. 2011, 30, 296–309. [Google Scholar] [CrossRef]
- Rosi-Marshall, E.J.; Vallis, K.L.; Baxter, C.V.; Davis, J.M. Retesting a prediction of the river continuum concept: Autochthonous versus allochthonous resources in the diets of invertebrates. Freshwater Sci. 2016, 35, 534–543. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Res. Appl. 2006, 22, 123–147. [Google Scholar] [CrossRef]
- Tornwall, B.; Sokol, E.R.; Skelton, J.; Brown, B.L. Trends in stream biodiversity research since the river continuum concept. Diversity 2015, 7, 16–35. [Google Scholar] [CrossRef]
- Webster, J.R. Spiraling down the river continuum: Stream ecology and the u-shaped curve. J. N. Am. Benthol. Soc. 2007, 26, 375–389. [Google Scholar] [CrossRef]
- Cao, Y.; Stodola, A.; Douglass, S.; Shasteen, D.; Cummings, K.; Holtrop, A. Modelling and mapping the distribution, diversity and abundance of freshwater mussels (family unionidae) in wadeable streams of illinois, USA. Freshwater Biol. 2015, 60, 1379–1397. [Google Scholar] [CrossRef]
- Heino, J.; Mykrä, H.; Muotka, T. Temporal variability of nestedness and idiosyncratic species in stream insect assemblages. Divers. Distrib. 2009, 15, 198–206. [Google Scholar] [CrossRef]
- Osborne, L.L.; Wiley, M.J. Influence of tibutary spatial position on the structure of warmwater fish communities. Can. J. Fish. Aquat.Sci. 1992, 49, 671–681. [Google Scholar] [CrossRef]
- Stenger-Kovács, C.; Tóth, L.; Tóth, F.; Hajnal, É.; Padisák, J. Stream order-dependent diversity metrics of epilithic diatom assemblages. Hydrobiologia 2014, 721, 67–75. [Google Scholar] [CrossRef]
- Fernandes, I.M.; Lourenço, L.S.; Ota, R.P.; Moreira, M.M.M.; Zawadzki, C.H. Effects of local and regional factors on the fish assemblage structure in meridional amazonian streams. Environ. Biol. Fish. 2013, 96, 837–848. [Google Scholar] [CrossRef]
- Rahel, F.J.; Hubert, W.A. Fish assemblages and habitat gradients in a rocky mountain—Great plains stream: Biotic zonation and additive patterns of community change. Trans. Am. Fish. Soc. 1991, 120, 319–332. [Google Scholar] [CrossRef]
- Angermeier, P.L.; Schlosser, I.J. Species-area relationship for stream fishes. Ecology 1989, 70, 1450–1462. [Google Scholar] [CrossRef]
- Anjos, M.B.D.; Zuanon, J. Sampling effort and fish species richness in small terra firme forest streams of central amazonia, Brazil. Neotrop. Ichthyol. 2007, 5, 45–52. [Google Scholar] [CrossRef]
- Couto, T.B.D.A.; Aquino, P.D.P.U.D. Structure and integrity of fish assemblages in streams associated to conservation units in central brazil. Neotrop. Ichthyol. 2011, 9, 445–454. [Google Scholar] [CrossRef]
- Heino, J.; Paasivirta, L. Unravelling the determinants of stream midge biodiversity in a boreal drainage basin. Freshwater Biol. 2008, 53, 884–896. [Google Scholar] [CrossRef]
- Paller, M.H. Relationships between fish assemblage structure and stream order in south carolina coastal plain streams. Trans. Am. Fish. Soc. 1994, 123, 150–161. [Google Scholar] [CrossRef]
