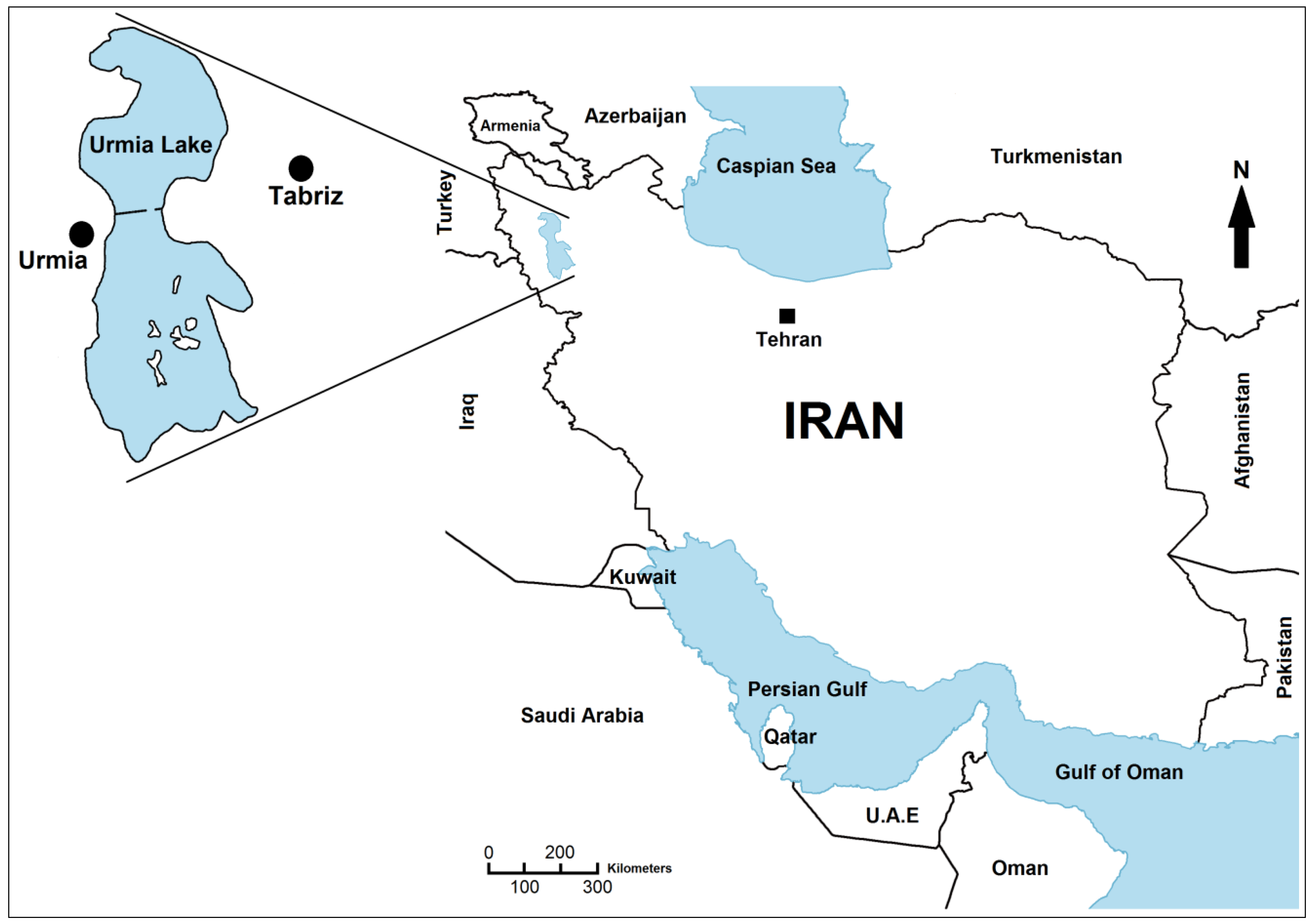
1. Introduction
Urmia Lake is the 20th largest lake in the world. The surface area of this salt lake varies between 2300 to 6000 km
2 (
Figure 1) for two main reasons. First, its hydrology largely depends on precipitation in the catchment area. Secondly, extremely gentle slopes of its marginal plains lead to inundation of large areas even by small water level rises. The average altitude is 1274 m above sea level and the salinity ranges between 120 g/L and more than 300 g/L. 102 islands have been recorded in this lake. Today, only the Islami (Shahi) Peninsula is inhabited by humans, but the “
Limit of the World from the East to the West”
—the oldest Persian geography book
—written by anonymous author(s) in 982 A.D., described Kaboudan Island as suitable for human settlement [
1].
Figure 1.
Geographical localisation of Urmia Lake in the northwestern of Iran.
Figure 1.
Geographical localisation of Urmia Lake in the northwestern of Iran.
