Abstract
We carried out an intensive sampling survey in ancient Lake Ohrid (Macedonia/Albania), covering all seasons, to determine total species number, relative species abundances and spatial distribution of Ostracoda. We identified 32 living species that belong to seven families (Candonidae, Ilyocyprididae, Cyprididae, Leptocytheridae, Limnocytheridae, Cytherideidae, and Darwinulidae) and 15 genera (Candona, Fabaeformiscandona, Candonopsis, Cypria, Cyclocypris, Ilyocypris, Eucypris, Prionocypris, Bradleystrandesia, Herpetocypris, Dolerocypris, Amnicythere, Paralimnocythere, Cytherissa, and Darwinula). Six additional species were identified from empty carapaces and valves. Dominant families in Lake Ohrid were Candonidae and Limnocytheridae, representing 53% and 16% of all species, respectively. Prevalence of species flocks in these two families confirms the “young” ancient status of the lake. Amnicythere displays a preference for oligo-haline to meso-haline waters, but some species are found in saline environments, which suggests Lake Ohrid has a marine history. Recent studies, however, indicate fluvial/glaciofluvial deposition at the onset of Lake Ohrid sedimentation. Candona is the most diverse genus in Lake Ohrid, represented by 12 living species. Paralimnocythere is represented by five living species and all other genera are represented by one or two species. Reports of Candona bimucronata, Ilyocypris bradyi, Eucypris virens, Eucypris sp., Prionocypris zenkeri, Bradleystrandesia reticulate, Herpetocypris sp. 2, and Dolerocypris sinensis are firsts for this lake. Living ostracodes were collected at the maximum water depth (280 m) in the lake (Candona hadzistei, C. marginatoides, C. media, C. ovalis, C. vidua, Fabaeformiscandona krstici, Cypria lacustris, C. obliqua and Amnicythere karamani). Cypria lacustris was overall the most abundant species and Cypria obliqua displayed the highest abundance at 280 m water depth. Principal environmental variables that influence ostracode distributions in Lake Ohrid are water depth and conductivity. In general, species richness, diversity and evenness were greater in waters <60 m deep, with highest values often found in the littoral zone, at depths <30 m. Candonids, however, displayed highest diversity in the sublittoral (30–50 m) and profundal (50–280 m) zones. The most frequent species encountered are taxa endemic to the lake (14 living species), which have a wide depth range (≤280 m), and display higher abundance with greater water depth. Non-endemic species were rare, limited to water depths <50 m, and were found mainly in the north part of the lake where anthropogenic pressure is high. Several cosmopolitan species were encountered for the first time, which suggests that these widespread species are new arrivals that may replace endemics as human impacts increase.
1. Introduction
Ancient lakes possess long continental archives of past climate and environmental changes and are hotspots of biodiversity and speciation dynamics for endemic taxa [,,,,]. The Balkan region is a biogeographically diverse area characterized by ancient lakes. Besides well-known Lake Ohrid, Lakes Prespa, Skutari, Pamvotis, and Dojran are also ancient lakes [,,,]. Lake Ohrid hosts a highly diverse endemic freshwater fauna and flora []. Its geologic age and mode of formation remain controversial []. Lake Ohrid was selected for drilling by the International Continental Scientific Drilling Program (ICDP) because of its high potential as a paleoenvironmental and paleoclimatic archive []. Lake Ohrid possesses >220 endemic faunal species [], most belonging to the Amphipoda, Gastropoda, Isopoda, Ostracoda, Porifera, and Tricladida []. Stankovič [], Salemaa [], Frogley and Preece [], and Albrecht and Wilke [] suggested that 33 (63%) of the 52 described ostracode species from Lake Ohrid and its catchment are endemic. Petkovski (2005, unpubl. data) listed 41 species for Lake Ohrid proper, excluding the catchment. Early works on Ostracoda focused on taxonomy [,,,,,,,,,,] and there is little information on species ecological preferences, distribution within the lake, life cycles, or habitats. Mikulić and Pljakić [] published the only paper that presents the spatial distribution of one genus, Candona, in Lake Ohrid. Greatest Candona diversity occurred between 20 m and 50 m water depth.
Ostracodes are microcrustaceans, generally ≤3 mm long, and are one of the few microfossil groups preserved in the last two interglacial sediment sequences from the lake [,]. Belmecheri et al. [] and Wagner et al. [] used ostracode fossil assemblages in sediment cores to infer past climate and environmental changes. Belmecheri et al. [] emphasized that most ostracode data came from the littoral zone and that, in general, the lake was under-sampled and taxonomic revision was needed. We carried out four field campaigns in Lake Ohrid to identify species that occur in the lake today [,]. In a previous study [], the relationship between ostracode species in Lake Ohrid and environmental variables was explored. The main objective was to gather autecological information for all species and determine species temperature tolerances, a prerequisite for paleoenvironmental reconstructions []. In this study, we investigated the spatial distribution of each taxon and ostracode diversity and species richness in the lake. This information will improve the potential for using Ostracoda as bioindicators of climate and environment, and will help to interpret fossil species assemblages from sediment cores to be recovered by the Lake Ohrid Drilling Project.
Study Region
Lake Ohrid lies along the border between Macedonia and Albania, at 695 m a.s.l. The maximum water depth (293 m) is found in the southeast part of the basin. The lake has a surface area of 360 km2 and a volume of 50.7 km3 [,]. The steep-sided basin is located in a tectonic graben system of Pliocene to Pleistocene age [] and is surrounded by mountains that reach ~1500 m a.s.l. to the west (“Mokra” mountain chain) and >1750 m a.s.l to the east (“Galičica” mountain chain) []. The Mokra is composed of serpentine (peridotites) overlain by Triassic limestone, whereas the Galičica consists mainly of Triassic limestone []. The timing of lake formation is still unclear and a subject of debate. Stankovič [] suggested a 2–3 Ma age for the tectonic formation of the basin, whereas Spirkovski et al. [] proposed an age of 4–10 Ma. Recent studies by Wagner et al. [] and Lindhorst et al. [] suggested a younger age (1.9 Ma).
An equally important question relates to the origin of the lake’s water. Albrecht and Wilke [] offered four hypotheses for the origin of Lake Ohrid. Hypothesis 1 (“Mesohellenic Trough” hypothesis) suggests that the Mesohellenic Trough was a narrow basin that extended from northern Greece northwest to the Korca Basin during the late Eocene and the middle Miocene and that modern Lake Ohrid filled by connection to the Aegean Basin. Hypothesis 2 (“Tethys” hypothesis) suggests that Lake Ohrid was part of a system of lacustrine basins that separated from the Tethys during the early and middle Pliocene. A connection between the Ohrid Basin and the Pannonian Basin via a series of freshwater lakes during the middle to late Miocene is postulated in hypothesis 3 (“Lake Pannon” hypothesis). Hypothesis 4 (“De novo” hypothesis) proposes that Lake Ohrid formed de novo in a dry polje, from springs or rivers. Albrecht and Wilke [] regard the de novo scenario as the most tenable. Stankovič [] notes that the fauna and flora of Lake Ohrid belong to freshwaters and that there is no evidence for marine or brackish immigrants in Lake Ohrid.
The Lake Ohrid catchment is influenced by Mediterranean climate, and less so by continental climate []. For the period 1961–1990, minimum air temperature was −5.7 °C, maximum air temperature was 31.5 °C, and average annual air temperature was 11.1 °C. Ice cover never formed []. Maximum precipitation occurs in December and March, and the late summer is dry []. Mean annual precipitation averages about 750 mm [].
Lake Ohrid is an oligomictic lake that presents complete overturn roughly every seventh winter. The top 150–200 m of the water column display the usual seasonal stratification of typical deep temperate lakes. Deeper water temperatures show a small depth gradient between −0.02 and 0.15 °C per 100 m. Stratification of the water column below 150 m is controlled by salinity [].
Numerous karstic springs contribute approximately 53% to the net hydrologic input, with the balance equally divided between river inflows and direct precipitation []. Spring inflows influence ostracode species distribution and ecology because they supply the cool, oxygen-rich water favored by all endemic forms in Lake Ohrid [,].
Vegetation of the Ohrid region is of the sub-Mediterranean type and belongs to the Quercetalia pubescentis order, especially in the zone of Quercus cerris forests. Quercetum macedonicae EM prov., Pinetum pallasianae macedonicum EM, and Carpinetum orientalis macedonicum associations occupy the lower belt of the Ohrid Basin (700–1000 m a.s.l.). The entire Ohrid region has, however, been largely deforested, giving rise to another characteristic vegetation consisting of small trees and shrubs, such as Pistacia terebinthus and Asparagus tenuifolius [].
It has been suggested that the subaqueous springs create a high number of ecological niches and spring water has been important in the evolution of the Lake Ohrid ecosystem [,]. Furthermore, small-scale differences in sediment composition may be responsible for the patchiness in sub-structuring of benthic communities. This heterogeneity may also be linked to evolutionary processes. Grain-size distribution, metal concentrations, and concentrations of particular mineral phases are closely tied to catchment geology and transport processes, and show strong spatial variability in lake surface sediments [].
Little information is available about the biodiversity of higher aquatic plants [], but the macrophyte diversity in the lake is apparently relatively low. Four major zones (belts) of macrophyte vegetation are found: Cladophora, Phragmites, Potamogeton and Chara []. The Chara belt is the best studied zone and extends into the lower littoral, to about 20 m depth [].
2. Sampling Survey
We carried out four field campaigns covering all seasons (March/April 2009, August/September 2009, February 2010, and June 2010) [], during which we collected a total of 187 surface sediment samples from the littoral zone to 280 m depth (Figure 1). Sampling efforts focused on water depths to 235 m, but additional samples were recovered from 270 m to 280 m. Physical and chemical variables and sediment information for each sampling site are found at [].
In areas of the lake where sediment is coarse, samples were retrieved with an exhauster, spoon net, UWITEC gravity corer, Ekman grab, dredge or sediment-suction device. The sediment suction device consisted of a metal pipe to which a 5-m-long flexible hose was attached. Sediment was drawn into a 125-µm-mesh sieve, using a manual membrane pump. In areas where sediment is finer, samples were collected with a gravity multi-corer (three cores, each with a diameter of 11 cm). The uppermost 2 cm of each core (190 cm3) was washed through a 125-µm-mesh sieve immediately after collection. All sieve residues were preserved in 95–99% ethanol.