- Yan, Y.; He, S.; Chu, L.; Xiang, X.; Jia, Y.; Toa, J.; Chen, Y. Spatial and temporal variation of fish assemblages in a subtropical small stream of the huangshan mountain. Curr. Zool. 2010, 56, 670–677. [Google Scholar]
- Desmond, J.S.; Zedler, J.B.; Williams, G.D. Fish use of tidal creek habitats in two southern california salt marshes. Ecol. Eng. 2000, 14, 233–252. [Google Scholar] [CrossRef]
- Gustafson, M.P. Effects of thermal regime on mayfly assemblages in mountain streams. Hydrobiologia 2008, 605, 235–246. [Google Scholar] [CrossRef]
- Kalaninová, D.; Bulánková, E.; Šporka, F. Caddisflies (trichoptera) as good indicators of environmental stress in mountain lotic ecosystems. Biologia 2014, 69, 1030–1045. [Google Scholar] [CrossRef]
- Grossman, G.D.; Ratajczak, R.E.; Farr, M.D.; Wagner, C.M.; Petty, J.T. Why there are fewer fish upstream. Am. Fish. Soc. Symp. 2010, 73, 63–81. [Google Scholar]
- Horwitz, R.J. Temporal variability patterns and the distributional patterns of stream fishes. Ecol. Monogr. 1978, 48, 307–321. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Hughes, R.M. Longitudinal zonation of pacific northwest (USA) fish assemblages and the species-discharge relationship. Copeia 2008, 2008, 311–321. [Google Scholar] [CrossRef]
- Taylor, C.M.; Warren, M.L. Dynamics in species composition of stream fish assemblages: Environmental variability and nested subsets. Ecology 2001, 82, 2320–2330. [Google Scholar] [CrossRef]
- Clarviate Analytics. Web of Science. Available online: http://apps.webofknowledge.com/ (accessed on 7 May 2017).
- Seaber, P.R.; Kapinos, F.P.; Knapp, G.L. Hydrologic Unit Maps; United States Geological Survey Water-Supply Paper 2294; US Geological Survey: Anchorage, AK, USA, 1987.
- Poff, N.L.; Olden, J.D.; Vieira, N.K.M.; Finn, D.S.; Simmons, M.P.; Kondratieff, B.C. Functional trait niches of north american lotic insects: Traits-based ecological applications in light of phylogenetic relationships. J. N. Am. Benthol. Soc. 2006, 25, 730–755. [Google Scholar] [CrossRef]
- Frimpong, E.A.; Angermeier, P.L. Fish traits: A database of ecological and life-history traits of freshwater fishes of the united states. Fisheries 2009, 34, 487–495. [Google Scholar] [CrossRef]
- Botta-Dukát, Z. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Rao, C.R. Diversity and dissimilarity coefficients: A unified approach. Theor. Pop. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Shipley, B. Fd: Measuring Functional Diversity (FD) from Multiple Traits, and Other Tools for Functional Ecology, R Package Version 1.0–11. Available online: https://rdrr.io/rforge/FD/ (accessed on 31 May 2017).
- Charney, N.; Record, S.; Charney, M.N. Package ‘Vegetarian’. Available online: https://cran.r-project.org/web/packages/vegetarian/vegetarian.pdf (accessed on 20 July 2009).
- De Mendiburu, F. Package ‘Agricolae’: Statistical Procedures for Agricultural Research, R Package Version 1.2–4. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 12 June 2016).