Contrary to widespread opinion, Urmia Lake is a second hypersaline lake with an active food web compared to the Dead Sea (with a salinity of >340 g/L), which includes bacteria, archaea, algae, protozoa and ciliates [
2,
3,
4]. Urmia Lake is saltier than the Aral Sea (>100 g/L) [
5] and Karabogaz Gol (40−100 g/L in the deep zone, 170−250 g/L in the northwest and southwest parts of the bay) [
6]. The lake hosts diverse bacterial communities, hyperhalophilous phytoplanktons, and notably the macrozooplankton crustacean, the brine shrimp
Artemia urmiana. Thus, with regard to its ecological significance, unique biodiversity and the presence of indigenous communities, Urmia Lake has been recognized as a Protected Field since 1967 and was designated as a National Park in 1976 as one of 59 biosphere reserves by UNESCO [
7]. In 1975, it was also registered in the Ramsar Convention on Wetlands as a wetland of international importance [
7]. Although the lake is a UNESCO biosphere reserve, several development projects have had detrimental consequences for biodiversity.
The lake is divided into north and south parts, separated by a dike-type causeway, which has a small gap (1400 m) that allows a limited exchange of water between the two sides [
7] (
Figure 1). About 35 dams have been built on 21 permanent rivers flowing to the lake [
8], which restrict the influx of fresh water. In the last decade, Urmia Lake has been affected by a transformation in the hydrological regime, due to climate instability and the construction of dams [
9]. Water resource development projects are diverting enormous quantities of fresh water and preventing a replenishment of lake water, which is lost to evaporation [
10]. The intensive development of agriculture during the last decades and the resultant over-exploitation of groundwater have also deprived the lake of one of its main water input resources.
A progressive drought has caused fundamental changes in the physio–chemical composition of the lake-the salinity exceeds >300 g/L, the surface area has decreased to less than 2366 km
2, the lake level has decreased to 1271 m, the volume of water has decreased from 42 to 22 billion m
3 between 1995 and 2010, and the water depth has decreased to 6 m, whereas reservoirs of dams have increased from 1.624 billion m
3 to 3.568 billion m
3 [
9,
11,
12].
Climate and anthropogenic alterations are recognized as crucial factors in population declines and even the risk of extinctions in most ecosystems [
13,
14]. A decrease of population density would ultimately limit dispersal, as well as gene flow, among populations. This will eventually lead to the loss of genetic diversity [
15]. On the other hand, an increased rate of dispersal can also disturb local genetic adaptation [
16]. At present, the severe drought which happened in 2003–2004 is jeopardizing the biodiversity of the lake [
17].
In this review, we have summarized the available information on the biodiversity of the lake ranging from bacteria and fungi to plants and animals. Apparently, the existing information is incomplete and more detailed future studies are required for a complete inventory of the biodiversity of Urmia Lake.
2. Archaebacteria and Bacteria
Urmia Lake harbours a diverse group of bacterial species (
Table 1). Two pathogenic bacteria
Clostridium perfringens and
Enterococcus faecalis have been identified in the lake water and particularly in the estuary sediments. As these bacteria constitute the natural flora of the human digestive tract, their presence in the lake water and sediments suggests that they have originated from the inflow of urban waste water into Urmia Lake [
18,
19].
In Urmia Lake, the muds contain green sulphur-bacteria, purple sulphur-bacteria and ferro-bacteria [
19,
20]. Halophilic archaebacteria can usually synthesize red and pink pigments in response to environmental stress [
21,
22,
23]. During the summer of 2008, the water of Urmia Lake around Kaboudan Island changed from blue, its normal colour, to red [
24]. This was the first report of this event that may be attributed to the bloom of Archaebacteria or of
Dunaliella or both (see [
25]).
Table 1.
List of Archaebacteria and Bacteria from Urmia Lake.
Table 1.
List of Archaebacteria and Bacteria from Urmia Lake.