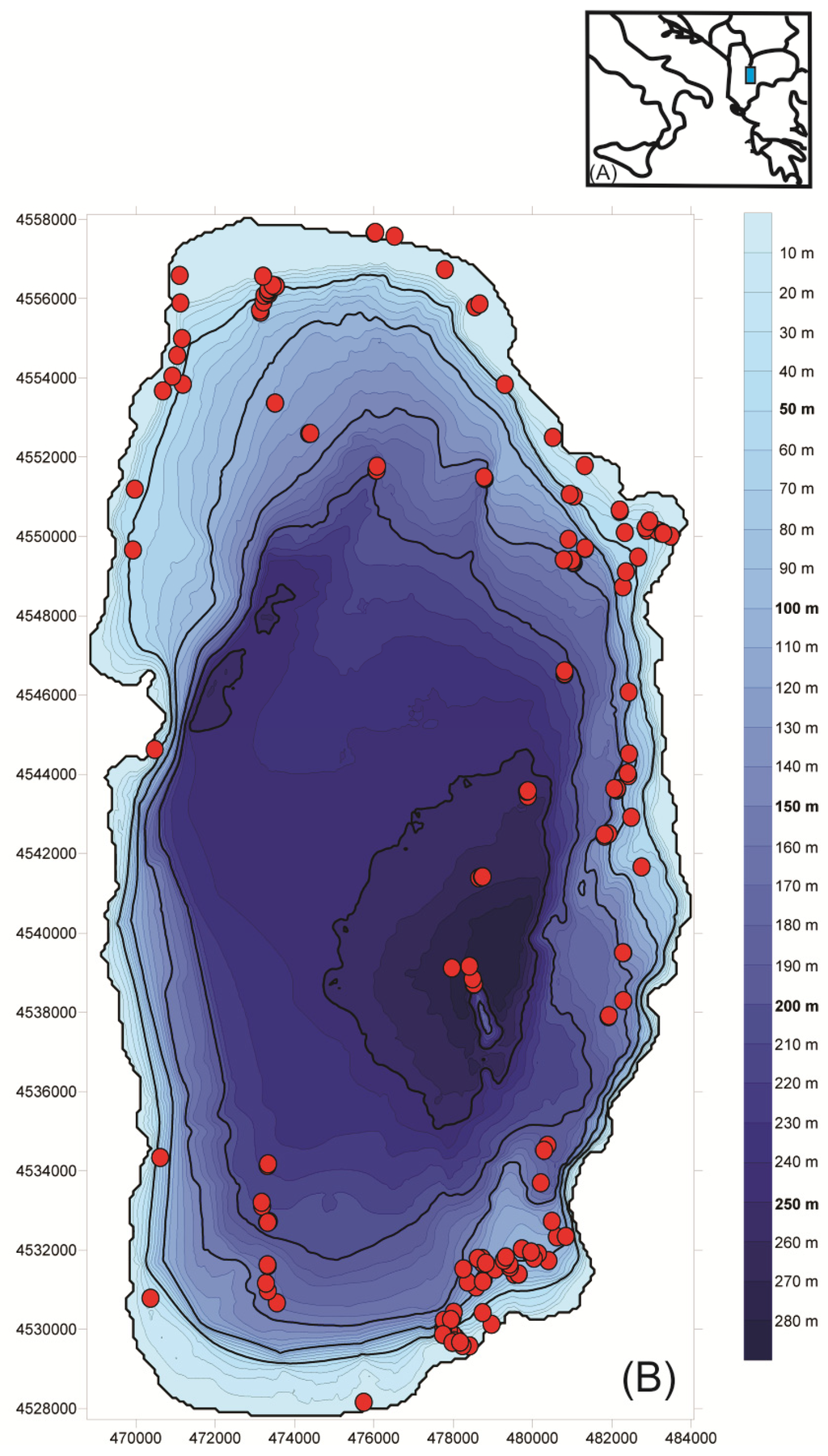
Figure 1.
Inset map showing (A) the location of Lake Ohrid (rectangle) on the border of Macedonia/Albania and (B) a bathymetric map of the lake, displaying the sediment sampling sites.
In the laboratory, ostracodes were picked from the residues with fine brushes, using a stereo microscope (Leica MZ 7.5) with a magnification up to 50×. Valves and soft bodies were separated and soft parts were dissected and mounted in Hydro-MatrixTM (Micro Tech Lab, Graz, Austria). The taxonomic works of Klie [,,,], Holmes [], Meisch [], Mikulić [], Namiotko et al. [], Petkovski [,,,,] and Petkovski et al. [] were used to identify species. Digital images of valves were taken with a light microscope (DM5000B; camera type Leica DFC320, Leica Microsystems GmbH, Wetzlar, Germany). Scanning Electron Microscope (SEM) pictures of ostracode valves and carapaces were taken at the Zoological Museum of the University of Hamburg, with a Scanning electron microscope LEO 1525 (Carl Zeiss Inc., Jena, Germany) and at the University of Cologne, with a CamScan-CS-44 (CamScan, Cranberry Township, PA, USA).
A total of 187 samples were included in numerical analyses, of which 27 samples contained no ostracodes. Furthermore, juvenile ostracodes that could not be identified to species level, were omitted from interpretation. Ostracode relative abundances are found at [].
Species richness (number of species in a sample, S) is indicated for all samples, whereas the Shannon diversity index (H) [] and the index of evenness (E) [] were calculated only for samples taken with the gravity multicorer. Adult counts, i.e., valves, empty carapaces and individuals with soft parts were included in analyses. Sample abundances per unit volume (190 cm−3) were only determined for the multi-corer samples. Species richness (S), diversity (H) and evenness (E) were modeled as a function of the spatial coordinates using a non-parametric, locally weighted regression (loess) with a span of 0.5 []. Additionally, abundance of species present in 10 or more sites was generalized as a function of collection depth and conductivity through non-parametric smoothing regressions []. All statistical analysis was performed in R [].
3. Results and Discussion
Reported numbers of ostracode species in Lake Ohrid differ among authors, suggesting the need for a taxonomic review. Early taxonomic work began in the 1930s and is compiled in Table 1. It is difficult, however, to ascertain the number of specimens collected and analyzed in those early studies. Holmes [] suspected that his species list was incomplete because spring and summer taxa might have already disappeared by the time he visited the lake between 1 August and 10 September 1935. Furthermore, he collected specimens from a restricted area of the lake. Our study is the first to sample during all seasons of the year.
3.1. Ostracode Diversity
We collected living specimens of 32 species belonging to seven families (Candonidae, Ilyocyprididae, Cyprididae, Leptocytheridae, Limnocytheridae, Cytherideidae, and Darwinulidae) (Box 1 and Figure 2, Figure 3, Figure 4 and Figure 5) []. Six additional species were identified from only hard parts: Candona expansa, C. holmesi, C. marginata, C. triangulata, Cypria ophtalmica, and Paralimnocythere umbonata. These species were excluded from consideration because valves and empty carapaces may have been transported to the sampling locations and, therefore, may not represent living species at the sites. Fifty-three percent of the ostracode species belong to Candonidae and 16% to Limnocytheridae. Only one species belonging to Cytherideidae (Cytherissa lacustris) was found, which is typical for ancient lakes. Other Cytherissa species are endemic to Lake Baikal []. Our results from the younger, though ancient Lake Ohrid agree with Frogley et al. [], in that they reported that “relatively younger” ancient lakes (0.75–8 Ma old) typically display high faunal diversity, with species flocks of Limnocytheridae and Candonidae being the most common groups. Such “younger” ancient lakes, with the same dominant subfamilies found in Lake Ohrid, include among others, Lakes Malawi, Biwa and Titicaca. Older lakes such Tanganyika (9–12 Ma) and Baikal (25–35 Ma) are truly ancient both geologically and biologically and most species flocks belong to Cytherideidae and Candonidae.
Box 1. Taxonomic ranking of ostracode species collected from Lake Ohrid. Species marked with asterisk (*) were identified using valves or empty carapaces, species endemic to the lake are marked with an open circle (°) (modified from []).
Class Ostracoda Latreille, 1806
Order Podocopida Sars, 1866
Suborder Podocopina Sars, 1866
Infraorder Cypridocopina Jones, 1901
Superfamily Cypridoidea Baird, 1845
Family Candonidae Kaufmann, 1900
Subfamily Candoninae Kaufmann, 1900
Genus Candona s. str. Baird, 1845
Candona bimucronata Klie, 1937
*°Candona expansa Mikulić, 1961
°Candona goricensis Mikulić, 1961
°Candona hadzistei Petkovski et al., 2002
°Candona hartmanni Petkovski, 1969
*°Candona holmesi Petkovski, 1960
°Candona litoralis Mikulić, 1961
°Candona margaritana Mikulić, 1961
*°Candona marginata Klie, 1942
Candona marginatoides Petkovski, 1960
°Candona media Klie, 1939
°Candona ohrida Holmes, 1937
°Candona ovalis Mikulić, 1961
°Candona trapeziformis Klie, 1939
*°Candona triangulata Klie, 1939
°Candona vidua Klie, 1942
Genus Fabaeformiscandona Krstić, 1972
°Fabaeformiscandona krstici (Petkovski, 1969)
Genus Candonopsis Vávra, 1891
Candonopsis kingsleii (Brady & Robertson, 1870)
Subfamily Cyclocypridinae Kaufmann, 1900
Genus Cypria Zenker, 1854
Cypria lacustris Sars, 1890
Cypria obliqua Klie, 1939
*Cypria ophtalmica (Jurine, 1820)
Genus Cyclocypris Brady & Norman, 1889
Cyclocypris ovum (Jurine, 1820)
Family Ilyocyprididae Kaufmann, 1900
Subfamily Ilyocypridinae Kaufmann, 1900
Genus Ilyocypris Brady & Norman, 1889
Ilyocypris bradyi Sars, 1890
Family Cyprididae Baird, 1845
Subfamily Eucypridinae Bronshtein, 1947
Genus Eucypris Vávra, 1891
Eucypris virens (Jurine, 1820)
Eucypris sp.
Genus Prionocypris Brady & Norman, 1896
Prionocypris zenkeri (Chyzer & Toth, 1858)
Subfamily Cypricercinae McKenzie, 1971
Genus Bradleystrandesia Broodbakker, 1983
Bradleystrandesia reticulata (Zaddach, 1844)
Subfamily Herpetocypridinae Kaufmann, 1900
Genus Herpetocypris Brady & Norman, 1889
Herpetocypris sp.
Herpetocypris sp.2
Subfamily Dolerocypridinae Triebel, 1961
Genus Dolerocypris Kaufmann, 1900
Dolerocypris sinensis (Sars, 1903)
Superfamily Cytheroidea Baird, 1850
Family Leptocytheridae Hanai, 1957
Subfamily Leptocytherinae Hanai, 1957
Genus Amnicythere Devoto, 1965
Amnicythere karamani (Klie, 1939)
Family Limnocytherinae Klie, 1938
Subfamily Limnocytherinae Klie, 1938
Genus Paralimnocythere Carbonnel, 1965
Paralimnocythere alata (Klie, 1939)
Paralimnocythere georgevitschi (Petkovski, 1960)
Paralimnocythere karamani (Petkovski, 1960)
Paralimnocythere ochridense (Klie, 1934)
Paralimnocythere slavei Petkovski, 1969
*Paralimnocythere umbonata (Klie, 1939)
Family Cytherideidae Sars, 1925
Genus Cytherissa Sars, 1925
Cytherissa lacustris (Sars, 1863)
Infraorder Darwinulocopina Sohn, 1988
Superfamily Darwinuloidea Brady & Norman, 1889
Family Darwinuloidae Brady & Norman, 1889
Genus Darwinula Brady & Robertson, 1885
Darwinula stevensoni (Brady & Robertson, 1870)
The 32 ostracode species found alive belong to 15 genera (Box 1 and Figure 2, Figure 3, Figure 4 and Figure 5). The most diverse genus in the lake was Candona, with 12 species identified from live individuals and four species from valves and empty carapaces (C. expansa, C. holmesi, C. marginata and C. triangulata). Besides the genus Candona, the subfamily Candoninae was represented by the genera Candonopsis (C. kingsleii) and Fabaeformiscandona (F. krstici). The second most diverse genus was Paralimnocythere. We found five living species (P. alata, P. georgevitschi, P. karamani, P. ochridense, and P. slavei), but only shells of P. umbonata. The genus Cypria included two living species (C. lacustris and C. obliqua) and one species identified from valves and empty carapaces (C. ophtalmica). All other genera were represented by one or two species. A distinctive feature of the ostracode fauna in this lake is the occurrence of the species Amnicythere karamani. Species of the genus Amnicythere normally live in oligo-haline to meso-haline waters and marine environments [], but some occur in springs, rivers and lakes []. Although the de novo hypothesis has been suggested as the most probable explanation for the lake’s origin, the presence of Amnicythere suggests a marine origin for the lake []. Recent studies, however, indicate fluvial/glaciofluvial sedimentation at the onset of Lake Ohrid [], suggesting other reasons for the presence of this species in the lake. According to Namiotko et al. [], this species probably derived from Lake Pannon species that colonized lakes in southern Europe through a stepping-stone process that enabled adaptation to the freshwater environment.
