Conservation of Protists: The Krauthügel Pond in Austria
Abstract
:1. Introduction


2. Study Area and Methods
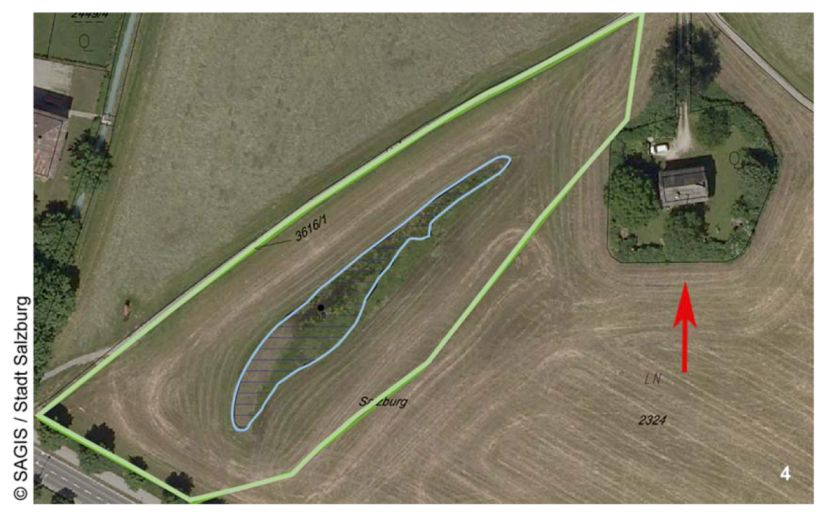
2.1. The Krauthügel Pond
2.2. Methods
3. Faunistics of the Ciliate Community of the Krauthügel Pond
3.1. Remarkable Species
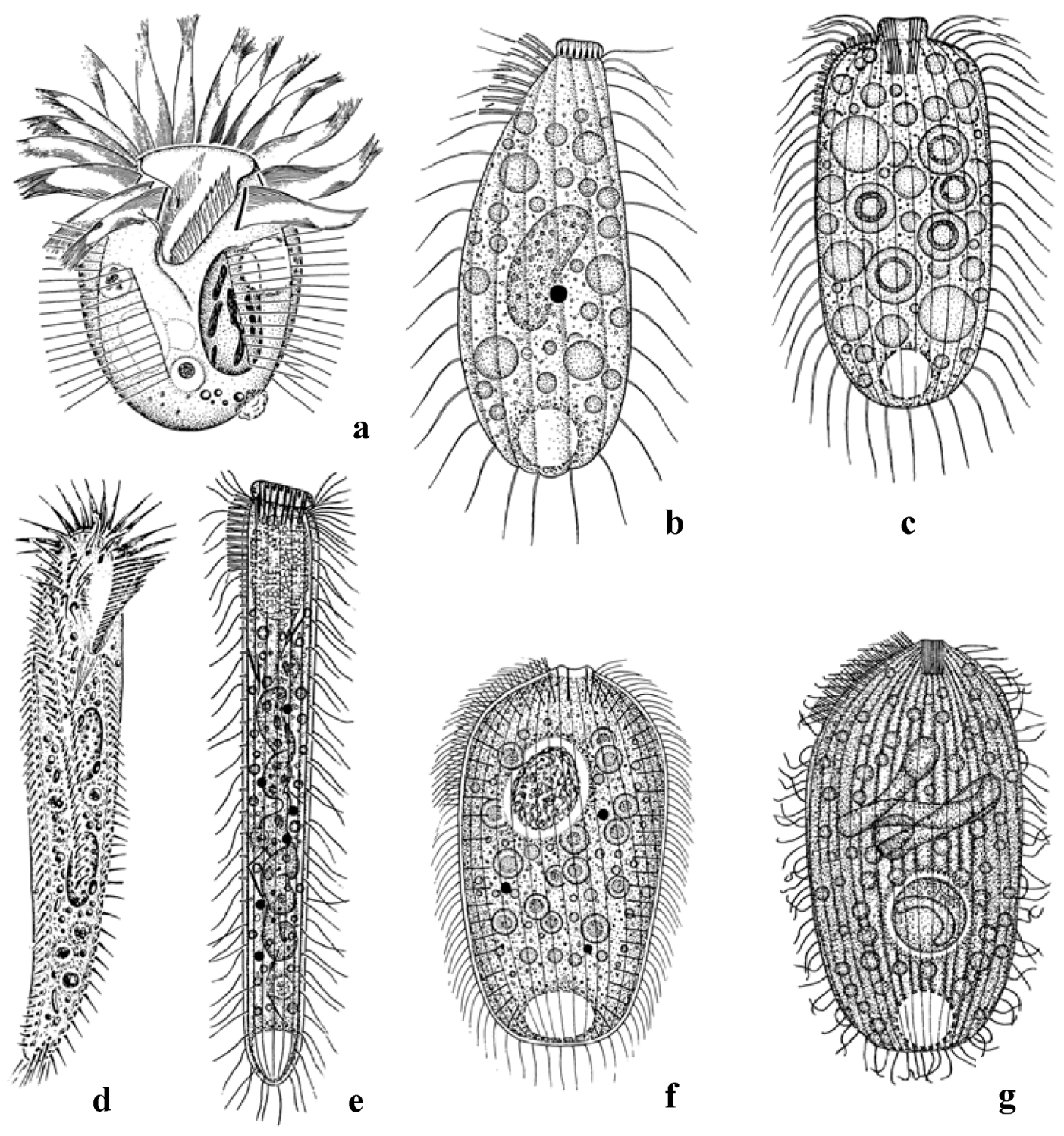
3.2. Species Numbers and Community Structure
| Taxa | |
|---|---|
| * | Acropisthium mutabile Perty, 1852 |
| Actinorhabdos trichocystiferus Foissner, 1984 | |
| Amphileptus pleurosigma (Stokes, 1884) Foissner, 1984 | |
| ** | Apertospathula implicata (Kahl, 1930) Foissner & Oertel, 2009 |
| ** | Apodileptus visscheri visscheri (Dragesco, 1963) Vďaĉný & Foissner, 2012 |
| Arcuospathidium cooperi Foissner, 1996 | |
| Askenasia volvox (Eichwald, 1852) Kahl, 1930 | |
| Aspidisca cicada (Mueller, 1786) Claparède & Lachmann, 1858 | |
| Balantidion pellucidum Eberhard, 1862 | |
| Bilamellophrya hawaiensis Foissner, Agatha & Berger, 2002 | |
| Blepharisma steini Kahl, 1932 | |
| Blepharisma undulans Stein, 1867 | |
| Bresslaua insidiatrix Claff, Dewey & Kidder, 1941 | |
| Bryometopus atypicus Foissner, 1980 | |
| Bryometopus pseudochilodon Kahl, 1932 | |
| Bursaria truncatella Mueller, 1773 | |
| Bursellopsis truncata (Kahl, 1927) Corliss, 1960 | |
| Chilodonella uncinata (Ehrenberg, 1838) Strand, 1928 | |
| Chilodontopsis depressa (Perty, 1852) Blochmann, 1895 | |
| Cinetochilum margaritaceum (Ehrenberg, 1831) Perty, 1849 | |
| Colpoda aspera Kahl, 1926 | |
| Colpoda cucullus (Mueller, 1773) Gmelin, 1790 | |
| Colpoda inflata (Stokes, 1884) Kahl, 1931 | |
| Colpoda lucida Greeff, 1888 | |
| Colpoda maupasi Enriques, 1908 | |
| Colpodidium bradburyarum Foissner, Agatha & Berger, 2002 | |
| Colpodidium caudatum Wilbert, 1982 | |
| Colpodidium microstoma Foissner, Agatha & Berger, 2002 | |
| Coriplites grandis Oertel et al., 2008 | |
| Cyclidium glaucoma Mueller, 1773 | |
| Cyrtolophosis minor Vuxanovici, 1963 | |
| *** | Cyrtolophosis mucicola Stokes, 1885 |
| Deviata bacilliformis (Gelei, 1954) Eigner, 1995 | |
| *** | Didinium nasutum (Mueller, 1773) Stein, 1859 |
| *** | Dileptus anatinus Golińska, 1971 |
| Dileptus margaritifer (Ehrenberg, 1833) Dujardin, 1841 | |
| Drepanomonas pauciciliata Foissner, 1987 | |
| *** | Enchelydium piliforme (Kahl, 1930) Foissner, 1984 |
| Enchelyodon anulatus Foissner, 1984 | |
| Engelmanniella mobilis (Engelmann, 1862) Foissner, 1982 | |
| Epispathidium ascendens (Wenzel, 1955) Foissner, 1987 | |
| Epistylis alpestris Foissner, 1978 | |
| Euplotes affinis (Dujardin, 1841) Kahl, 1932 | |
| Euplotes muscicola Kahl, 1932 | |
| ** | Frontonia angusta angusta Kahl, 1931 |
| Furgasonia rubens (Perty, 1852) Jankowski, 1964 | |
| ** | Furgasonia theresae (Fabre-Domergue, 1889) Foissner, Agatha & Berger, 2002 |