| Domain | Phylum | Class | Order | Family | Genus | Species | Reference |
|---|
| Archaea | Euryarchaeota | Halobacteria | Halobacteriales | Halobacteriaceae | Haloarcula | sp. | [23,26] |
| Halobacterium | sp. | [23] |
| Haloferax | sp. | [22] |
| Halorubrum | sp. | [23] |
| Bacteria | Actinobacteria | Actinobacteria | Actinomycetales | Microbacteriaceae | Microbacterium | sp. | [27] |
| Micrococcaceae | Kocuria | sp. | [27] |
| Micrococcus | sp. | [27] |
| Sanguibacteraceae | Sanguibacter | sp. | [27] |
| Bacteroidetes | Cytophagia | Cytophagales | Cytophagaceae | Adhaeribacter | sp. | [27] |
| Pontibacter | sp. | [27] |
| Flammeovirgaceae | Cesiribacter | sp. | [27] |
| Incertae sedis | Incertae sedis | Rhodothermaceae | Salinibacter | sp. | [27] |
| Cyanobacteria | Cyanophyceae | Chroococcales | Chroococcaceae | Chroococcus | sp. | [28] |
| Microcystaceae | Anacystis | sp. | [28] |
| Gloeocapsa | sp. | [29,30] |
| Spirulinaceae | Spirulina | S. maxima | [30] |
| Nostocales | Nostocaceae | Anabaena | sp. | [28,31] |
| Nostoc | sp. | [29,30] |
| Oscillatoriales | Oscillatoriaceae | Lyngbya | sp. | [28] |
| Oscillatoria | sp. | [28,29,30,31,32] |
| Synechococcales | Synechococcaceae | Synechococcus | sp. | [28] |
| Bacteria | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | B. licheniformis | [33] |
| sp. | [27,34,35,36] |
| Gracilibacillus | sp. | [27] |
| Halobacillus | sp. | [27,34,36,37] |
| Oceanobacillus | sp. | [27] |
| Piscibacillus | sp. | [27] |
| Pontibacillus | sp. | [27] |
| Thalassobacillus | sp. | [27,34] |
| Virgibacillus | V. halodenitrificans | [38] |
| sp. | [37] |
| Planococcaceae | Planococcus | sp. | [27] |
| Staphylococcaceae | Staphylococcus | sp. | [27] |
| Lactobacillales | Carnobacteriaceae | Alkalibacterium | sp. | [27] |
| Enterococcaceae | Enterococcus | E. faecalis * | [18] |
| Clostridia | Clostridiales | Clostridiaceae | Clostridium | C. perfringens | [18] |
| Proteobacteria | Alphaproteobacteria | Caulobacterales | Caulobacteraceae | Brevundimonas | sp. | [27] |
| Rhodobacterales | Rhodobacteraceae | Paracoccus | sp. | [27] |
| Gammaproteobacteria | Alteromonadales | Alteromonadaceae | Marinobacter | sp. H57B71 | [36] |
| Marinobacter | sp. | [27,34,36,37] |
| Idiomarinaceae | Idiomarina | sp. Y24 | [36] |
| Enterobacteriales | Enterobacteriaceae | Plesiomonas | P. shigelloides | [39] |
| Providencia | sp. | [27] |
| Bacteria | Proteobacteria | Gammaproteobacteria | Oceanospirillales | Hahellaceae | Halospina | H. denitrificans | [36] |
| Halomonadaceae | Chromohalobacter | C. salexigens | [40] |
| Halomonas | H. ventosae | [36,41] |
| H. sediminis YIM C248 | [36] |
| sp. | [37] |
| Halovibrio | H. denitrificans | [36] |
| Salicola | sp. | [27,36] |
| Pseudomonadales | Pseudomonadaceae | Pseudomonas | sp. | [36] |
| Vibrionales | Vibrionaceae | Vibrio | V. mimicus | [39,42] |
| V. alginolyticus | [39,42] |
| V. fluvialis | [39,42] |
| Xanthomonadales | Xanthomonadaceae | Lysobacter | sp. | [27] |
4. Phytoplankton
The algal flora of Urmia Lake was studied by Plattner in 1960 [
47], who reported
Enteromorpha intestinalis as a macroscopic alga [
18,
20].
E. intestinalis produces considerable amounts of β-carotene, a red-coloured carotenoid with antioxidant properties [
20]. Urmia Lake contains a diverse assemblage of phytoplankton species, with
Dunaliella as the major species (more than 95% of the total phytoplankton in number) and an important fraction of diatoms such as
Navicula and
Nitzschia [
31].
Dunaliella is a green halophilic alga which bears two flagellates and produces high amounts of β-carotene. This phytoplankton is the major food source for
Artemia in the Urmia Lake. It seems that phytoplankton populations in the lake benefit from the increased salinity that has reduced the number of other species and favoured the dominance of
Dunaliella. Usually, in the early spring, especially in Golmankhane Port, a bloom of
Dunaliella occurs near the shore line which changes the water colour to yellow (see [
48]). A current list of phytoplankton composition in Urmia Lake is given in
Table 3.
Table 3.
List of phytoplankton species from Urmia Lake.
Table 3.
List of phytoplankton species from Urmia Lake.
| Phylum | Class | Order | Family | Genus | Species | Reference |
|---|
| Heterokontophyta | Bacillariophyceae | Bacillariales | Bacillariaceae | Nitzschia | sp. | [28,31] |
| Cymbellales | Cymbellaceae | Cymbella | sp. | [31,32] |
| Naviculales | Naviculaceae | Amphiprora | sp. | [28,31] |
| Navicula | sp. | [28,29,31] |
| Pleurosigmataceae | Gyrosigma | sp. | [31] |
| Pinnulariaceae | Pinnularia | sp. | [31] |
| Surirellales | Surirellaceae | Cymatopleura | sp. | [31] |
| Surirella | sp. | [31] |
| Thalassiophysales | Catenulaceae | Amphora | sp. | [28] |
| Coscinodiscophyceae | Thalassiosirales | Stephanodiscaceae | Cyclotella | sp. | [31,32] |
| Fragilariophyceae | Fragilariales | Fragilariaceae | Diatoma | sp. | [31] |
| Synedra | sp. | [31] |
| Chlorophyta | Chlorophyceae | Sphaeropleales | Selenastraceae | Ankistrodesmus | sp. | [28,31] |
| Volvocales | Dunaliellaceae | Dunaliella | sp. | [28,31,32] |
| Volvocaceae | Pandorina | sp. | [28] |
| Ulvophyceae | Ulotrichales | Monostromataceae | Monostroma | sp. | [28] |
| Ulvales | Ulvaceae | Enteromorpha | E. intestinalis | [47] |
5. Land Plants
Because of the diversity of soils and topography, Urmia Lake islands possess a diverse flora.