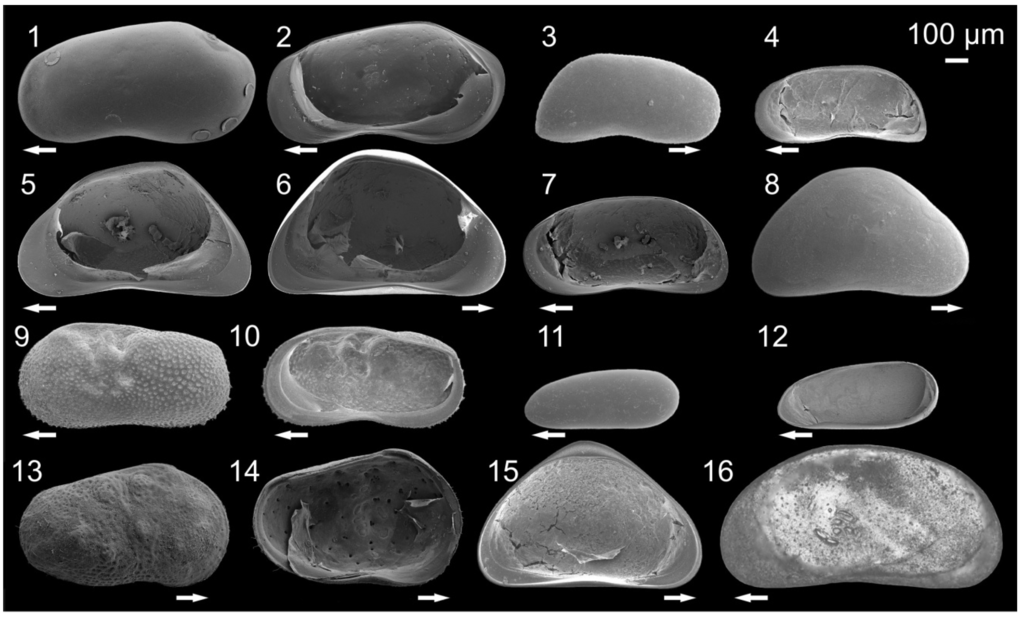
Figure 2.
Pictures of ostracodes from Lake Ohrid: Fabaeformiscandona krstici (1) Left valve (LV), external view, male; F. krstici (2) Right valve (RV), internal view, male; Candona hadzistei (3) RV, external view, male; C. hadzistei (4) RV, internal view, female; Candona margaritana (5) RV, internal view, female; C. margaritana (6) LV, internal view, female; C. marginata (7) RV, internal view, female; Candona goricensis (8) RV, external view, female; Ilyocypris bradyi (9) LV, external view, female; I. bradyi (10) RV, internal view, female; Darwinula stevensoni (11) LV, external view, female; D. stevensoni (12) RV, internal view, female; Cytherissa lacustris (13) RV, external view, female; C. lacustris (14) LV, internal view, female; Candona goricensis (15) LV, internal view, female; Herpetocypris sp. 2 (16) LV, external view, female. Arrows point to anterior (modified from []).
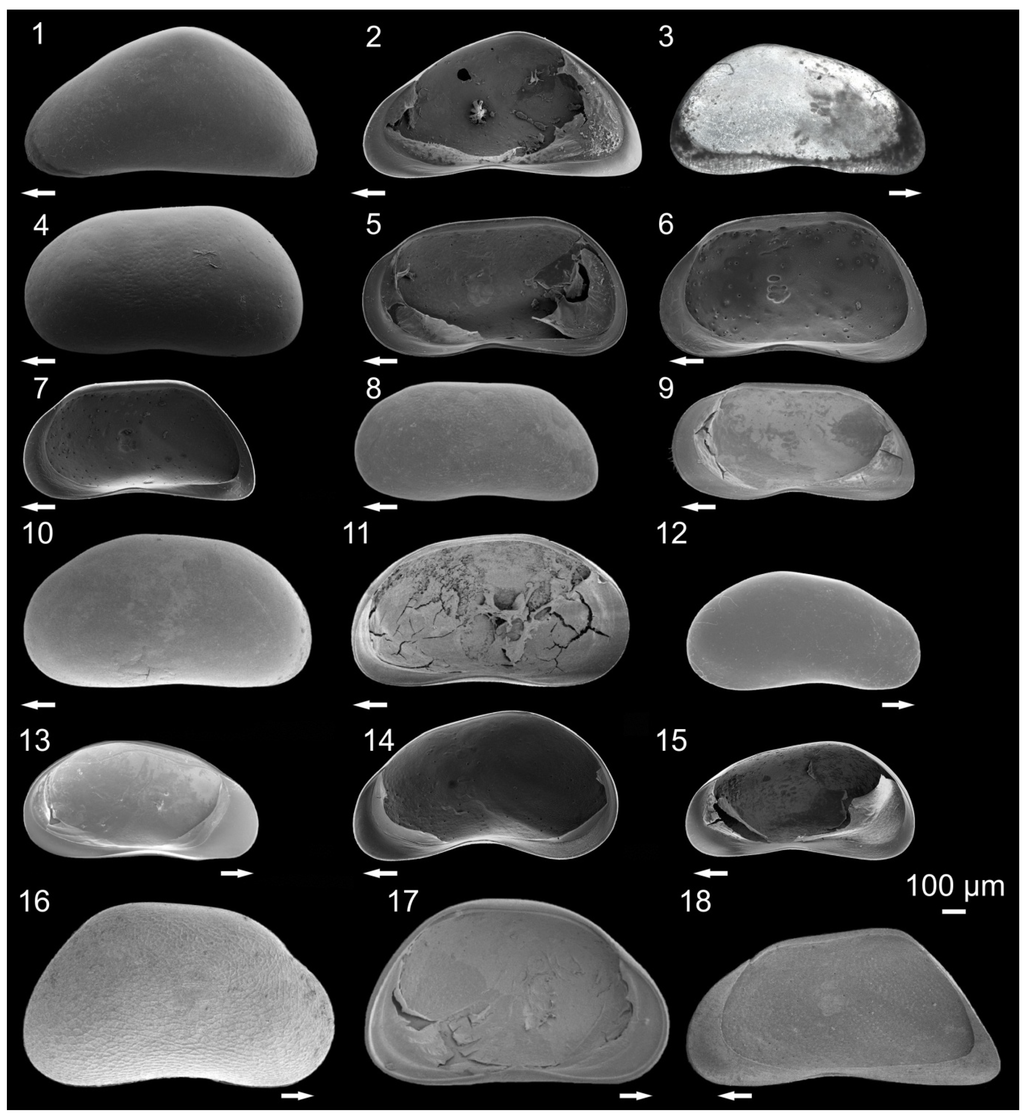
Figure 3.
Pictures of ostracodes from Lake Ohrid: Candona triangulata (1) Left valve (LV), external view, female; C. triangulata (2) Right valve (RV), internal view, female; Candona litoralis (3) RV, external view, male; Candona media (4) LV, external view, female; C. media (5) RV, internal view, female; Candona trapeziformis (6) RV, internal view, male; C.trapeziformis (7) RV, internal view, female; Candona vidua (8) LV, external view, female; C. vidua (9) RV, internal view, female; Candona ovalis (10) LV, external view, female; C. ovalis (11) LV, internal view, female; Candonopsis kingsleii (12) RV, external view, male; C. kingsleii (13) LV, internal view, male; Candona hartmanni (14) RV, internal view, male; C. hartmanni (15) RV, internal view, female; Candona ohrida (16) RV, external view, male; C. ohrida (17) LV, internal view, male; Candona holmesi (18) RV, internal view, female. Arrows point to anterior part of valves (modified from []).
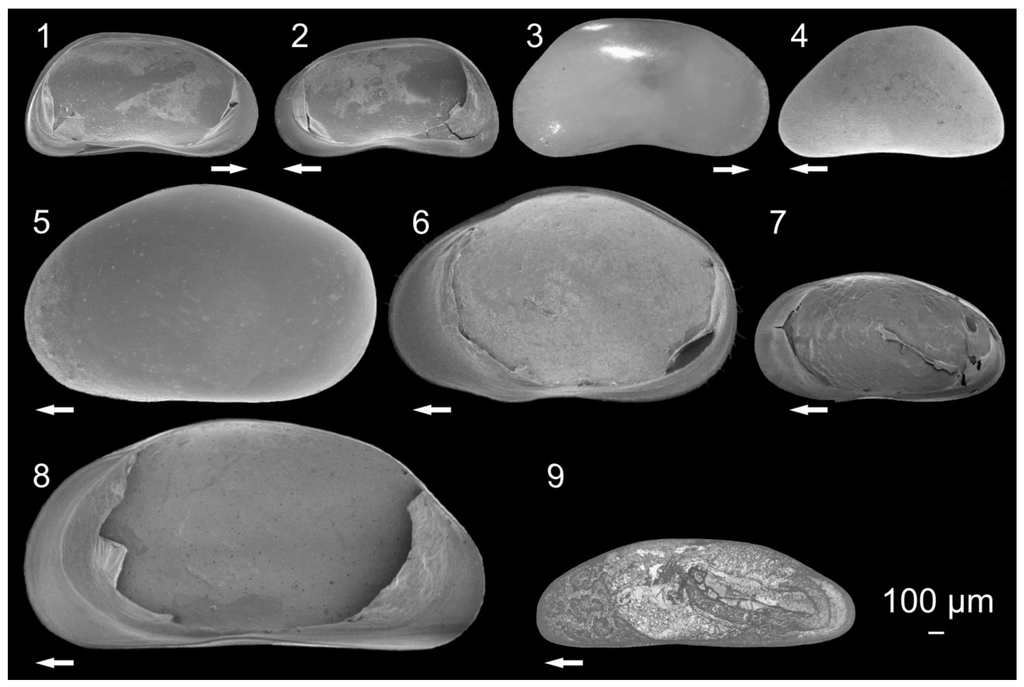
Figure 4.
Pictures of ostracodes from Lake Ohrid: Candona marginatoides (1) Left valve (LV), internal view, female; C. marginatoides (2) Right valve (RV), internal view, female; Candona bimucronata (3) RV, external view, female; Candona expansa (4) LV, external view, female; Eucypris virens (5) LV, external view, female; E. virens (6) RV, internal view, female; Bradleystrandesia reticulata (7) RV, internal view, female; Eucypris sp. (8) RV, internal view, female; Dolerocypris sinensis (9) LV, external view, female. Arrows point to anterior part of valves (modified from []).
It was previously uncertain how many ostracode species inhabit the lake and surrounding waters and earlier studies reported different species numbers. In a literature review, Martens [] reported 38 species from 13 genera in Lake Ohrid, but did not provide a species list. Martens and Schön [] mentioned that the lake possesses ~50 species. A total of 52 species, in the lake and its surrounding wetlands, is found in Frogley et al. [] and Griffiths and Frogley []. According to Frogley et al. [], endemic ostracodes include three species of Amnicythere (Leptocythere), four of Paralimnocythere, and 25 candonids. Petkovski (2005, unpubl. data) reduced the list of species (excluding the catchment area) to a total of 41, because some species were described several times under different names (Table 1). Such repeated description of some species under different names may be one reason we still miss some previously described ostracode species. It is also possible that some species have disappeared from the lake during the last few decades. We found eight living species that had never been reported in the lake: Candona bimucronata, Ilyocypris bradyi, Eucypris virens, Eucypris sp., Prionocypris zenkeri, Bradleystrandesia reticulata, Herpetocypris sp. 2, and Dolerocypris sinensis. Candona bimucronata, however, was found by Petkovski et al. [] in a small rheocrene stream northeast of the City of Struga at Lake Ohrid, and D. sinensis was encountered in a puddle in a meadow near Struga []. Ilyocypris bradyi and P. zenkeri were reported by Holmes from nearby wetlands [].