| Fuscheria nodosa salisburgensis Gabilondo & Foissner, 2009 | |
| Gastrostyla mystacea mystacea (Stein, 1859) Sterki, 1878 | |
| Grossglockneria acuta Foissner, 1980 | |
| Grossglockneria hyalina Foissner, 1985 | |
| *** | Halteria grandinella (Mueller, 1773) Dujardin, 1841 |
| Holophrya teres (Ehrenberg, 1833) Foissner, Berger & Kohmann, 1994 | |
| Holosticha stueberi Foissner, 1987 | |
| Homalogastra setosa Kahl, 1926 | |
| Kahliella simplex (Horváth, 1934) Corliss, 1960 | |
| Kahlilembus attenuatus (Smith, 1897) Foissner, Berger & Kohmann, 1994 | |
| Leptopharynx costatus Mermod, 1914 | |
| Maryna lichenicola (Gelei, 1950) Foissner, 1993 | |
| *** | Maryna ovata (Gelei, 1950) Foissner, 1993 |
| * | Maryna umbrellata (Gelei, 1950) Foissner, 1993 |
| Meseres corlissi Petz & Foissner, 1992 | |
| Metopus hasei Sondheim, 1929 | |
| Metopus inversus (Jankowski, 1964) Foissner & Agatha, 1999 | |
| Metopus minor Kahl, 1927 | |
| Metopus palaeformis Kahl, 1927 | |
| Monodinium balbiani Fabre-Domergue, 1888 | |
| Monomacrocaryon terrenum (Foissner, 1981) Vďaĉný et al., 2012 | |
| Mykophagophrys terricola (Foissner, 1985) Foissner, 1995 | |
| Odontochlamys alpestris Foissner, 1981 | |
| Opisthonecta minima Foissner, 1975 | |
| Opisthonecta bivacuolata Foissner, 1978 | |
| Oxytricha opisthomuscorum Foissner et al., 1991 | |
| Papillorhabdos multinucleatus Foissner, 1984 | |
| Paracolpoda steinii (Maupas, 1883) Lynn, 1978 | |
| Paramecium aurelia-complex | |
| Paramecium caudatum Ehrenberg, 1833 | |
| Paramphisiella caudata (Hemberger, 1985) Foissner, 1988 | |
| * | Perispira ovum Stein, 1859 |
| Plagiocampa difficilis Foissner, 1981 | |
| Platyophrya vorax Kahl, 1926 | |
| Podophrya fixa (Mueller, 1786) Ehrenberg, 1833 | |
| Protocyclidium terrenum Alekperov, 1993 | |
| ** | Protospathidium serpens (Kahl, 1930) Foissner, 1981 |
| ** | Pseudochilodonopsis algivora (Kahl, 1931) Foissner, 1979 |
| Pseudochilodonopsis piscatoris (Blochmann, 1895) Foissner, 1979 | |
| Pseudomicrothorax dubius (Maupas, 1883) Penard, 1922 | |
| Pseudoplatyophrya nana (Kahl, 1926) Foissner, 1980 | |
| Pseudouroleptus procerus Berger & Foissner, 1987 | |
| Pseudourostyla franzi Foissner, 1987 | |
| *** | Psilotricha succisa (Mueller, 1786) Foissner, 1983 |
| Rostrophryides africana Foissner, 1987 | |
| Sagittaria hyalina Foissner, Czapik & Wiakowski. 1981 | |
| Sathrophilus muscorum (Kahl, 1931) Corliss, 1960 | |
| Semispathidium pulchrum Foissner, Hess & Al-Rasheid, 2010 | |
| Spathidium claviforme Kahl, 1930 | |
| Spathidium procerum Kahl, 1930 | |
| Spathidium puteolagri Baumeister in Kahl (1930) | |
| Spathidium rusticanum Foissner, 1981 | |
| Spathidium spathula (Mueller, 1773) Moody, 1912 | |
| Steinia platystoma (Ehrenberg, 1831) Diesing, 1866 | |
| Steinia sphagnicola Foissner, 1989 | |
| Sterkiella histriomuscorum (Foissner, Blatterer, Berger & | |