Table 4 shows the checklist of plants in the Urmia Lake National Park. The halophilous vegetation around Urmia Lake displays an interesting gradient of salinity-tolerance, ranging from annual obligatory hygro-halophytic communities on lake marshes dominated by
Salicornia spp. up to hydrophytic communities dominated by
Alisma plantago-aquatica. The latter species grows on the margins of salty and brackish water marshes where the fresh groundwater dilutes the salt contents of the soil [
49,
50]. The constant occurrence of plant communities in this habitat may suggest that salt-water inundation plays the main role in plant distribution. Inundation seems to act mainly through increasing soil moisture and affecting soluble salt contents to levels suitable for life.
Several cryptic species in the area still need to be identified. This is the case for a rare liverwort
Riella aff.
cossoniana, whose spores have only recently been discovered in ancient and recent sediments of the lake [
50].
Table 4.
List of plants on islands and in salt marshes of Urmia Lake.
Table 4.
List of plants on islands and in salt marshes of Urmia Lake.
| Order | Family | Genus | Species | Reference |
|---|
| Alismatales | Alismataceae | Alisma | A. plantago-aquatica | [49] |
| Araceae | Arum | sp. | [51] |
| Butomaceae | Butomus | B. umbellatus | [49] |
| Apiales | Apiaceae | Alococarpum | A. erianthum | [51,52] |
| Bupleurum | B. falcatum | [51,52] |
| B. gerardii | [51,52] |
| Eryngium | E. billardieri | [51,52] |
| Malabaila | M. secacul | [51,52] |
| Pimpinella | P. tragium | [51,52] |
| Scandix | S. stellata | [51,52] |
| Torilis | T. leptophylla | [51,52] |
| Zosima | Z. absinthifolia | [51,52] |
| Asparagales | Amaryllidaceae | Allium | A. akaka | [51,52] |
| sp. | [51] |
| Asparagaceae | Bellevalia | sp. | [51,52] |
| Leopoldia | L. caucasica1 | [51,52] |
| Iridaceae | Crocus | sp. | [51,52] |
| Iris | I. barnumae | [51,52] |
| I. spuria | [49] |
| sp. | [51] |
| Ixioliriaceae | Ixiolirion | I. tataricum | [52] |
| Orchidaceae | Dactylorhiza | D. umbrosa | [49] |
| Xanthorrhoeaceae | Eremurus | E. spectabilis | [51,52] |
| Asterales | Asteraceae | Amberboa | A. nana | [51,52] |
| Artemisia | A. fragrans | [45,49,53] |
| A. haussknechtii | [51,52] |
| sp. | [51] |
| Aster | A. tripolium | [49] |
| Centaurea | C. aucheri | [51,52] |
| C. ustulata | [51,52] |
| C. virgata | [51,52] |
| Cirsium | C. alatum | [49] |
| Cousinia | sp. | [51,52] |
| Crepis | sp. | [51,52] |
| Crupina | C. vulgaris | [51,52] |
| Echinops | E. orientalis | [45] |
| sp. | [51,52] |
| Garhadiolus | G. angulosus | [52] |
| Helichrysum | H. rubicundum | [51,52] |
| Inula | I. aucheriana | [49] |
| Koelpinia | K. linearis | [49] |
| Asterales | Asteraceae | Lactuca | L. serriola | [53] |
| L. undulata | [51,52] |
| Picris | P. kotschyi | [51,52] |
| Saussurea | S. salsa | [49] |
| Scorzonera | S. laciniata | [49] |
| Senecio | S. vernalis | [45] |
| sp. | [51,52] |
| Steptorhamphus | S. tuberosus | [52] |
| Taraxacum | sp. | [49] |
| Tragopogon | T. graminifolius | [49] |
| T. marginatus | [51,52] |
| Xeranthemum | X. squarrosum | [51,52] |
| Boraginales | Boraginaceae | Anchusa | A. arvensis | [51,52] |
| Buglossoides | B. arvensis | [52] |
| Heliotropium | H. samoliflorum | [49,51,52] |
| Heterocaryum | H. laevigatum | [52] |
| Moltkia | M. longiflora | [51,52] |
| Nonnea | N. caspica | [51,52] |
| Brassicales | Brassicaceae | Aethionema | A. carneum | [51,52] |
| Alyssum | A. linifolium | [45] |
| A. murale | [51,52] |
| A. szovitsianum | [51,52] |
| sp. | [51,52] |
| Descurainia | D. sophia | [45] |
| Erysimum | E. sisymbrioides | [49] |
| Hutchinsia | H. procumbens | [53] |
| Isatis | I. buschiana | [51,52] |
| Lepidium | L. aucheri | [49] |
| L. cartilagineum | [49,53] |
| Malcolmia | sp. | [51,52] |