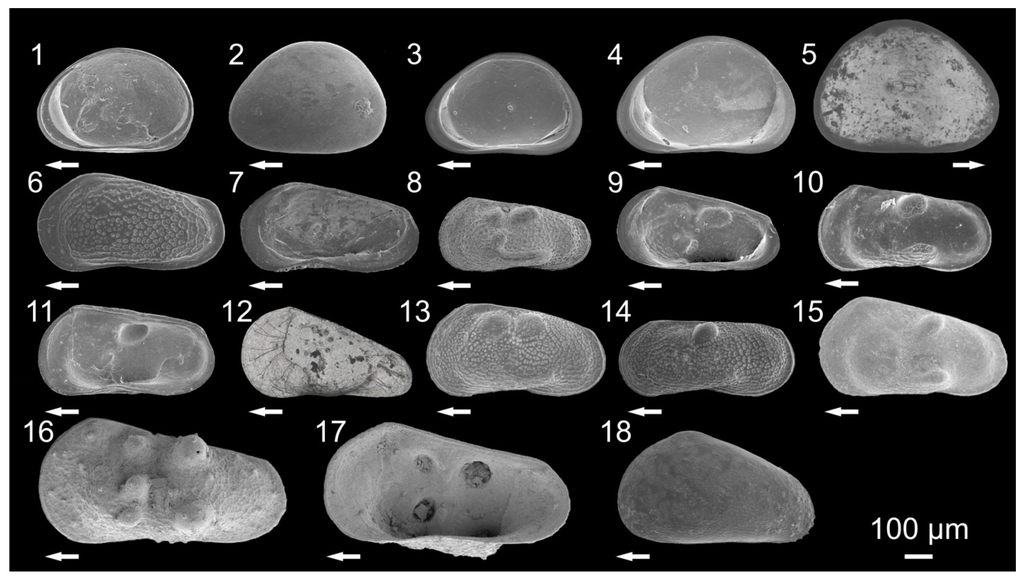
Figure 5.
Pictures of ostracodes from Lake Ohrid: Cyclocypris ovum (1) Right valve (RV), internal view, female; Cypria obliqua (2) Left valve (LV), external view, female; C. obliqua (3) RV, internal view, female; C. lacustris (4) RV, internal view, female; Cypria ophtalmica (5) LV, internal view, isolated valve; Amnicythere karamani (6) LV, external view, female; A. karamani (7) RV, internal view, female; Paralimnocythere ochridense (8) LV, external view, male; P. ochridense (9) RV, internal view, female; Paralimnocythere karamani (10) LV, external view, female; P.karamani (11) RV, internal view, female; Paralimnocythere alata (12) LV, external view, female; Paralimnocythere slavei (13) LV, external view, female; P. slavei (14) LV, external view, male; Paralimnocythere georgevitschi (15) LV, external view, female; Paralimnocythere umbonata (16) LV, external view, female; P. umbonata (17) RV, internal view, female; Prionocypris zenkeri (16) LV, external view, juvenile. Arrows point to anterior part of valves (modified from []).

Table 1.
Checklist of ostracode species from Lake Ohrid, including synonymy. Comparison with the ostracode fauna reported in this paper and by [] (+living species, * valves or empty carapaces), species reported previously in the lake from other authors, and Petkovski (2005, unpubl. data).
| Ostracode Species in Lake Ohrid | This Paper | Other Authors | Petkovski (2005, Unpubl. Data) |
|---|---|---|---|
| Amnicythere angulata | (Klie, 1939b) | + | |
| Amnicythere karamani | + | (Klie, 1939b) | + |
| Amnicythere prespensis | (Petkovski & Keyser, 1992) | + | |
| Amnicythere proboscidea | (Klie, 1939b) | + | |
| Bradleystrandesia reticulata | + | ||
| Candona acricauda | Mikulić, 1961 | synonym of Candona holmesi | |
| Candona alta | Klie, 1939a | Candona neglecta (review needed) | |
| Candona bimucronata | + | ||
| Candona candida | Holmes, 1937 | ||
| Candona compressa | Klie, 1942 | Pseudocandona compressa | |
| Candona cristatella | Klie, 1939a | synonym of Candona ohrida | |
| Candona depressa | Klie, 1939a | + | |
| Candona expansa | * | Mikulić,1961 | + |
| Candona fabaeformis | Klie, 1939a | Eucandona (Fabaeformiscandona) krstici | |
| Candona formosa | Mikulić,1961 | + | |
| Candona goricensis | + | Mikulić,1961 | + |
| Candona hadzistei | + | Petkovski et al. 2002 | + |
| Candona hartmanni | + | Petkovski, 1969a | + |
| Candona holmesi | * | Petkovski, 1960a | + |
| Candona jordeae | Petkovski et al. 2002 | + | |
| Candona litoralis | + | Mikulić,1961 | + |
| Candona lucida | Petkovski, 1969a | renamed in Candona jordeae | |
| Candona lychnitis | Petkovski, 1969a | + | |
| Candona macedonica | Mikulić,1961 | + | |
| Candona margaritana | + | Mikulić,1961 | |
| Candona marginata | * | Klie, 1942 | + |
| Candona marginatoides | + | Petkovski, 1960b | + |
| Candona media | + | Klie, 1939a | Candona neglecta (review needed) |
| Candona neglecta | Holmes, 1937 | + | |
| Candona ohrida | + | Holmes, 1937 | + |
| Candona ovalis | + | Mikulić,1961 | + |
| Candona parvula | Mikulić,1961 | renamed in Candona hadzistei | |
| Candona sp. | Petkovski, 2005 (unpub. data) | + | |
| Candona sublitoralis | Mikulić,1961 | synonym of Candona ohrida | |
| Candona trapeziformis | + | Klie, 1939a | + |
| Candona triangulata | * | Klie, 1939a | + |
| Candona vidua | + | Klie, 1942 | + |
| Candonopsis kingsleii | + | Klie, 1942 | + |
| Cyclocypris ovum | + | Klie, 1939a | + |
| Cypria lacustris | + | Petkovski, 1960b | + |
| Cypria obliqua | + | Klie, 1939a | + |
| Cypria ophtalmica | * | Holmes, 1937 | |
| Cytherissa lacustris | + | Klie, 1942 | + |
| Darwinula stevensoni | + | Holmes, 1937 | + |
| Dolerocypris sinensis | + | ||
| Eucypris virens | + | ||
| Eucypris sp. | + | ||
| Fabaeformiscandona krstici | + | Petkovski, 1969a | + as Eucandona (Fabaeformiscandona) krstici |
| Herpetocypris sp. 2 | + | ||
| Ilyocypris bradyi | + | ||
| Paralimnocythere alta | + | Klie, 1939b | + |
| Paralimnocythere georgevitschi | + | (Petkovski, 1960c) | + |
| Paralimnocythere karamani | + | (Petkovski, 1960c) | + |
| Paralimnocythere ochridense | + | (Klie, 1934) | + |
| Paralimnocythere slavei | + | (Petkovski, 1969b) | + |
| Paralimnocythere umbonata | * | (Klie, 1939b) | + |
| Physocypria sp. (Physocypria kraepelini?) | Holmes, 1937 | Physocypria kliei? (review needed) | |
| Prionocypris zenkeri | + | ||
| Pseudocandona compressa | + | ||
| Pseudocandona elongata | Holmes, 1937 | + | |
| Pseudocandona slavei | Petkovski, 1969a | + |
3.2. Spatial Distribution, Species Richness and Diversity of Ostracodes
Lorenschat and Schwalb [] studied the ecological preferences of ostracodes in Lake Ohrid and indicated that water depth and conductivity (salinity) are the two main environmental variables that determine their distribution. We therefore focused on these two variables, even though we were aware that ostracode distribution may be influenced by other variables. Many of the species were widely distributed in the lake (Figure 6, Figure 7 and Figure 8). Endemic species had a broad water-depth range and non-endemic species occurred mainly at shallow depths.
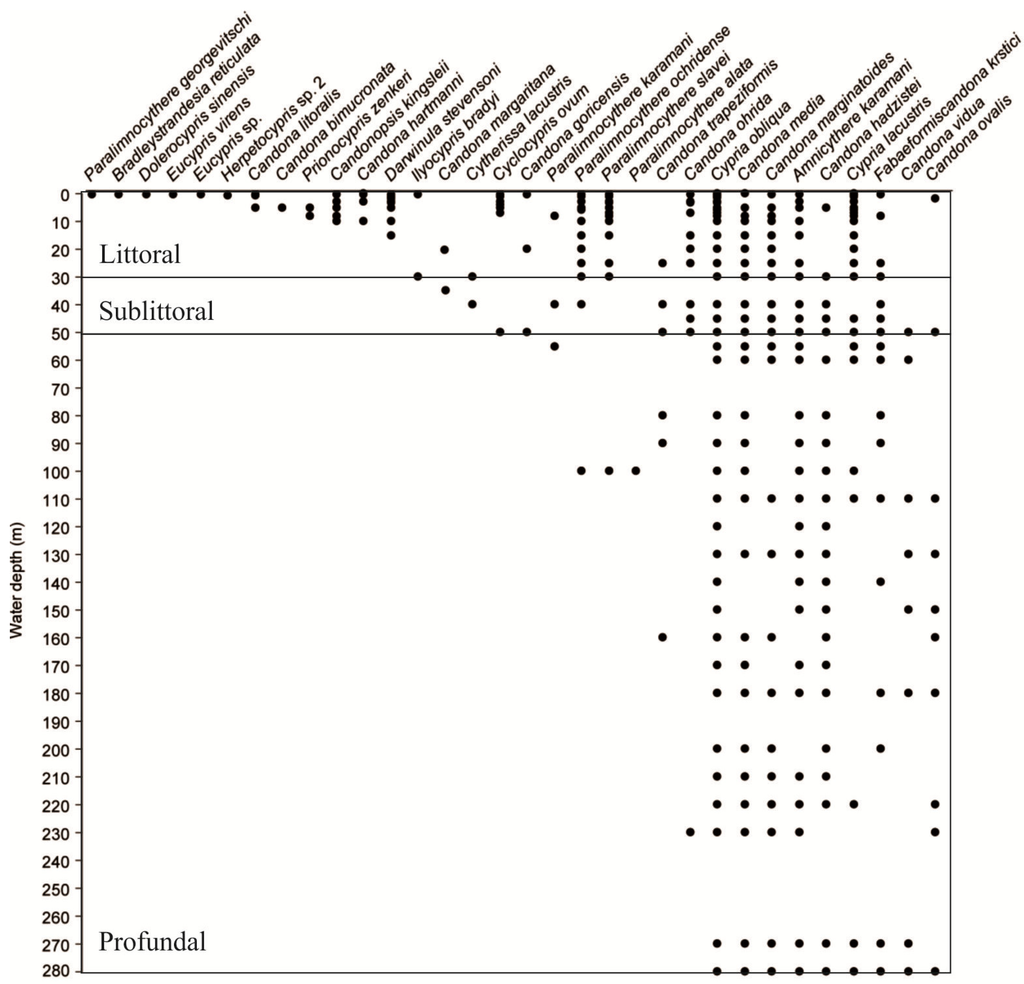
Figure 6.
Depth distributions of living ostracodes in Lake Ohrid.