| Kohmann, 1991) Foissner, Blatterer, Berger & Kohmann, 1991 | |
| Strobilidium caudatum (Fromentel, 1876) Foissner, 1987 | |
| Stylonychia mytilus-complex | |
| Stylonychia notophora Stokes, 1885 | |
| Tetrahymena pyriformis-complex | |
| Tetrahymena rostrata (Kahl, 1926) Corliss, 1952 | |
| * | Tillina magna Gruber, 1880 |
| Tokophrya infusionum (Stein, 1859) Bütschli, 1889 | |
| Uroleptus gallina (Mueller, 1786) Foissner, Blatterer, Berger & Kohmann, 1991 | |
| Urosoma acuminata (Stokes, 1887) Kahl, 1932 | |
| Urosoma emarginata (Stokes, 1885) Berger, 1999 | |
| Urostyla grandis Ehrenberg, 1830 | |
| Urotricha farcta Claparède & Lachmann, 1859 | |
| Urotricha globosa Schewiakoff, 1892 | |
| Vorticella convallaria-complex | |
| Vorticella (Echinovorticella) echini (King, 1931) Foissner, Agatha & Berger, 2002 | |
| Vorticella infusionum-complex | |
| Vasicola ciliata Tatem, 1869 | |
| Woodruffides metabolicus (Johnson & Larson, 1938) Foissner, 1987 | |
| Epistylis sp. n.? | |
| Notohymena sp. n.? | |
| Odontochlamys n. sp.? | |
| Spathidium -like gen. n., sp. n. (description in preparation) | |
| Spathidium spp. | |
| Urotricha sp. n.? | |
| Epispathidium cf. amphoriforme | |
| Nassula spp. |
3.3. Distribution
3.4. Official Recognition
4. Discussion
4.1. Number of Ciliate Species in Other Well-Investigated Ephemeral Waters
| Brief site description | Numberof species | % newspecies | Authors | |
|---|---|---|---|---|
| 1. | Many ephemeral waters sampled across Central Europe a | 69 | 10 | [51] |
| 2. | Five ephemeral, eutrophic pasture ponds, each investigated several times during two years, on a mountain meadow in Hungary | 75 | 45 | [52,53,54] |
| 3. | 785 ephemeral waters, mainly road and meadow puddles in Bavaria, Germany | 132 | 12 | [55] |
| 4. | An arctic tundra pond sampled several times b | ~30 | 0? | [56] |
| 5. | 23 alpine, eutrophic pasture ponds in Austria, most investigated 1 to 2 times (but see next entry) | 107 | 33 | [57] |
| 6. | Many samples from pasture pond 1 in the series mentioned above | 54 | 17 | [57] |
| 7. | Four soil samples from a seasonally inundated grassland in New Zealand | 57 | 0? | [58] |
| 8. | Many samples from an experimental ricefield in Italy studied for four years | 60 | 0? | [59] |
| 9. | One sample from a few road puddles within the Bambatsi guest house area (Namibia, Southwest Africa) | 130 | 14 | [27] |
| 10. | Two meltwater ponds in southern Ontario (Canada) investigated weekly for 98 and 34 days, respectively | 145 | 0? | [60] |
| 11. | Nine Lajas (granitic lithotelmes in Venezuela), each investigated one time (Foissner, unpubl.) | 103 | 9 | |
| 12. | Present study | ~121 | 8 |
4.2. Conservation Arguments
4.2.1. Endemism
4.2.2. Type Locality and Landscape Diversity
4.3. A Benchmark for Future Biodiversity Characterization