| Neslia | N. apiculata | [51,52] |
| Capparaceae | Capparis | C. spinosa | [51,52] |
| Cleomaceae | Cleome | C. iberica | [51,52] |
| Caryophyllales | Amaranthaceae | Atriplex | A. aucheri | [51,52] |
| A. hastata | [49] |
| A. patula | [51,52] |
| A. tatarica | [49] |
| A.verrucifera | [45,49,53] |
| sp. | [51,52] |
| Camphorosma | C. monspeliaca | [49,53] |
| Chenopodium | C. murale | [51,52] |
| sp. | [51] |
| Climacoptera | C. crassa | [49] |
| Caryophyllales | Amaranthaceae | Halanthium | H. rarifolium | [49,52,53] |
| Halocnemum | H. strobilaceum | [49,52,53] |
| Halopeplis | H. pygmaea | [49] |
| Halostachys | H. caspica | [49,51,52] |
| Kalidium | K. caspicum | [49] |
| Noaea | N. mucronata | [51,52] |
| Petrosimonia | P. brachiata | [49,53] |
| P. glauca | [49] |
| Salicornia | S. maritime2 | [45,49,51,52,53] |
| Salsola | S. crassa | [51,52,53] |
| S. dendroides | [51,52] |
| S. kali | [45] |
| S. laricina | [51,52] |
| S. soda | [49,53] |
| S. verrucosa | [51,52] |
| Suaeda | S. acuminata | [52] |
| S. altissima | [45,49,53] |
| S. crassifolia | [53] |
| S. maritima | [49,53] |
| S. microphylla | [49,51,52] |
| sp. | [51] |
| Caryophyllaceae | Acanthophyllum | A. mucronatum | [51,52] |
| Cerastium | C. inflatum | [51,52] |
| Dianthus | D. orientalis | [51,52] |
| sp. | [51,52] |
| Gypsophila | sp. | [51] |
| Minuartia | M. hamata | [51,52] |
| Paronychia | P. kurdica | [51,52] |
| Saponaria | S. viscosa | [51,52] |
| Silene | S. conoidea | [45,51,52] |
| S. marshallii | [51,52] |
| S. spergulifolia | [51,52] |
| sp. | [51,52] |
| Spergularia | S. marina | [49,53] |
| S. salina3 | [49,53] |
| Velezia | V. rigida | [51,52] |
| Frankeniaceae | Frankenia | F. hirsuta | [49,51,52,53] |
| F. pulverulenta | [49,51,52,53] |
| Plumbaginaceae | Acantholimon | sp. | [51,52] |
| Limonium | L. bellidifolium | [49] |
| L. carnosum | [49] |
| L. caspium | [51,52] |
| L. gmelinii | [49] |
| L. meyeri | [49,52,53] |
| Psylliostachys | P. leptostachya | [49,53] |
| Caryophyllales | Plumbaginaceae | Psylliostachys | P. spicata | [49] |
| Polygonaceae | Atraphaxis | A. spinosa | [51,52] |
| Polygonum | P. aviculare | [51,52] |
| Rumex | R. conglomeratus | [45,49] |
| R. crispus | [49,51,52] |
| R. tuberosus | [51,52] |
| Tamaricaceae | Reaumuria | R. cistoides | [52] |
| Tamarix | T. kotschyi | [49] |
| T. octandra | [49] |
| T. octandra | [49] |
| T. ramosissima | [49,51,52] |
| Dipsacales | Dipsacaceae | Dipsacus | sp. | [45] |
| Pterocephalus | P. canus | [52] |
| Scabiosa | S. rotata | [52] |
| sp. | [52] |
| Valerianaceae | Valerianella | V. amblyotis | [52] |
| V. coronata | [52] |
| V. oxyrrhyncha | [52] |
| V. vesicaria | [52] |
| Ephedrales | Ephedraceae | Ephedra | E. procera | [51,52] |
| Ericales | Primulaceae | Androsace | A. maxima | [52] |
| Fabales | Fabaceae | Alhagi | A. pseudalhagi | [49,53] |
| sp. | [51] |
| Astragalus | A. eriocarpus | [51,52] |
| A. oxyglottis | [51,52] |
| sp. | [45,51,52] |
| Glycyrrhiza | sp. | [51] |
| Lotus | L. tenuis | [49] |
| Medicago | M. radiata | [51,52] |
| M. rigidula | [51,52] |
| M. sativa | [51,52] |
| Trifolium | T. arvense | [51,52] |
| T. fragiferum | [49] |
| Trigonella | T. asteroides | [51,52] |
| T. filipes | [51,52] |
| T. monantha | [51,52] |
| T. spruneriana | [51,52] |
| Vicia | V. michauxii | [51,52] |
| Gentianales | Rubiaceae | Callipeltis | C. cucullaris | [51,52] |
| Crucianella | C. gilanica | [51,52] |
| C. latifolia | [51,52] |
| Galium | G. aparine | [51,52] |
| G. verticillatum | [51,52] |
| Gentianales | Rubiaceae | Rubia | sp. | [51,52] |
| Geraniales | Geraniaceae | Erodium | E. ciconium | [52] |
| E. cicutarium | [51,52] |
| E. oxyrrhynchum | [51,52] |
| Geranium | G. rotundifolium | [51,52] |
| Lamiales | Lamiaceae | Eremostachys | E. moluccelloides | [52] |
| Hymenocrater | H. bituminosus | [52] |