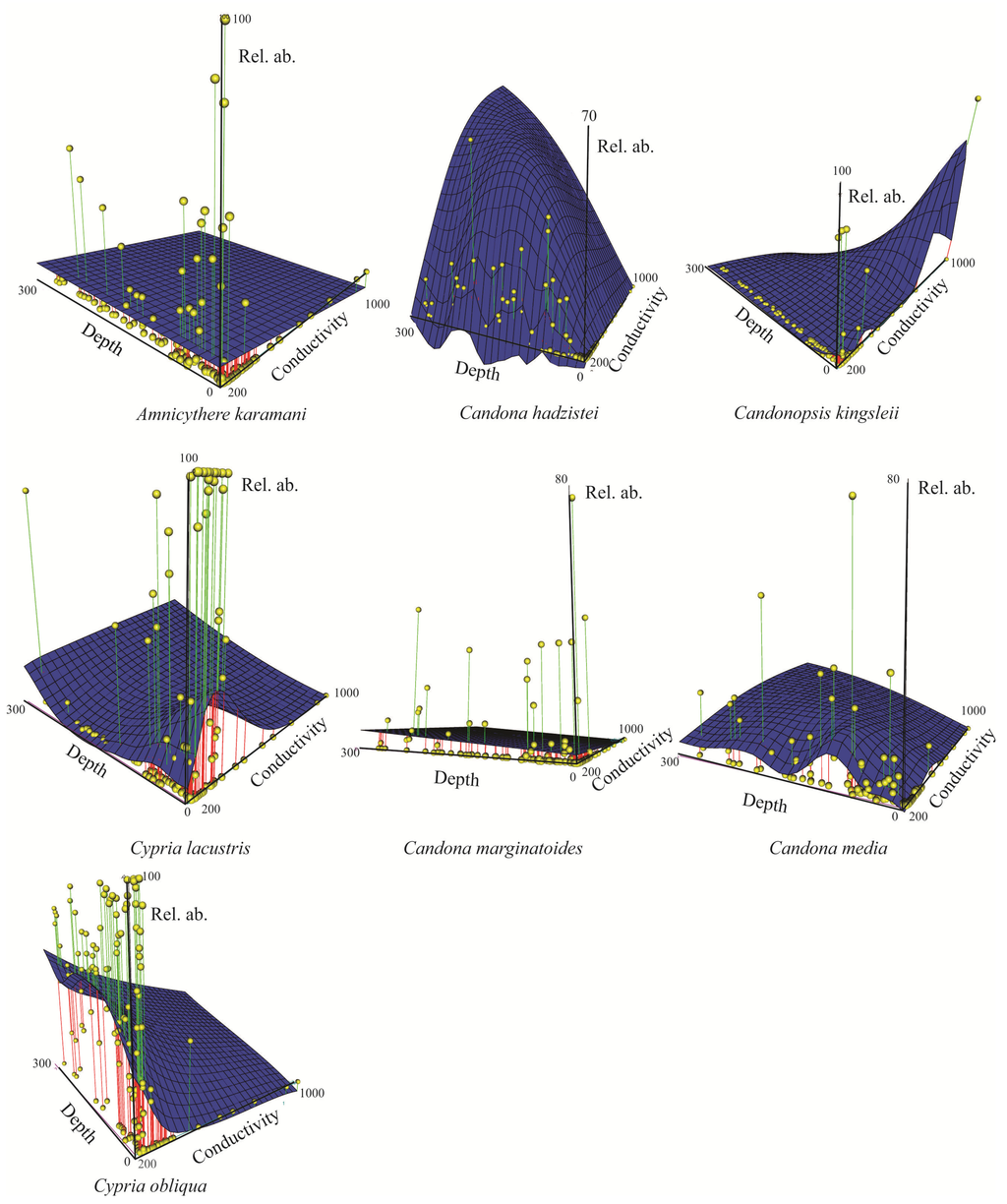
Figure 7.
3D scatter plot of species relative abundance (%) as a function of depth (m) and water conductivity (μS cm−1) reported for species collection sites. Vertical lines and spheres indicate the species relative abundances. Blue surface shows modeled abundance through smooth regression. Only species present in ≥10 sites are shown.
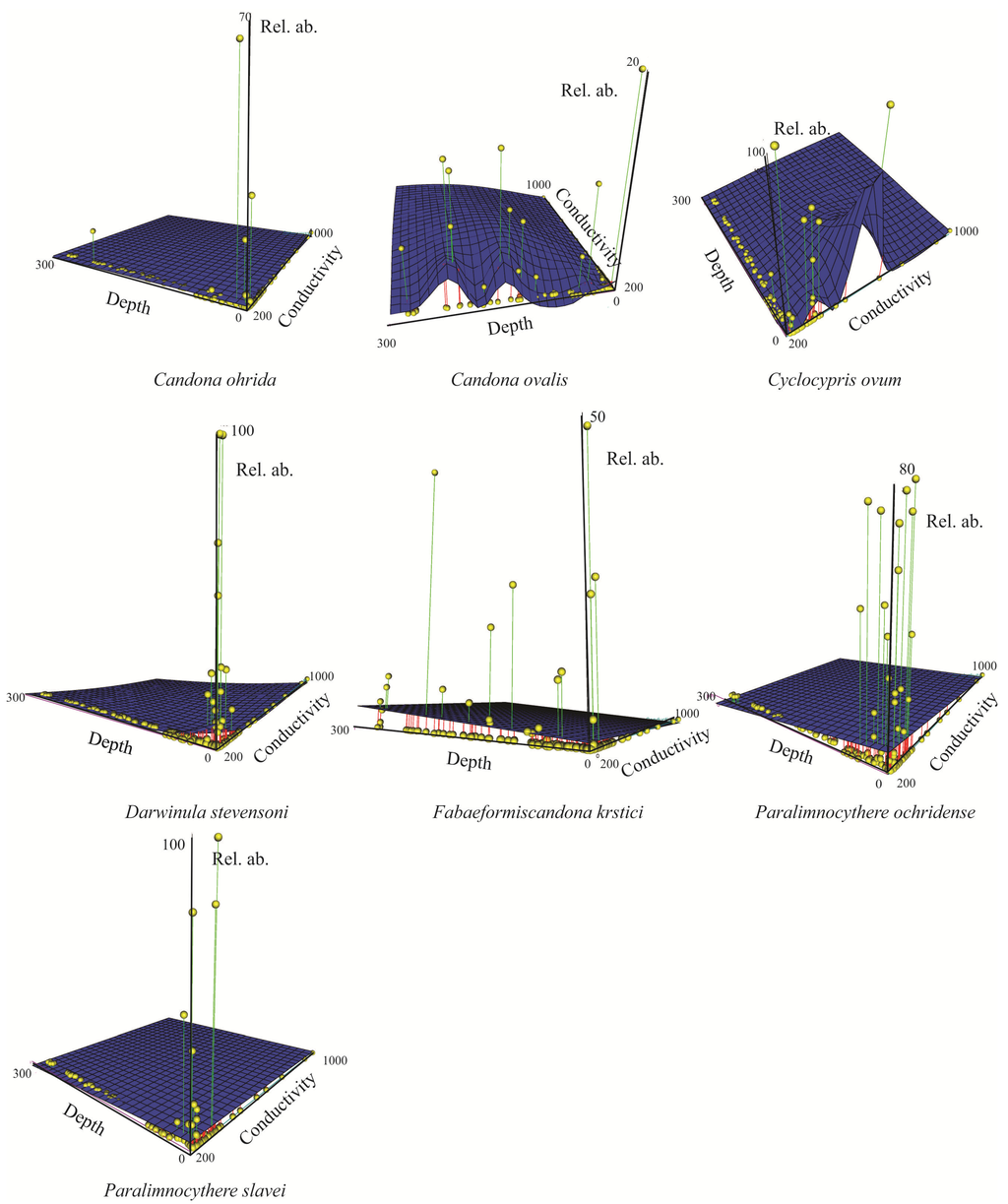
Figure 8.
3D scatter plot of species relative abundance (%) as a function of depth (m) and water conductivity (μS cm−1) reported for species collection sites. Vertical lines and spheres indicate the species relative abundances. Blue surface shows modeled abundance through smooth regression. Only species present in ≥10 sites are shown.
3.2.1. Spatial Distribution
Species of Paralimnocythere generally have highest abundances in the north part of the lake and P. ochridense showed the highest abundance of all species from this genus (777 individuals 190 cm−3). Only the two endemic species, Paralimnocythere alata and P. georgevitschi, are restricted to the north part of the lake, and five non-endemic species were also found only in the north part of the lake. Dolerocypris sinensis, Eucypris virens, Eucypris sp., and Bradleystrandesia reticulata occurred in the northeast part of the lake, which is affected by agricultural land use. Candona hartmanni was found by Petkovski [] along the muddy northeast shore of the lake. Prionocypris zenkeri inhabited only the northwest part of the lake, near the springs of Kališta, and Candona bimucronata colonized exclusively the Bay of Ohrid. Cytherissa lacustris occurred only in the area influenced by the springs of Sveti Naum and Herpetocypris sp. 2 was found in the littoral area near the street in the village of Peštani. Empty carapaces of Candona expansa were found only in the northern part of the lake and valves and empty carapaces of Candona holmesi were recovered at 14 sampling locations distributed around the entire lake. Valves and empty carapaces of Candona marginata and Cypria ophtalmica were found in only one sample from the eastern shore. Dead individuals of Candona triangulata occurred in three samples from the eastern shore and remains of Paralimnocythere umbonata were found in one sample from the western shore of the lake. In the southern part of the lake, P. ochridense showed high abundance in one sample taken in the area of Sveti Naum (516 individuals 190 cm−3). Otherwise, abundances for other species ranged between 1 and 9 individuals/190 cm3 in the southern part of the basin. Species richness and the diversity and evenness indexes were higher in the northern and eastern parts of the lake (Figure 9, Figure 10 and Figure 11).
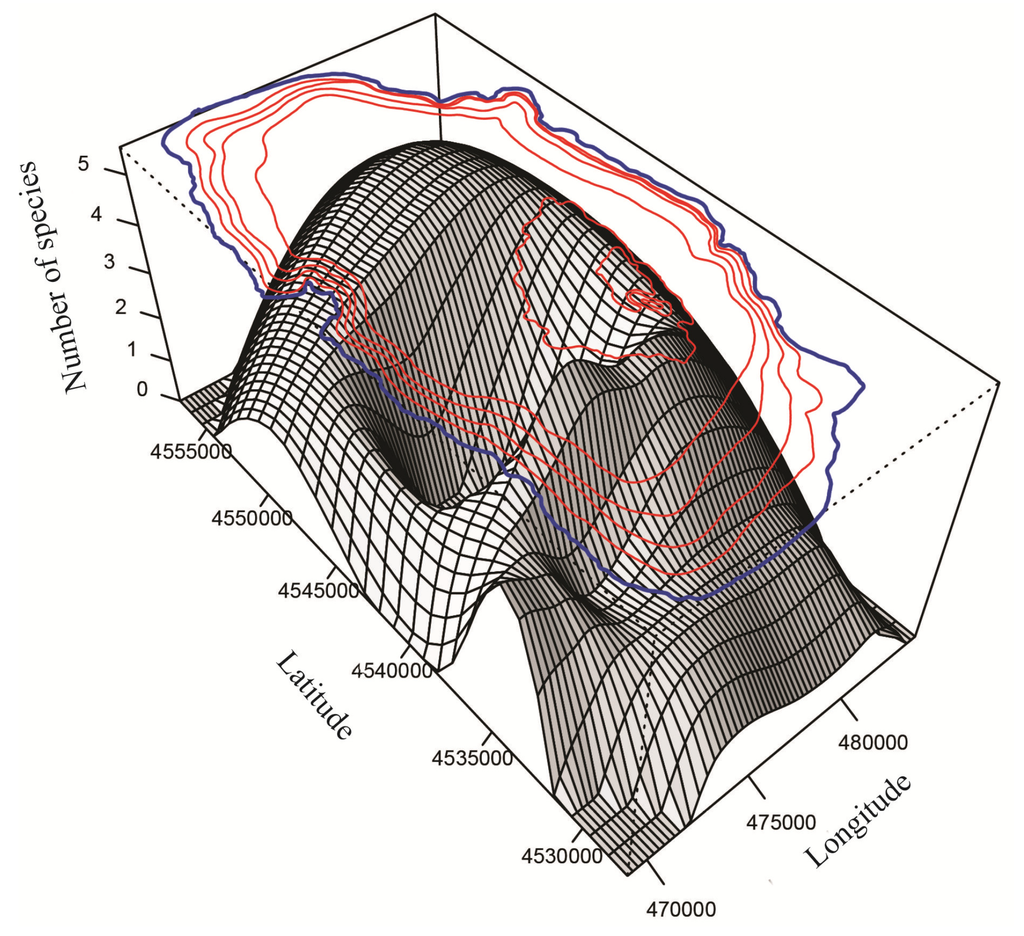
Figure 9.