4.4. Taxonomy Informs Conservation
5. Conclusions
Acknowledgements
References
- Finlay, B.J.; Corliss, J.O.; Esteban, G.; Fenchel, T. Biodiversity at the microbial level: the number of free-living ciliates in the biosphere. Q. Rev. Biol. 1996, 71, 221–237. [Google Scholar]
- Finlay, B.J.; Esteban, G.F.; Fenchel, T. Protist diversity is different? Protist 2004, 155, 15–22. [Google Scholar] [CrossRef]
- Markert, B.A.; Breure, A.M.; Zechmeister, H.G. Bioindicators & Biomonitors Principles, Concepts and Applications; Elsevier: Amsterdam, the Netherlands, 2003; p. 997. [Google Scholar]
- Cotterill, F.P.D.; Al-Rasheid, K.; Foissner, W. Conservation of protists: Is it needed at all? Biodivers. Conserv. 2008, 17, 427–443. [Google Scholar] [CrossRef]
- Likens, G.E.; Lindenmayer, D.B. Integrating approaches leads to more effective conservation of biodiversity. Biodivers. Conserv. 2012, 21, 3323–3341. [Google Scholar] [CrossRef]
- Foissner, W.P. Diversity and Geographical Distribution. Biodivers. Conserv. 2008, 17, 235–343. [Google Scholar] [CrossRef]
- Fontaneto, D. Biogeography of Microscopic Organisms. Is Everything Small Everywhere? Cambridge University Press: Cambridge, UK, 2011; p. 365. [Google Scholar]
- Foissner, W. Protist diversity and distribution: Some basic considerations. Biodivers. Conserv. 2008, 17, 235–242. [Google Scholar] [CrossRef]
- Cotterill, F.P.D.; Foissner, W. A pervasive denigration of natural history misconstrues how biodiversity inventories and taxonomy underpin scientific knowledge. Biodivers. Conserv. 2010, 19, 291–303. [Google Scholar] [CrossRef]
- Parnell, J.; Hole, M.; Boyce, A.J.; Spinks, S.; Bowden, S. Heavy metal, sex and granites: Crustal differentiation and bioavailability in the mid-Proterozoic. Geology 2012, 40, 751–754. [Google Scholar] [CrossRef]
- Weisse, T.; Strüder-Kypke, C.; Berger, H.; Foissner, W. Genetic, morphological, and ecological diversity of spatially separated clones of Meseres corlissi Petz & Foissner, 1992 (Ciliophora, Spirotrichea). J. Eukaryot. Microbiol. 2008, 55, 257–270. [Google Scholar] [CrossRef]
- Foissner, W. Soil protozoa: fundamental problems, ecological significance, adaptations in ciliates and testaceans, bioindicators, and guide to the literature. Progr. Protistol. 1987, 2, 69–212. [Google Scholar]
- Foissner, W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Europ. J. Protistol. 1991, 27, 313–330. [Google Scholar] [CrossRef]
- Perty, M. Zur Kenntniss kleinster Lebensformen nach Bau, Funktionen, Systematik, mit Specialverzeichnis der in der Schweiz beobachteten. Jent & Reinert: Bern, Switzerland, 1852; p. 228. [Google Scholar]
- Foissner, W. Infraciliatur, Silberliniensystem und Biometrie einiger neuer und wenig bekannter terrestrischer, limnischer und mariner Ciliaten (Protozoa: Ciliophora) aus den Klassen Kinetofragminophora, Colpodea und Polyhymenophora. Stapfia 1984, 12, 1–165. [Google Scholar]