| Lamium | L. amplexicaule | [52] |
| Mentha | M. longifolia | [49] |
| Salvia | S. ceratophylla | [51,52] |
| S. hydrangea | [51,52] |
| S. multicaulis | [51,52] |
| S. reuteriana | [51,52] |
| sp. | [51,52] |
| Scutellaria | S. theobromina | [51,52] |
| Sideritis | S. montana | [51,52] |
| Teucrium | T. polium | [51,52] |
| Thymus | T. fedtschenkoi | [51,52] |
| Ziziphora | Z. capitata | [51,52] |
| Z. tenuior | [51,52] |
| Orobanchaceae | Orobanche | sp. | [52] |
| Plantaginaceae | Linaria | L. micrantha | [51,52] |
| L. simplex | [51,52] |
| Plantago | P. major | [49] |
| P. maritima | [49,53] |
| Veronica | V. anagallis-aquatica | [49] |
| V. beccabunga | [49] |
| Scrophulariaceae | Scrophularia | S. variegata | [51,52] |
| Verbascum | V. nudicaule | [52] |
| V. songaricum | [51,52] |
| V. thapsus | [51,52] |
| Liliales | Liliaceae | Gagea | G. reticulata | [52] |
| Tulipa | T. montana | [51,52] |
| Malpighiales | Euphorbiaceae | Euphorbia | E. heteradenia | [51,52] |
| E. myrsinites | [51,52] |
| E. phymatosperma | [51,52] |
| E. szovitsii | [52] |
| Hypericaceae | Hypericum | H. hyssopifolium | [52] |
| H. scabrum | [52] |
| Phyllanthaceae | Andrachne | A. aspera | [52] |
| Violaceae | Viola | V. rupestris | [51,52] |
| Malvales | Cistaceae | Helianthemum | H. ledifolium | [51,52] |
| Thymelaeaceae | Daphne | D. mucronata | [52] |
| Malvales | Thymelaeaceae | Diarthron | D. vesiculosum | [52] |
| Myrtales | Onagraceae | Chamerion | C. angustifolium | [49] |
| Pinales | Cupressaceae | Juniperus | J. excelsa | [51,52] |
| Poales | Cyperaceae | Bolboschoenus | B. maritimus | [49] |
| Carex | C. distans | [49] |
| C. divisa | [49] |
| sp. | [51,52] |
| Cyperus | C. fuscus | [49] |
| C. laevigatus | [49] |
| Eleocharis | E. palustris | [49] |
| Juncaceae | Juncus | J. acutus | [49] |
| J. gerardii | [49] |
| J. heldreichianus | [49] |
| J. inflexus | [49] |
| J. maritimus | [49] |
| Poaceae | Aegilops | A. columnaris | [51] |
| A. triuncialis | [51,52] |
| Aeluropus | A. littoralis | [45,49,52,53] |
| Agropyrum | A. elongatum | [49] |
| Agrostis | A. stolonifera | [49] |
| Alopecurus | A. arundinaceus | [49] |
| Arrhenatherum | A. kotschyi | [52] |
| Avena | A. fatua | [51,52] |
| sp. | [51] |
| Beckmannia | B. eruciformis | [49] |
| Bromus | B. danthoniae | [51,52] |
| B. scoparius | [45] |
| B. tectorum | [52] |
| Catabrosa | C. aquatica | [49] |
| Crypsis | C. schoenoides | [49] |
| C. vaginiflora | [49] |
| Cynodon | C. dactylon | [49] |
| Eremopyrum | E. triticeum | [49] |
| F. arundinacea | [49] |
| Festuca | sp. | [51] |
| Gaudinopsis | G. macra | [52] |
| Hordeum | H. geniculatum | [49] |
| H. leporinum | [52] |
| H. spontaneum | [52] |
| sp. | [51] |
| Melica | M. jacquemontii | [52] |
| Nardurus | N. subulatus | [52] |
| Parapholis | P. incurva | [49] |
| Phleum | sp. | [51] |
| Phragmites | P. australis | [45,49,51,52] |
| Poa | P. bulbosa | [51,52] |
| P. persica4 | [52] |
| Poales | Poaceae | Polypogon | P. trivialis | [49] |
| P. monspeliensis | [49] |
| P. semiverticillata | [49] |
| Puccinellia | P. bulbosa | [49,53] |
| P. distans | [49] |
| Sclerochloa | S. dura | [49] |
| Stipa | S. barbata | [45,51,52] |
| Taeniatherum | T. crinitum | [52] |
| Zingeria | Z. trichopoda | [49] |
| Ranunculales | Berberidaceae | Berberis | B. integerrima | [51,52] |
| Leontice | L. leontopetalum | [52] |
| Papaveraceae | Fumaria | F. parviflora | [51] |
| F. vaillantii | [51,52] |
| Glaucium5 | sp. | [51] |
| Papaver | P. argemone | [51,52] |
| P. glaucium | [52] |
| sp. | [51,52] |
| Ranunculaceae | Adonis | A. aestivalis | [51,52] |
| Batrachium | B. trichophyllum | [49] |
| Consolida | sp. | [51] |
| Delphinium | D. quercetorum | [51,52] |
| Ranunculus | sp. | [51,52] |
| Thalictrum | T. isopyroides | [51,52] |
| T. sultanabadense | [51,52] |