Spatially modeled species richness (S) in Lake Ohrid.

Figure 10.
Spatially modeled species diversity (H) in Lake Ohrid.
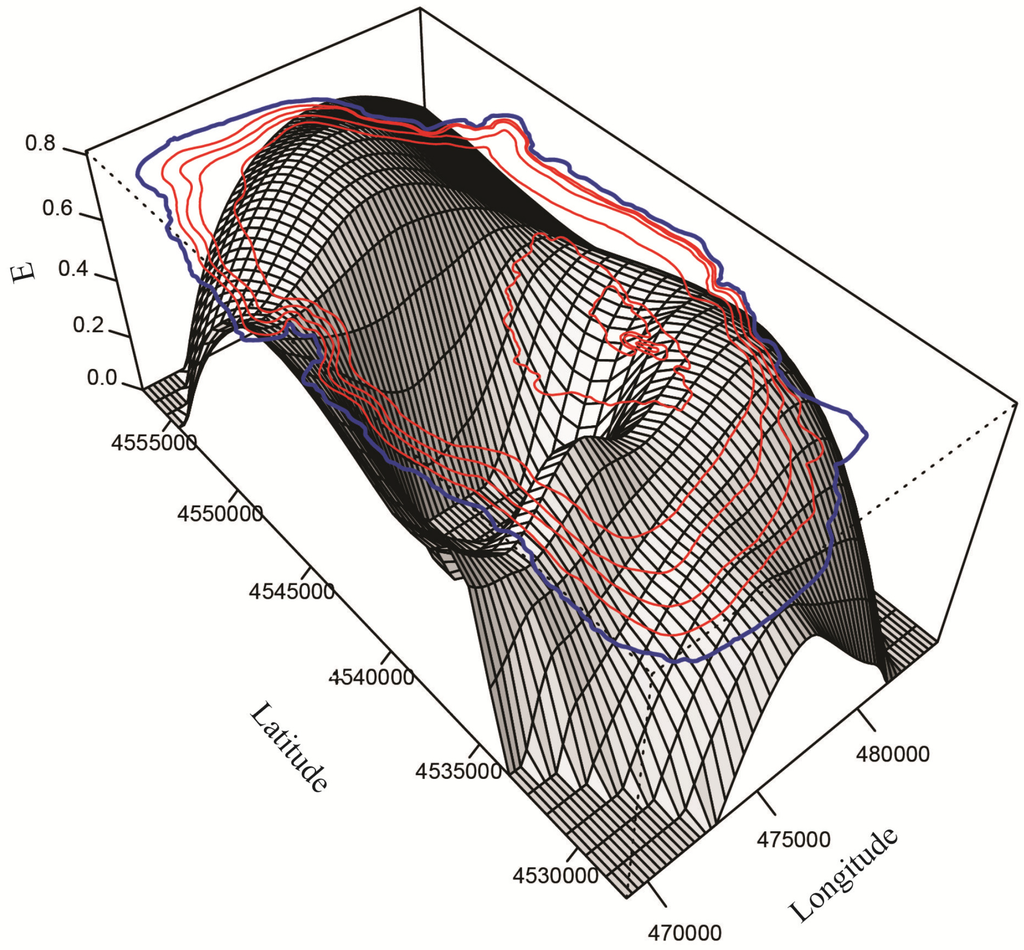
Figure 11.
Spatially modeled species evenness (E) in Lake Ohrid.
3.2.2. Vertical Distribution
Figure 7 and Figure 8 display 3D plots of the relative abundance, water depth and conductivity data corresponding to the species collection sites. Our studies are the first in Lake Ohrid that sampled down to 280 m water depth [,]. We found that some species were restricted to littoral, sublittoral or profundal zones, whereas other species had wider distributions (littoral-sublittoral zones or littoral-profundal zones, Figure 6, Figure 7 and Figure 8).
3.2.2.1. Littoral Zone (≤30 m)
Three species of Candona (C. litoralis, C. bimucronata and C. hartmanni) occurred only in the littoral zone (Figure 6), which we define as water depths to 30 m because we found submerged aquatic vegetation (Chara and Potamogeton) down to this depth during all sampling campaigns. Besides the three species of Candona, P. georgevitschi, C. litoralis, C. bimucronata, and C. hartmanni, and nine other species inhabited only the littoral zone (Figure 6). The “real littoral Candona species” C. litoralis was found by Mikulić [] down to 20 m water depth, and C. bimucronata was reported by Griffiths and Brancelj [] from 13 m to 15 m in Lake Bohinj (Slovenia). Highest abundance of all Ohrid species was displayed by C. lacustris: 2468 individuals 190 cm−3 at 9 m water depth and 1910 individuals 190 cm−3 at 0.3 m (Figure 7). Highest abundances of this species were reported mainly in waters with low conductivities. Paralimnocythere georgevitschi was found only at 0.3 m water depth. Paralimnocythere ochridense showed the highest abundance of all species from this genus (777 individuals 190 cm−3) at 30 m water depth and in collection sites with low conductivity (Figure 8), and also displayed high abundance in a sample from the area of Sveti Naum taken at 7 m depth (516 individuals 190 cm−3). Klie found this species in the littoral of Lake Ohrid [] and empty carapaces down to 10 m []. Petkovski [] reported P. slavei from the littoral zone. Ilyocypris bradyi appeared near the springs of Sveti Naum at 30 m water depth (14 and 9 individuals 190 cm−3) and at 0.5 m (1 individual 190 cm−3). Meisch [] suggested that individuals of I. bradyi found in lakes are usually discharged from nearby springs. Darwinula stevensoni occurred in water depths between 0.2 m and 15 m, where conductivities were low (Figure 6 and Figure 8) and highest abundance was at 0.4 m water depth (30 individuals 190 cm−3). Holmes et al. [] found D. stevensoni in water depths from 7 m to 10 m. According to Meisch [] this species lives at depths of 0–12 m, with a maximum abundance at 6 m. We confirmed that Candonopsis kingsleii prefers the littoral zone of lakes []. It was collected at 0.3 and 10.2 m water depth (Figure 6), with a maximum abundance (202 individuals 190 cm−3) at 3 m depth. It seems to tolerate water conductivities up to 1000 μS cm−1 (Figure 7). Klie [] reported C. kingsleii in Lake Ohrid, but the location and water depth were not indicated. Prionocypris zenkeri was found in two samples from 5 m and 8.7 m water depth near the northwest shore, but abundances were very low (1 and 2 individuals 190 cm−3) (Figure 6). This area near the village of Kališta is affected by subaquatic and surface springs. This species is known to occur in waters connected to springs and the interstitial habitat of streams, and occasionally enters lakes from nearby streams []. Eucypris virens was found at 0.3 and 0.5 m water depth in the northeastern part of the lake, as were D. sinensis (0.3 m depth), Eucypris sp. (0.3 m depth) and B. reticulata (0.5 m depth) (Figure 6). None of these species had been reported in the lake before. Eucypris virens, D. sinensis and B. reticulata are species that colonize temporary waters in open fields [] and it cannot be ruled out that these species immigrated in waters from the northeast part of the Lake Ohrid watershed, which is affected by agricultural land use. We found Herpetocypris sp. 2 in one sample from 0.7 m depth. In general, highest species richness was found in the littoral zone (Figure 9), especially in the Bay of Ohrid and near the springs of Kališta. High species richness, diversity and evenness characterize the littoral zone, especially in the northern and eastern parts of the lake (Figure 9, Figure 10 and Figure 11).
3.2.2.2. Sublittoral (30–50 m)
Few species are restricted to the sublittoral zone. Candona margaritana was found down to 35 m and C. goricensis down to 50 m. Candona margaritana colonized water depths ranging from 20 m to 35 m, but Mikulić [] found it between 20 m to 100 m. Cytherissa lacustris was present in our samples from 30 and 40 m water depth and maximum abundance occurred at 30 m (94 individuals 190 cm−3) (Figure 6). Klie [] reported that it occurs in the littoral zone of lakes, but prefers deeper water, and according to Meisch [], it inhabits the sublittoral and profundal zones of cold, deep lakes and the littoral and sublittoral zones of high-altitude, alpine lakes. Similar to the littoral zone, high species richness, diversity and evenness were reported for the sublittoral zone, especially at 30 m and 50 m water depth.
3.2.2.3. Profundal (50–280 m)
Candona trapeziformis was found at a maximum depth of 163 m and C. ohrida, which seems to prefer low conductivities, at a maximum depth of 232 m (Figure 8). All other species of Candona and the genera Fabaeformiscandona, Cypria and Amnicythere occurred down to the maximum depth of 280 m. Comparison of our data to those from previous sampling campaigns is difficult because sampling during the earlier projects was restricted to water depths between the littoral and 160 m. One deep sample, from a water depth of 240 m, was analyzed by Klie []. Candona vidua occurred in our samples from 50 m to 280 m. Klie [] encountered it at water depths of 40, 160, and 240 m and Mikulić and Pljakić [] found it between 20 m and 100 m. Ostracode abundances in our study were low at 280 m water depth. The most frequent species was C. obliqua (95 individuals 190 cm−3), followed by C. lacustris (57, 18 and 7 individuals 190 cm−3 in three samples), which prefers conductivities <500 μS cm−1 (Figure 7). All other species at 280 m water depth showed low abundances (1–4 individuals 190 cm−3). Samples collected near Sveti Naum displayed high species richness. Below 50 m water depth, species richness, diversity and evenness are relatively low, although there are peaks at 110, 135, 200, 220 and 280 m water depth (Figure 9, Figure 10 and Figure 11).
3.2.2.4. Littoral-Sublittoral Taxa (≤50 m)
We found C. goricensis in the littoral and sublittoral zone (0.5, 20, and 50 m), whereas Mikulić [] found this species only between 20 m and 50 m water depth. Candona media was found at 40 and 100 m water depth by Klie [] and between 20 m and 100 m by Mikulić and Pljakić []. This species shows a broad water-depth distribution and a preference for low conductivities (Figure 7). Candona ovalis was discovered by Mikulić [] only between 20 m and 100 m water depth, and our results suggest that it prefers waters with low conductivities. Paralimnocythere karamani inhabited water depths to only 54 m. This species and P. georgevitschi (a littoral species) were reported from Lake Ohrid by Petkovski [], but sample depths were not reported. Cyclocypris ovum appeared in water depths from 0.3 m to 50 m (Figure 6), with a maximum abundance at 5.5 m (394 individuals 190 cm−3). Preferred conductivity is <700 μS cm−1. Klie [] found this species in the lake down to 10 m water depth, and according to Meisch [] it is most common in the littoral zone of lakes, but also occurs down to water depths of 70 m []. As indicated, the littoral and sublittoral zones are characterized by high species richness, diversity and evenness (Figure 9, Figure 10 and Figure 11).
3.2.2.5. Wide Depth-Range Taxa
The genera Candona, Fabaeformiscandona, Cypria and Amnicythere showed the greatest water-depth ranges (Figure 6). Candona trapeziformis occurred in our samples from 25 m to 163 m water depth. Klie [] indicated only “great depth” for locations where it was encountered and Mikulić and Pljakić [] found this species between 20 m and 100 m. Candona ohrida was present between 0.3 m and 232 m water depth. Highest abundances were seen in shallow waters and indicated a preference for water with low conductivity (Figure 8). Holmes [] collected C. ohrida in a shallow pool on a marsh near Lake Ohrid and Mikulić [] reported it (as C. sublitoralis, Table 1) between 20 m and 50 m. We found four species of Candona that inhabited the lake from the littoral zone down to 280 m water depth: C. hadzistei, C. marginatoides, C. media, and C. ovalis. These species also showed a preference for low conductivities (Figure 7). Mikulić [] reported C. hadzistei (as C. parvula, Table 1) in the littoral, sublittoral, and profundal zones. Depth preferences for C. marginatoides were not reported in previous studies. F. krstici was collected by us from 0.2 m down to 280 m water depth (Figure 6 and Figure 8), whereas Petkovski [] reported F. krstici from the transition between the littoral and the sublittoral zone. We found C. lacustris and C. obliqua from 0.1 m down to 280 m depth. Cypria lacustris was reported by Petkovski [] in deeper parts of the lake and C. obliqua by Klie [] at 40, 100, and 120 m water depth. Amnicythere karamani inhabited water depths from 0.4 down to 280 m (Figure 6 and Figure 7) and Klie [] collected this species only at 40 m. This species was found in waters with high conductivities (Figure 7). Most species of Paralimnocythere occurred down to a maximum water depth of 100 m (Figure 6), but P. alata appeared only in one sample taken from 100 m. Klie [] found this species at 120 m water depth, but also at 40 m. We discovered maximum abundances of P. ochridense and P. slavei in the littoral zone, but we also found individuals down to water depths of 100 m (Figure 6, Figure 8). Generally, the diversity of candonids was higher in the sublittoral and profundal zones and lower in the littoral zone. This partially confirmed the results of Mikulić and Pljakić [] who also found highest candonid diversity in the sublittoral zone.