- Kahl, A. Neue und ergänzende Beobachtungen holotricher Infusorien. II. Arch. Protistenk. 1930, 70, 313–416. [Google Scholar]
- Foissner, W.; Oertel, A. Morphology and ciliary pattern of some rare haptorid ciliates, with a description of the new family Kamburophryidae (Protists, Haptoria). Europ. J. Protistol. 2009, 45, 205–218. [Google Scholar] [CrossRef]
- Dragesco, J. Revision du genre Dileptus., Dujardin 1871 (Ciliata Holotricha) (systématique, cytologie, biologie). Bull. Biol. Fr. Belg. 1963, 97, 103–145. [Google Scholar]
- Vd’ačný, P.; Foissner, W. Monograph of the dileptids (Protista, Ciliophora, Rhynchostomatia). Denisia 2012, 31, 1–529. [Google Scholar]
- Stokes, A.C. Some new infusoria. Am. Nat. 1885, 19, 433–443. [Google Scholar]
- Foissner, W. Neue und wenig bekannte hypotriche und colpodide Ciliaten (Protozoa: Ciliophora) aus Böden und Moosen. Zool. Beitr. N. F. 1987, 31, 187–282. [Google Scholar]
- Müller, O.F. Vermium Terrestrium Et Fluviatilium, Seu Animalium Infusorium, Helminthicorum Et Testaceorum, Non Marinorum, Succincta Historia. Heineck & Faber: Havniae, Denmark & Lipsiae, Germany, 1773; p. 135. [Google Scholar]
- Stein, F. Charakteristik neuer Infusorien-Gattungen. Lotos 1859, 9, 2–5. [Google Scholar]
- Golińska, K. Comparative studies on the morphology of Dileptus anatinus sp. n. (Holotricha, Gymnostomata). Acta. Protozool. 1971, 8, 367–378. [Google Scholar]
- Kahl, A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 1. Allgemeiner Teil und Prostomata. Tierwelt. Dtl. 1930, 18, 1–180. [Google Scholar]
- Kahl, A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil behandelten Prostomata. Tierwelt. Dtl. 1931, 21, 181–398. [Google Scholar]
- Foissner, W.; Agatha, S.; Berger, H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Denisia 2002, 5, 1–1459. [Google Scholar]
- Fabre-Domergue, P.L. Sur une nouvelle forme de colpode (Colpoda. henneguyi) et sur un flagellé pélagique. Annls. Microgr. 1889, 2, 351–357. [Google Scholar]
- Foissner, W. Morphologie und Infraciliatur einiger neuer und wenig bekannter terrestrischer und limnischer Ciliaten (Protozoa, Ciliophora). Sber. Akad. Wiss. Wien. 1989, 196, 173–247. [Google Scholar]
- Gabilondo, R.; Foissner, W. Four new fuscheriid soil ciliates (Ciliophora: Haptorida) from four biogeographic regions. Acta. Protozool. 2009, 48, 1–24. [Google Scholar]
- Dujardin, F. Histoire Naturelle Des Zoophytes Infusoire; Suites à Buffon: Paris, France, 1841; p. 684. [Google Scholar]
- Petz, W.; Foissner, W. Morphology and morphogenesis of Strobilidium. caudatum (Fromentel), Meseres corlissi n. sp., Halteria grandinella (Müller), and Strombidium rehwaldi n. sp., and a proposed phylogenetic system for oligotrichid ciliates (Protozoa, Ciliophora). J. Protozool. 1992, 39, 159–176. [Google Scholar]