| Rosales | Cannabaceae | Celtis | C. glabrata | [51,52] |
| Moraceae | Ficus | F. carica | [51] |
| Rhamnaceae | Rhamnus | R. pallasii | [51] |
| Rosaceae | Amygdalus | A. trichamygdalus | [51,52] |
| Cerasus | C. microcarpa | [51,52] |
| Cotoneaster | sp. | [51,52] |
| Potentilla | P. recta | [49] |
| Prunus | sp. | [51] |
| Urticaceae | Parietaria | P. judaica | [51,52] |
| Sapindales | Anacardiaceae | Pistacia | P. atlantica | [51,52] |
| Nitrariaceae | Nitraria | N. sibirica | [51,52] |
| Peganum | P. harmala | [51,52] |
| Tetradiclis | T. tenella | [52] |
| Rutaceae | Haplophyllum | H. perforatum | [52] |
| Sapindaceae | Acer | A. monspessulanum | [51,52] |
| Saxifragales | Crassulaceae | Rosularia | R. persica | [52] |
| Sedum | S. hispanicum | [52] |
| Sphaerocarpales | Riellaceae | Riella | aff.
Cossoniana 6 | [50] |
| Solanales | Convolvulaceae | Convolvulus | C. lineatus | [51,53] |
| Cressa | C. cretica | [49] |
| Solanales | Convolvulaceae | Cuscuta | sp. | [51] |
| Solanaceae | Hyoscyamus | H. pusillus | [51,52] |
| Lycium | L. ruthenicum | [49,51,52] |
| Solanum | sp. | [51] |
| Zygophyllales | Zygophyllaceae | Zygophyllum | Z. fabago | [51,52] |
6. Brine Shrimp Artemia
The brine shrimp
Artemia (Crustacea: Anostraca) is a small crustacean adapted to hypersaline habitats, of Urmia Lake. It was first reported from the lake in 982, more than one thousand years ago [
54]. In Curzon’s published work “Persia and the Persian Question” (1892, Volume i, p. 533), he described
Artemia as a species of small “Jelly-Fish” [
55,
56]. In the first reports of A. Günther and R.T Günther, it was interpreted as a “Medusa”, “a species of
Branchipus” [
57] and “the
Artemia group of varieties of the
Branchipus type” [
58]. Finally, Günther pointed out that this bisexual species belongs to the genus
Artemia [
59] (p. 509), and the species was nomenclatured as
Artemia urmiana [
60] (p. 395).
Barigozzi
et al. (1987) [
61] reported the existence of only parthenogenetic populations of
Artemia based on a single sample from Urmia Lake. However, another study of
Artemia cysts (which are encysted gastrula embryos) collected from the western shore-line of Urmia Lake in 1987 revealed that Urmia Lake has both bisexual and parthenogenetic populations in the surrounding lagoons, especially on the western shore of the lake [
62]. Sediment cores suggest that parthenogenetic populations of
Artemia have lived in Urmia Lake for at least 5000 years [
11]; however, the oldest remains of
Artemia that have been recovered from a sediment core from this lake are 200,000 years old [
63]. The two parthenogenetic
Artemia populations from the Urmia Lake basin (those in the coastal lagoons and interior of the lake) have been tentatively attributed to two morphotypes that are not yet completely separated [
64]. Several studies have shown that biometrical, morphometrical and genetic variations exist in
A. urmiana [
65,
66,
67]. The results indicate that ecological speciation is an ongoing process in Urmia Lake [
67].
11. Mammals
Some Armenian sheep,
Ovis orientalis gmilini, were transferred to Kaboudan Island in 1895 and 1906 by one of the governors of Azerbaijan [
84]. In 1970 and 1971, in order to control the population of Armenian sheep, two leopards,
Panthera pardus, were introduced on Kaboudan Island; however, their corpses were found in 1982. During 1993, 49, and in 1995, 98 Armenian Sheep were transferred from Kaboudan Island to the Islami Peninsula [
51,
75,
85].
Fifty-two Persian fallow deer,
Dama dama mesopotamica, were transferred to Ashk Island between 1977 and 1989. Also in 1989, six of these deer (three males and three females) were introduced to Kaboudan Island in order to study their ecology [
51,
75,
85].
Table 8 lists the Mammals of Urmia Lake National Park.
Table 8.