- Townsend, C.R. The patch dynamics concept of stream community ecology. J. N. Am. Benthol. Soc. 1989, 8, 36–50. [Google Scholar] [CrossRef]
- Minshall, G.W.; Brock, J.T.; Lapoint, T.W. Characterization and dynamics of benthic organic-matter and invertebrate functional feeding group relationships in the upper salmon river, idaho (USA). Int. Rev. Gesamten Hydrobiol. 1982, 67, 793–820. [Google Scholar]
- Hubackova, L.; Radkova, V.; Bojkova, J.; Syrovatka, V.; Polaskova, V.; Schenkova, J.; Horsak, M. Diversity patterns of aquatic specialists and generalists: Contrasts among two spring-fen mesohabitats and nearby streams. Biologia 2016, 71, 678–687. [Google Scholar] [CrossRef]
- Heino, J. Functional biodiversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams. Freshwater Biol. 2005, 50, 1578–1587. [Google Scholar] [CrossRef]
- Arscott, D.B.; Tockner, K.; Ward, J. Thermal heterogeneity along a braided floodplain river (tagliamento river, northeastern italy). Can. J. Fish. Aquat.Sci. 2001, 58, 2359–2373. [Google Scholar] [CrossRef]
- Brown, B.L.; Swan, C.M.; Auerbach, D.A.; Grant, E.H.C.; Hitt, N.P.; Maloney, K.O.; Patrick, C. Metacommunity theory as a multispecies, multiscale framework for studying the influence of river network structure on riverine communities and ecosystems. J. N. Am. Benthol. Soc. 2011, 30, 310–327. [Google Scholar] [CrossRef]
- Grönroos, M.; Heino, J.; Siqueira, T.; Landeiro, V.L.; Kotanen, J.; Bini, L.M. Metacommunity structuring in stream networks: Roles of dispersal mode, distance type, and regional environmental context. Ecol. Evol. 2013, 3, 4473–4487. [Google Scholar] [CrossRef] [PubMed]
- Bilton, D.T.; Freeland, J.R.; Okamura, B. Dispersal in freshwater invertebrates. Annu. Rev. Ecol. Syst. 2001, 32, 159–181. [Google Scholar] [CrossRef]
- Mackay, R.J. Colonization by lotic macroinvertebrates: A review of processes and patterns. Can. J. Fish. Aquat. Sci. 1992, 49, 617–628. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Stoll, S.; Sundermann, A.; Haase, P. Dispersal distance and the pool of taxa, but not barriers, determine the colonisation of restored river reaches by benthic invertebrates. Freshwater Biol. 2014, 59, 1843–1855. [Google Scholar] [CrossRef]
- Vander Vorste, R.; Malard, F.; Datry, T. Is drift the primary process promoting the resilience of river invertebrate communities? A manipulative field experiment in an intermittent alluvial river. Freshwater Biol. 2016, 61, 1276–1292. [Google Scholar] [CrossRef]
- Stoffels, R.J.; Clarke, K.R.; Linklater, D.S. Temporal dynamics of a local fish community are strongly affected by immigration from the surrounding metacommunity. Ecol. Evol. 2015, 5, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.E.; McIntosh, A.R. Do isolation and local habitat jointly limit the structure of stream invertebrate assemblages? Freshwater Biol. 2013, 58, 128–141. [Google Scholar] [CrossRef]
- McHugh, P.A.; Thompson, R.M.; Greig, H.S.; Warburton, H.J.; McIntosh, A.R. Habitat size influences food web structure in drying streams. Ecography 2015, 38, 700–712. [Google Scholar] [CrossRef]
- Xenopoulos, M.A.; Lodge, D.M. Going with the flow: Using species-discharge relationships to forecast losses in fish biodiversity. Ecology 2006, 87, 1907–1914. [Google Scholar] [CrossRef]
- Al-Shami, S.A.; Heino, J.; Che Salmah, M.R.; Abu Hassan, A.; Suhaila, A.H.; Madrus, M.R. Drivers of beta diversity of macroinvertebrate communities in tropical forest streams. Freshwater Biol. 2013, 58, 1126–1137. [Google Scholar] [CrossRef]
- Kaelin, K.; Altermatt, F. Landscape-level predictions of diversity in river networks reveal opposing patterns for different groups of macroinvertebrates. Aquat. Ecol. 2016, 50, 283–295. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Louhi, P.; Mykrä, H.; Paavola, R.; Huusko, A.; Vehanen, T.; Mäki-Petäys, A.; Muotka, T. Twenty years of stream restoration in finland: Little response by benthic macroinvertebrate communities. Ecol. Appl. 2011, 21, 1950–1961. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, A.; Stoll, S.; Haase, P. River restoration success depends on the species pool of the immediate surroundings. Ecol. Appl. 2011, 21, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.; Downes, B.J. A landscape-scale field experiment reveals the importance of dispersal in a resource-limited metacommunity. Ecology 2017, 98, 565–575. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).