- Gelei, J. Die Marynidae der Sodagewässer in der Nähe von Szeged XIV Beitrag zur Ziliatenfauna Ungarns. Hidrol. Közl. 1950, 30, 107–119, 157–158. [Google Scholar]
- Foissner, W. Protozoenfauna. Volume 4/1: Colpodea (Ciliophora.); Fischer: Stuttgart, Germany, 1993; pp. I–X, 798. [Google Scholar]
- Stein, F. Charakteristik neuer Infusorien-Gattungen. Lotos 1859, 9, 57–60. [Google Scholar]
- Foissner, W. Morphologie und Taxonomie einiger neuer und wenig bekannter kinetofragminophorer Ciliaten (Protozoa: Ciliophora) aus alpinen Böden. Zool. Jb. Syst. 1981, 108, 264–297. [Google Scholar]
- Xu, K.; Foissner, W. Descriptions of Protospathidium serpens (Kahl, 1930) and P. fraterculum n. sp. (Ciliophora, Haptoria), two species based on different resting cyst morphology. J. Eukaryot. Microbiol. 2005, 52, 298–309. [Google Scholar] [CrossRef]
- Foissner, W. Ökologische und systematische Studien über das Neuston alpiner Kleingewässer, mit besonderer Berücksichtigung der Ciliaten. Int. Revue ges. Hydrobiol. 1979, 64, 99–140. [Google Scholar] [CrossRef]
- Foissner, W. Taxonomie und Ökologie einiger Ciliaten (Protozoa, Ciliophora) des Saprobiensystems. II. Familie Chilodonellidae. Hydrobiologia 1988, 162, 21–45. [Google Scholar] [CrossRef]
- Berger, H.; Foissner, W. Morphology and biometry of some soil hypotrichs (Protozoa: Ciliophora). Zool. Jb. Syst. 1987, 114, 193–239. [Google Scholar]
- Foissner, W. An updated compilation of world soil ciliates (Protozoa, Ciliophora), with ecological notes, new records, and descriptions of new species. Europ. J. Protistol. 1998, 34, 195–235. [Google Scholar] [CrossRef]
- Müller, O.F. Animalcula Infusoria Fluviatilia Et Marina, Quae Detexit, Systematice Descripsit Et Ad Vivum Delineari Curavit; N. Mölleri: Hauniae, Denmark, 1786; p. 367. [Google Scholar]
- Foissner, W. Morphologie und Morphogenese von Psilotricha succisa (O.F. Müller, 1786) nov. comb. (Ciliophora, Hypotrichida). Protistologica 1983, 19, 479–493. [Google Scholar]
- Foissner, W.; Hess, S.; Al-Rasheid, K.A.S. Two vicariant Semispathidium species from tropical Africa and central Europe: S. fraterculum nov. spec. and S. pulchrum nov. spec. (Ciliophora, Haptorida). Europ. J. Protistol. 2010, 46, 61–73. [Google Scholar] [CrossRef]
- Gruber, A. Neue Infusorien. Z. Wiss. Zool. 1880, 33, 439–466. [Google Scholar]
- Foissner, W.; Berger, H.; Blatterer, H.; Kohmann, F. Taxonomische und Ökologische Revision der Ciliaten des Saprobiensystems Band I–IV; Bayerisches Landesamtes für Wasserwirtschaft: Deggendorf, Germany, 1995; pp. 1–540. [Google Scholar]
- Foissner, W.; Berger, H.; Schaumburg, J. Identification and Ecology of Limnetic Plankton Ciliates; Bayerisches Landesamtes für Wasserwirtschaft: Deggendorf, Germany, 1999; p. 793. [Google Scholar]