3.3. Biogeography and Anthropogenic Impact on Ostracode Distribution
The endemic ostracode species in Lake Ohrid are highlighted in Box 1. Fourteen of 32 collected species displayed well-preserved soft parts, and five were identified only from hard parts. Martens [] reported 38 species of which 25 species (66%) were endemic, and mentioned that two thirds of the Ohrid endemics belong to the family Candonidae. Our findings indicate 44% of the Ohrid ostracodes are endemic and 79% of the endemics (11 species) belong to the Candonidae. Candona marginatoides, C. obliqua, A. karamani and P. ochridense occur in Lakes Ohrid and Prespa []. Darwinula stevensoni is the only truly cosmopolitan species in the lake. Candonopsis kingsleii, Cyclocypris ovum, I. bradyi, E. virens, B. reticulata, and C. lacustris are found in the Holarctic, D. sinensis in the Palaearctic (Eurasia), and P. zenkeri in Europe and Asia Minor (Turkey) []. Paralimnocythere karamani is known from Lakes Ohrid and Prespa and was also found in a spring and a river in the Skadar Valley, Montenegro []. Candona bimucronata inhabits waters in the montane, subalpine and high alpine areas of Macedonia and Montenegro and it is also common in the mountains of Bosnia, Kosovo, and Slovenia [,]. Cypria lacustris was described from areas including Iran, Bulgaria, Montenegro, Serbia, Croatia and Slovenia []. Meisch [] mentioned that C. lacustris and C. ophtalmica appear to be extreme forms of a single species that is variable in autecology, carapace shape, structure of the penis, male clasping organs and all genital processes of the female. We propose that C. lacustris and C. ophtalmica are two different species because the genital processes of the female specimens differ; C. ophtalmica has two processes and C. lacustris has only one. But because the differences between processes show variations, it will be necessary to carry out DNA analysis to determine if this is one or two taxa. Cypria ophtalmica is a nearly cosmopolitan species, absent only from Australia.
Our findings regarding the diversity of endemic species contradict the results of Martens []. He reported that endemic diversity is higher in the littoral zone than in the sublittoral or profundal zones of ancient lakes. We found cosmopolitan species and those distributed in the Holarctic or the Palaearctic in <50 m water depth, whereas species endemic to the lake occurred mostly in deeper water. Few lakes in the world possess living ostracodes at great water depths, which highlights the importance of this finding. Live specimens were also reported at water depths >40 m in Lake Petén Itzá, Guatemala []. Pérez et al. [,] also showed that water depth and conductivity are the factors that most influence species distribution in water bodies on the neotropical Yucatán Peninsula.
Ostracodes in Lake Ohrid show a distribution pattern similar to that for Gastropoda [], in that the most abundant snail species are endemic and non-endemic species are relatively rare. Endemic snail species displayed a broad water-depth range, whereas non-endemic species were largely restricted to shallow waters, down to only ~5 m. Furthermore, most of the widespread species, which were previously unknown from the lake, occur mainly at the north end where the two largest Macedonian cities are located (Ohrid and Struga), and where much land is in agriculture. This may be evidence for increasing anthropogenic pressure on the lake, because cosmopolitan species often replace rare endemic taxa []. Kostoski et al. [] also pointed out that anthropogenic pressure threatens endemic species. Pieri et al. [] found that wastewater discharge in the Ledra River Basin (NE Italy) systematically causes replacement of local, rare ostracode species by common species. Because all the cosmopolitan species found in this survey were not reported in previous studies, we suspect that recent increases in anthropogenic pressures, combined with the wider tolerance ranges of cosmopolitan species, is leading to replacement of endemic species by widespread taxa in the littoral zone. Endemic ostracode species were relatively evenly distributed in the lake and did not show preferences for areas influenced by spring inflows, as mentioned by Matzinger et al. [] and Matter et al. [].
4. Conclusions
Lake Ohrid is characterized by high ostracode species richness. The most speciose genus in the lake is Candona (12 living species) and the second most prominent genus is Paralimnocythere (five living species). The “young” ancient lake status was confirmed by the prevalence of species flocks of Limnocytherinae and Candonidae. Cypria lacustris displayed the highest abundances (2468 individuals 190 cm−3) among encountered species. Species abundances at 280 m water depth were relatively low, with highest values of 95 individuals 190 cm−3 (C. obliqua). Ostracode species living at 280 m water depth include C. obliqua, C. hadzistei, C. marginatoides, C. media, C. ovalis, C. vidua, F. krstici, C. lacustris, and A. karamani. Highest species richness was detected at 5 m water depth (15 species). Lower species richness in deep waters may reflect extreme environmental conditions (e.g., low oxygen content), and higher richness in the littoral zone may be a consequence of high O2 and diverse microhabitats. The water-depth distribution of ostracodes in the lake is probably a consequence of multiple interacting factors including oxygen concentration, water temperature, bottom substrate and food availability. In addition, ostracodes adapt to the environment by being mobile. Eight species were detected in the lake for the first time: C. bimucronata, I. bradyi, E. virens, Eucypris sp., P. zenkeri, B. reticulata, Herpetocypris sp. 2, and D. sinensis. These species and all the other cosmopolitan species found in the lake are restricted to water depths <50 m. However, given the increasing anthropogenic pressure on Lake Ohrid and the large tolerance ranges of some cosmopolitan species, we expect that endemic ostracodes will be progressively replaced by widespread species. The presence of A. karamani may be important for providing insights into the origin of Lake Ohrid. Although Amnicythere species usually inhabit oligo-haline to meso-haline waters, some species are marine, which may suggest a marine origin for the lake. Recent studies, however, indicate fluvial/glaciofluvial sedimentation at the onset of Lake Ohrid.
Acknowledgments
We are grateful to Trajan Petkovski (Macedonian Museum of Natural History, Skopje) and Burkhard Scharf (Bremen) for support with ostracode taxonomy, Dietmar Keyser (Zoologisches Institut und Zoologisches Museum, Hamburg), Martin Wessels (Institut für Seenforschung, Langenargen) for equipment supply and Bernd Wagner and Hanna Ciesynski (Institut für Geologie und Mineralogie, Universität zu Köln) for the bathymetric map and SEM pictures, respectively. Special thanks go to Goce Kostoski, Sasho Trajanovski, and Zoran Brdarovski (Hydrobiological Institute, Ohrid) for their support and commitment during the field campaigns. We thank Bernd Wagner and anonymous reviewers for constructive comments that improved this manuscript. This project was funded by the Deutsche Forschungsgemeinschaft (Schw 671/11).
Author Contributions
This study was designed and conducted by Antje Schwalb and Julia Lorenschat. Julia Lorenschat organized and carried out the field trips, laboratory analysis (identification and counting), statistical analysis and manuscript writing. Antje Schwalb provided funding for the development of this study (DFG project) and made important revisions to the submitted manuscript. Liseth Pérez was the corresponding author and was in charge of the manuscript final corrections suggested by multiple reviewers. Alexander Correa-Metrio was responsible for all statistical analyses and graphs, and Ullrich von Bramann provided the lake bathymetric data. Mark Brenner contributed with manuscript editing and language improvement. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martens, K. Speciation in ancient lakes. Trends Ecol. Evol. 1997, 12, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Schön, I.; Martens, K. Adaptive, pre-adaptive and non-adaptive components of radiations in ancient lakes: A review. Org. Divers. Evol. 2004, 4, 137–156. [Google Scholar] [CrossRef]
- Albrecht, C.; Trajanovski, S.; Kuhn, K.; Streit, B.; Wilke, T. Rapid evolution of an ancient lake species flock: Freshwater limpets (gastropoda: Ancylidae) in the balkan lake Ohrid. Org. Divers. Evol. 2006, 6, 294–307. [Google Scholar] [CrossRef]
- Von Rintelen, T.; Glaubrecht, M. Rapid evolution of sessility in an endemic species flock of the freshwater bivalve Corbicula from ancient lakes on sulawesi, Indonesia. Biol. Lett. 2006, 2, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Lindhorst, K.; Vogel, H.; Krastel, S.; Wagner, B.; Hilgers, A.; Zander, A.; Schwenk, T.; Wessels, M.; Daut, G. Stratigraphic analysis of lake level fluctuations in lake Ohrid: An integration of high resolution hydro-acoustic data and sediment cores. Biogeosciences 2010, 7, 3651–3689. [Google Scholar] [CrossRef]
- Griffiths, H.I.; Reed, J.M.; Leng, M.J.; Ryan, S.; Petkovski, S. The recent palaeoecology and conservation status of balkan lake Dojran. Biol. Conserv. 2002, 104, 35–49. [Google Scholar] [CrossRef]
- Frogley, M.R.; Preece, R.C. A faunistic review of the modern and fossil molluscan fauna from lake pamvotis, ioannina, an ancient lake in NW Greece: Implications for endemism in the Balkans. In Balkan Biodiversity—Pattern and Process in the European Hotspot; Griffiths, H.I., Kryštufek, B., Reed, J.M., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2004; pp. 243–260. [Google Scholar]
- Reed, J.M.; Kryštufek, B.; Eastwood, W.J. The physical geography of the Balkans and nomenclature of place names. In Balkan Biodiversity—Pattern and Process in the European Hotspot; Griffiths, H.I., Kryštufek, B., Reed, J.M., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2004; pp. 9–22. [Google Scholar]
- Albrecht, C.; Hauffe, T.; Schreiber, K.; Trajanovski, S.; Wilke, T. Mollusc biodiversity and endemism in the potential ancient lake Trichonis, Greece. Malacologia 2009, 51, 357–375. [Google Scholar] [CrossRef]
- Albrecht, C.; Wilke, T. Ancient lake Ohrid: Biodiversity and evolution. Hydrobiologia 2008, 615, 103–140. [Google Scholar] [CrossRef]