List of mammals from Urmia Lake.
Table 8.
List of mammals from Urmia Lake.
| Order | Family | Genus | Species | Locality | Reference |
|---|
| Artiodactyla | Bovidae | Ovis | O. orientalis1 | Kaboudan Island and Islami Peninsula | [51,75,84,85] |
| Cervidae | Dama | D. dama2 | Kaboudan Island and Ashk Island | [51,85] |
| Carnivora | Canidae | Canis | C. aureus | the shores of Urmia Lake | [75] |
| C. lupus | the shores of Urmia Lake | [75] |
| Vulpes | V. vulpes | the shores of Urmia Lake | [75] |
| Felidae | Panthera | P. pardus 3 | Kaboudan Island | [51,75,85] |
| Mustelidae | Martes | M. foina | the shores of Urmia Lake | [75] |
| Mustela | M. nivalis | the shores of Urmia Lake | [75] |
| Chiroptera | Emballonuridae | Taphozous | T. nudiventris | some islands of Urmia Lake and the shores of Urmia Lake | [75] |
| Rhinolophidae | Rhinolophus | R. ferrumequinum | some islands of Urmia Lake and the shores of Urmia Lake | [75] |
| Vespertilionidae | Myotis | M. blythii | some islands of Urmia Lake and the shores of Urmia Lake | [75] |
| M. mystacinus | some islands of Urmia Lake and the shores of Urmia Lake | [75] |
| Pipistrellus | P. kuhlii | some islands of Urmia Lake and the shores of Urmia Lake | [75] |
| P. pipistrellus | some islands of Urmia Lake and the shores of Urmia Lake | [75] |
| P. savii | some islands of Urmia Lake andthe shores of Urmia Lake | [75] |
| Insectivora | Erinaceidae | Erinaceus | E. europaeus | the shores of Urmia Lake | [75] |
| Soricidae | Crocidura | C. russula | the shores of Urmia Lake | [75] |
| Lagomorpha | Leporidae | Lepus | L. capensis | the shores of Urmia Lake | [75] |
| Rodentia | Cricetidae | Arvicola | A. amphibious4 | the shores of Urmia Lake | [75] |
| Cricetulus | C. migratorius | the shores of Urmia Lake | [75] |
| Dipodidae | Allactaga | A. elater | the shores of Urmia Lake | [75] |
| Muridae | Apodemus | A. sylvaticus | the shores of Urmia Lake | [51,75] |
| Meriones | M. persicus | the shores of Urmia Lake | [75] |
| | | Mus | M. musculus | some islands of Urmia Lake and the shores of Urmia Lake | [51,75] |
12. Hidden Biodiversity
Our knowledge of the biodiversity of a given area or ecosystem is based on the species already discovered and described by biologists. However, the list of plants and animals is subject to change in that new species are discovered in the field, taxonomic revisions based on the application of molecular techniques are carried out, or both.
The case of
Riella aff.
cossoniana, whose spores have only recently been discovered in ancient and recent sediments of the lake, is worth mentioning [
50].
Riella is a liverwort extremely rarely encountered in the field, owing to the very particular ecological conditions needed for the germination of its spores. In Urmia Lake, the spores of this liverwort have been documented in the sediment archives since about 200,000 years ago, and they are also found in recent sediments, showing that the plant is still thriving in brackish water ponds and springs around the lake. However, despite its presence in sediments of salt lakes in many salt and brackish water wetlands, this plant has never been documented in Iran by botanists [
50]. Today,
Riella is considered an endangered plant in many places in semi-arid regions of the world (e.g., [
86]). The above example indicates that the conservation of aquatic ecosystems around the lake is of primordial importance for the conservation of such hidden components of the biodiversity.
13. Urmia Lake Hydrological Variations and their Impact on the Biodiversity
Palaeoecological and geochemical investigations of long sediment cores from Urmia Lake have revealed that lake levels underwent dramatic changes during the glacial-interglacial cycles of the Quaternary period [
17,
50,
87]. Based on the total thickness of the fluvio-lacustrine infill of the Urmia Lake basin, as revealed by geophysical explorations and the dated 100-m long core samples taken from the central basin, the age of the lake can be grossly estimated at >600,000 years. Pollen evidence shows that the aquatic vegetation composition and density has greatly fluctuated in response to these hydrological changes during the last 200,000 years. The most important development of aquatic vegetation took place during glacial, rather than interglacial periods (see Figure 8 in [
88] for details). During the Last Glacial (70 ka to 17 ka) and Penultimate Glacial (190 ka to 130 ka), numerous time intervals with extensive development of fresh- to brackish water macrophytic vegetation may be observed. These periods have been interpreted as high lake levels during which the lake chemistry became considerably less saline [
50]. Although the glacial periods are known as periods with a significant loss of biodiversity, they were times of a more diversified plant and most probably animal life within the lake and in its associated ecosystems. The presence of several peaks of brackish to fresh water dinoflagellates and
Pediastrum (green alga) indicate that the algal life also increased in response to the dilution of lake water [
17,
50,
87,
88].