- Oertel, A.; Wolf, K.; Al-Rasheid, K.; Foissner, W. Revision of the genus Coriplites Foissner, 1988 (Ciliophora: Haptorida), with description of Apocoriplites nov. gen. and three new species. Acta. Protozool. 2008, 47, 231–246. [Google Scholar]
- Stadt Salzburg. Amtsblatt 2012, 4, 1–7.
- Williams, D.D. The Biology of Temporary Waters; Oxford University Press: New York, NY, USA, 2006; p. 337. [Google Scholar]
- Spandl, H. Die Tierwelt vorübergehender Gewässer Mitteleuropas. Arch. Hydrobiol. 1926, 16, 74–132. [Google Scholar]
- Gelei, J. Über die Lebensgemeinschaft einiger temporärer Tümpel auf einer Bergwiese im Börzsönygebirge (Oberungarn) III Ciliaten. Acta. biol. hung. 1954, 5, 259–343. [Google Scholar]
- Gelei, J. Über die Lebensgemeinschaft einiger temporärer Tümpel auf einer Bergwiese im Börzsönygebirge (Oberungarn) I Die Tümpel. Acta. biol. hung. 1954, 5, 227–233. [Google Scholar]
- Gelei, J.; Megyeri, J.; Szabados, M.; Varga, L. Über die Lebensgemeinschaft einiger temporärer Tümpel auf einer Bergwiese im Börzsönygebirge (Oberungarn) VIII Allgemeine Betrachtungen. Acta. biol. hung. 1954, 5, 363–382. [Google Scholar]
- Dingfelder, J.H. Die Ciliaten vorübergehender Gewässer. Arch. Protistenk 1962, 105, 509–658. [Google Scholar]
- Fenchel, T. The quantitative importance of the benthic microfauna of an arctic tundra pond. Hydrobiologia. 1975, 46, 445–464. [Google Scholar] [CrossRef]
- Foissner, W. Artenbestand und Struktur der Ciliatenzönose in alpinen Kleingewässern (Hohe Tauern, Österreich). Arch. Protistenk. 1980, 123, 99–126. [Google Scholar] [CrossRef]
- Stout, J.D. The protozoan fauna of a seasonally inundated soil under grassland. Soil Biol. Biochem. 1984, 16, 121–125. [Google Scholar] [CrossRef]
- Madoni, P. The contribution of ciliated protozoa to plankton and benthos biomass in a European ricefield. J. Eukaryot. Microbiol. 1996, 43, 193–198. [Google Scholar] [CrossRef]
- Andrushchyshyn, O.; Magnusson, A.K.; Williams, D.D. Ciliate populations in temporary freshwater ponds: seasonal dynamics and influential factors. Freshwater Biol. 2003, 48, 548–564. [Google Scholar] [CrossRef]
- Bresslau, E. Über Protozoen aus Rasenaufgüssen. Verh. dt. zool. Ges. 1922, 27, 88–90. [Google Scholar]
- Foissner, W.; Kreutz, M. Systematic position and phylogenetic relationships of the genera Bursaridium, Paracondylostoma, Thylakidium, Bryometopus., and Bursaria. (Ciliophora: Colpodea). Acta Protozool. 1998, 37, 227–240. [Google Scholar]
- Townsend, C.R.; Harper, J.L.; Begon, M.E. Ökologie; Springer: Berlin, Germany, 2003; p. 647. [Google Scholar]
- ICZN (The International Commission on Zoological Nomenclature), International Code of Zoological Nomenclature; International Trust for Zoological Nomenclature: London, UK, 1999; p. 306.