- Trajanovski, S.; Albrecht, C.; Schreiber, K.; Schultheiß, R.; Stadler, T.; Benke, M.; Wilke, T. Testing the spatial and temporal framework of speciation in an ancient lake species flock: The leech genus Dina (hirudinea: Erpobdellidae) in lake Ohrid. Biogeosciences 2010, 7, 3387–3402. [Google Scholar] [CrossRef]
- Wagner, B.; Reicherter, K.; Daut, G.; Wessels, M.; Matzinger, A.; Schwalb, A.; Spirkovski, Z.; Sanxhaku, M. The potential of lake Ohrid for long-term palaeoenvironmental reconstructions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 259, 341–356. [Google Scholar] [CrossRef]
- Belmecheri, S.; Namiotko, T.; Robert, C.; von Grafenstein, U.; Danielopol, D.L. Climate controlled ostracod preservation in lake Ohrid (Albania, Macedonia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 277, 236–245. [Google Scholar] [CrossRef]
- Stankovič, S. The Balkan Lake Ohrid and Its Living World; W. Junk: Den Haag, The Netherlands, 1960; Volume IX, p. 357. [Google Scholar]
- Salemaa, H. Lake Ohrid. Arch. Hydrobiol. 1994, 44, 55–64. [Google Scholar]
- Klie, W. Zur kenntnis der ostracoden-gattung Limnocythere. Z Wiss Zool Abt A 1934, 3, 534–544. (In German) [Google Scholar]
- Klie, W. Studien über ostracoden aus dem Ohridsee: II. Limnocytherinae und Cytherinae. Arch. Hydrobiol. 1939, 35, 631–646. (In German) [Google Scholar]
- Klie, W. Studien über ostracoden aus dem Ohridsee: I. Candocyprinae. Arch. Hydrobiol. 1939, 35, 28–45. (In German) [Google Scholar]
- Klie, W. Studien über ostracoden aus dem Ohridsee: III. Erster nachtrag. Arch. Hydrobiol. 1942, 38, 254–259. (In German) [Google Scholar]
- Holmes, P.F. Ostracoda of lake Ohrid. Arch. Hydrobiol. 1937, 31, 484–500. [Google Scholar]
- Mikulić, F. Nove Candona vrste iz Ohridskog jezera. Bull Mus natn Hist nat 1961, 17, 87–107. (In German) [Google Scholar]
- Petkovski, T.K. Zur kenntnis der crustaceen des Prespasees. Fragm Balc. Mus. Maced. Sci. Nat. 1960, 3, 117–131. (In German) [Google Scholar]
- Petkovski, T.K. Zwei neue ostracoden aus dem Ohrid- und Prepasee. Izd Inst Piscic RP Maced 1960, 3, 57–66. (In German) [Google Scholar]
- Petkovski, T.K. Süsswasserostracoden aus Jugoslawien VII. Fragm Balc Mus Maced Sci. Nat. 1960, 3, 99–108. (In German) [Google Scholar]
- Petkovski, T.K. Zwei neue Limnocythere-arten aus Mazedonien (crustacea-ostracoda). Musei Maced. Sci. Nat. 1969, 12, 1–18. (In German) [Google Scholar]
- Petkovski, T.K. Einige neue und bemerkenswerte Candoninae aus dem Ohridsee und einigen anderen fundorten in Europa. Musei Maced. Sci. Nat. 1969, 11, 81–111. (In German) [Google Scholar]
- Mikulić, F.; Pljakić, M.A. Die merkmale der kvalitativen distribution der endemischen Candonaarten im Ochridsee. Ekologija 1970, 5, 101–115. (In German) [Google Scholar]
- Wagner, B.; Lotter, A.; Nowaczyk, N.; Reed, J.; Schwalb, A.; Sulpizio, R.; Valsecchi, V.; Wessels, M.; Zanchetta, G. A 40,000-year record of environmental change from ancient lake Ohrid (Albania and Macedonia). J. Paleolimnol. 2009, 41, 407–430. [Google Scholar] [CrossRef]
- Lorenschat, J.; Scharf, B.; Petkovski, T.; Viehberg, F.; Schwalb, A. Scientific collaboration on past speciation conditions in Ohrid (SCOPSCO): Recent and fossil ostracods from lake Ohrid as indicators of past environments: A coupled ecological and molecular genetic approach with deep-time perspective. Joannea Geologie und Palaontologie 2011, 11, 113–115. [Google Scholar]
- Lorenschat, J.; Schwalb, A. Autecology of the extant ostracod fauna of Lake Ohrid and adjacent waters—A key to paleoenvironmental reconstruction. Belg. J. Zool. 2013, 143, 42–68. [Google Scholar]
- Lindhorst, K.; Krastel, S.; Reicherter, K.; Stipp, M.; Wagner, B.; Schwenk, T. Sedimentary and Tectonic Evolution of Lake Ohrid (Macedonia/Albania). Basin Res. 2014. [Google Scholar] [CrossRef]
- Hauffe, T.; Albrecht, C.; Schreiber, K.; Birkhofer, K.; Trajanovski, S.; Wilke, T. Spatially explicit analysis of gastropod biodiversity in ancient Lake Ohrid. Biogeosciences 2011, 8, 175–188. [Google Scholar] [CrossRef]
- Spirkovski, Z.; Avramovski, O.; Kodzoman, A. Watershed management in the lake Ohrid region of Albania and Macedonia. Lakes Reserv. Res. Manag. 2001, 6, 237–242. [Google Scholar] [CrossRef]
- Wagner, B.; Wilke, T.; Krastel, S.; Zanchetta, G.; Sulpizio, R.; Reicherter, K.; Leng, M.; Grazhdani, A.; Trajanovski, S.; Levkov, Z.; Reed, J.; Wonik, T. Deep drilling of ancient Lake Ohrid capture over 1 million years of evolution and global climate cycling. EOS Trans. 2014, 95, 25–26. [Google Scholar] [CrossRef]
- Leng, M.J.; Baneschi, I.; Zanchetta, G.; Jex, C.N.; Wagner, B.; Vogel, H. Late quaternary palaeoenvironmental reconstruction from lakes Ohrid and Prespa (Macedonia/Albania border) using stable isotopes. Biogeosciences 2010, 7, 3109–3122. [Google Scholar] [CrossRef]
- Popovska, C.; Bonacci, O. Basic data on the hydrology of lakes Ohrid and Prespa. Hydrol. Process 2007, 21, 658–664. [Google Scholar] [CrossRef]
- Matzinger, A.; Spirkovski, Z.; Patceva, S.; Wüest, A. Sensitivity of ancient lake Ohrid to local anthropogenic impacts and global warming. J. Great Lakes Res. 2006, 32, 158–179. [Google Scholar] [CrossRef]
- Matter, M.; Anselmetti, F.S.; Jordanoska, B.; Wagner, B.; Wessels, M.; Wüest, A. Carbonate sedimentation and effects of eutrophication observed at the kališta subaquatic springs in lake Ohrid (Macedonia). Biogeosciences 2010, 7, 4715–4747. [Google Scholar] [CrossRef]
- Vogel, H.; Wessels, M.; Albrecht, C.; Stich, H.B.; Wagner, B. Spatial variability of recent sedimentation in lake Ohrid (Albania/Macedonia)—A complex interplay of natural and anthropogenic factors and their possible impact on biodiversity patterns. Biogeosciences 2010, 7, 3333–3342. [Google Scholar] [CrossRef]
- Talevska, M.; Petrovic, D.; Milosevic, D.; Talevski, T.; Maric, D.; Talevska, A. Biodiversity of macrophyte vegetation from lake Prespa, lake Ohrid and lake Skadar. Biotech. Biotech. Equip. 2007, 23, 931–935. [Google Scholar] [CrossRef]
- Data Publisher for Earth and Environmental Science. Available online: http://doi.pangaea.de/doi:10.1594/PANGAEA.819871?format=html (accessed on 3 July 2014).
- Meisch, C. Freshwater Ostracoda of Western and Central Europe; Spektrum Akademischer Verlag: Berlin, Germany, 2000; p. 522. [Google Scholar]
- Namiotko, T.; Danielopol, D.L.; Belmecheri, S.; Gross, M.; von Grafenstein, U. On the Leptocytheridae Ostracods of the Long-Lived Lake Ohrid: A Reappraisal of their Taxonomic Assignment and Biogeographic Origin. Int. Rev. Hydrobiol. 2012, 97, 356–374. [Google Scholar] [CrossRef]
- Petkovski, T.K.; Scharf, B.; Keyser, D. New and little known species of the genus Candona (Crustacea, Ostracoda) from Macedonia and other Balkan areas. Limnologica 2002, 32, 114–130. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology; Harper and Row Publishers: New York, NY, USA, 1989. [Google Scholar]
- Magurran, A. Ecological Diversity and Its Measurement; Princeton Universuty Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Cleveland, W.S.; Devlin, S.J. Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Wood, S. Generalized Additive Models: An Introduction with R, 1st ed.; Chapman and Hall: London, UK, 2006. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2009.
- Karanovic, I. Recent Freshwater Ostracods of the World. Crustacea, Ostracoda, Podocopida; Springer: Berlin, Germany, 2012; p. 608. [Google Scholar]
- Frogley, M.R.; Griffiths, H.I.; Martens, K.; Holmes, J.A.; Chivas, A.R. Modern and fossil ostracods from ancient lakes. In The Ostracoda: Applications in Quaternary Research; Holmes, J.A., Chivas, A.R., Eds.; American Geophysical Union: Washington, DC, USA, 2002; pp. 167–184. [Google Scholar]
- Gliozzi, E.; Grossi, F. Late messinian lago-mare ostracod palaeoecology: A correspondence analysis approach. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 264, 288–295. [Google Scholar] [CrossRef]
- Martens, K. Ostracod speciation in ancient lakes: A review. In Speciation in Ancient Lakes; Martens, K., Goddeeris, B., Coulter, G., Eds.; Archiv für Hydrobiologie: Stuttgart, Germany, 1994; Volume 44, pp. 203–222. [Google Scholar]
- Martens, K.; Schön, I. Crustacean biodiversity in ancient lakes: A review. Crustaceana 1999, 72, 899–910. [Google Scholar] [CrossRef]
- Griffiths, H.I.; Frogley, M.R. Fossil ostracods, faunistics and the evolution of regional biodiversity. In Balkan Biodiversity—Pattern and Process in the European Hotspot; Griffiths, H.I., Krystufek, B., Reed, J.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 261–272. [Google Scholar]
- Griffiths, H.I.; Brancelj, A. The (palaeo-)biogeography of Candona bimucronata: An unusual candonid ostracod from the Balkans. Crustaceana 1996, 69, 882–889. [Google Scholar] [CrossRef]
- Klie, W. Krebstiere Oder Crustacea III: Ostracoda, Muschelkrebse. Die Tierwelt Deutschlands und der Angrenzenden Meeresteile; Verlag Von Gustav Fischer: Jena, Germany, 1938; p. 230. (In German) [Google Scholar]
- Karanovic, I.; Petkovski, T.K. Two interesting ostracod species from Montenegro (SE Europe). Ann. Limnol. 1999, 35, 123–132. [Google Scholar] [CrossRef]
- Pérez, L.; Lorenschat, J.; Bugja, R.; Brenner, M.; Scharf, B.; Schwalb, A. Distribution, diversity and ecology of modern freshwater ostracodes (Crustacea), and hydrochemical characteristics of Lago Petén Itzá, Guatemala. J. Limnol. 2010, 69, 146–159. [Google Scholar] [CrossRef]
- Pérez, L.; Lorenschat, J.; Massaferro, J.; Pailles, C.; Sylvestre, F.; Hollwedel, W.; Brandorff, G.-O.; Brenner, M.; Islebe, G.; Lozano, M.S. Bioindicators of climate and trophic state in lowland and highland aquatic ecosystems of the Northern Neotropicos. Revista de Biología Tropical 2013, 61, 603–644. [Google Scholar] [PubMed]
- Pérez, L.; Frenzel, P.; Brenner, M.; Escobar, J.; Hoelzmann, P.; Scharf, B.; Schwalb, A. Late Quaternary (24–10 ka BP) environmental history of the Neotropical lowlands inferred from ostracodes in sediments of Lago Petén Itzá, Guatemala. J. Paleolimnol. 2011, 46, 59–74. [Google Scholar] [CrossRef]
- Külköylüoğlu, O.; Sari, N. Ecological characteristics of the freshwater ostracoda in Bolu region (Turkey). Hydrobiologia 2012, 688, 37–46. [Google Scholar] [CrossRef]
- Kostoski, G.; Albrecht, C.; Trajanovski, S.; Wilke, T. A freshwater biodiversity hotspot under pressure—Assessing threats and identifying conservation needs for ancient lake Ohrid. Biogeosciences 2010, 7, 3999–4015. [Google Scholar] [CrossRef]
- Pieri, V.; Vandekerkhove, J.; Goi, D. Ostracoda (Crustacea) as indicators for surface water quality: A case study from the ledra river basin (NE Italy). Hydrobiologia 2012, 688, 25–35. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).