- Foissner, W.; Wenzel, F. Life and legacy of an outstanding ciliate taxonomist, Alfred Kahl (1877–1946), including a facsimile of his forgotten monograph from 1943. Acta. Protozool. 2004, 43 (Suppl.), 3–69. [Google Scholar]
- Cotterill, F.P.D. Systematics, biological knowledge and environmental conservation. Biodivers. Conserv. 1995, 4, 183–205. [Google Scholar] [CrossRef]
- Cotterill, F.P.D. The Second Alexandrian Tragedy, and the fundamental relationship between biological collections and scientific knowledge. In The Values and Valuation of Natural Science Collections, Proceedings of Conference at the Manchester Museum, Manchester, UK, 19–21 April 1995; Nudds, J.R., Pettitt, C.R., Eds.; Geological Society: London, UK; pp. 217–241.
- Foissner, W. Neotypification of protists, especially ciliates (Protozoa, Ciliophora). Bull. Zool. Nom. 2002, 59, 165–169. [Google Scholar]
- Wu, D.; Wu, M.; Halpern, A.; Rusch, D.B.; Yooseph, S.; Frazier, M.; Venter, J.C.; Eisen, J.A. Stalking the fourth domain in metagenomic data: searching for, discovering, and interpreting novel, deep branches in marker gene phylogenetic trees. PLoS One 2011, 6, e18011. [Google Scholar]
- Hansen, A.J.; DeFries, R. Ecological mechanisms linking protected areas to surrounding lands. Ecol. Appl. 2007, 17, 974–988. [Google Scholar] [CrossRef]
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem. Biol. 1998, 5, R245–R249. [Google Scholar] [CrossRef]
- Morgan, J.L.; Darling, A.E.; Eisen, J.A. Metagenomic sequencing of an in vitro-simulated microbial community. PLoS One 2010, 5, e10209. [Google Scholar] [CrossRef]
- Foissner, W.; Chao, A.; Katz, L.A. Diversity and geographic distribution of ciliates (Protista: Ciliophora). Biodivers. Conserv. 2008, 17, 345–363. [Google Scholar] [CrossRef]
- Barberán, A.; Casamayor, E.O. Euxinic freshwater hypolimnia promote bacterial endemicity in continental areas. Microb. Ecol. 2011, 61, 465–472. [Google Scholar] [CrossRef]
- Griffith, G.W. Do we need a global strategy for microbial conservation? Trends Ecol. Evol. 2012, 27, 1–2. [Google Scholar] [CrossRef]
- Mace, G.M. The role of taxonomy in species conservation. Phil. Trans. R. Soc. Lond. B. 2004, 359, 711–719. [Google Scholar] [CrossRef]
- Bernardo, J. A critical appraisal of the meaning and diagnosability of cryptic evolutionary diversity, and its implications for conservation in the face of climate change. In Climate Change, Ecology and Systematics; Hodkinson, T.R., Jones, M.B., Waldren, S., Parnell, J.A.N., Eds.; Cambridge University Press, The Systematics Association: Cambridge, UK, 2011; pp. 380–438. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cotterill, F.P.D.; Augustin, H.; Medicus, R.; Foissner, W. Conservation of Protists: The Krauthügel Pond in Austria. Diversity 2013, 5, 374-392. https://doi.org/10.3390/d5020374
Cotterill FPD, Augustin H, Medicus R, Foissner W. Conservation of Protists: The Krauthügel Pond in Austria. Diversity. 2013; 5(2):374-392. https://doi.org/10.3390/d5020374
Chicago/Turabian StyleCotterill, Fenton P.D., Hannes Augustin, Reinhard Medicus, and Wilhelm Foissner. 2013. "Conservation of Protists: The Krauthügel Pond in Austria" Diversity 5, no. 2: 374-392. https://doi.org/10.3390/d5020374
APA StyleCotterill, F. P. D., Augustin, H., Medicus, R., & Foissner, W. (2013). Conservation of Protists: The Krauthügel Pond in Austria. Diversity, 5(2), 374-392. https://doi.org/10.3390/d5020374




