Multidimensional Scaling Analysis of Morphological Spike Traits in Local Wheat Genotypes from the Van Lake Basin
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
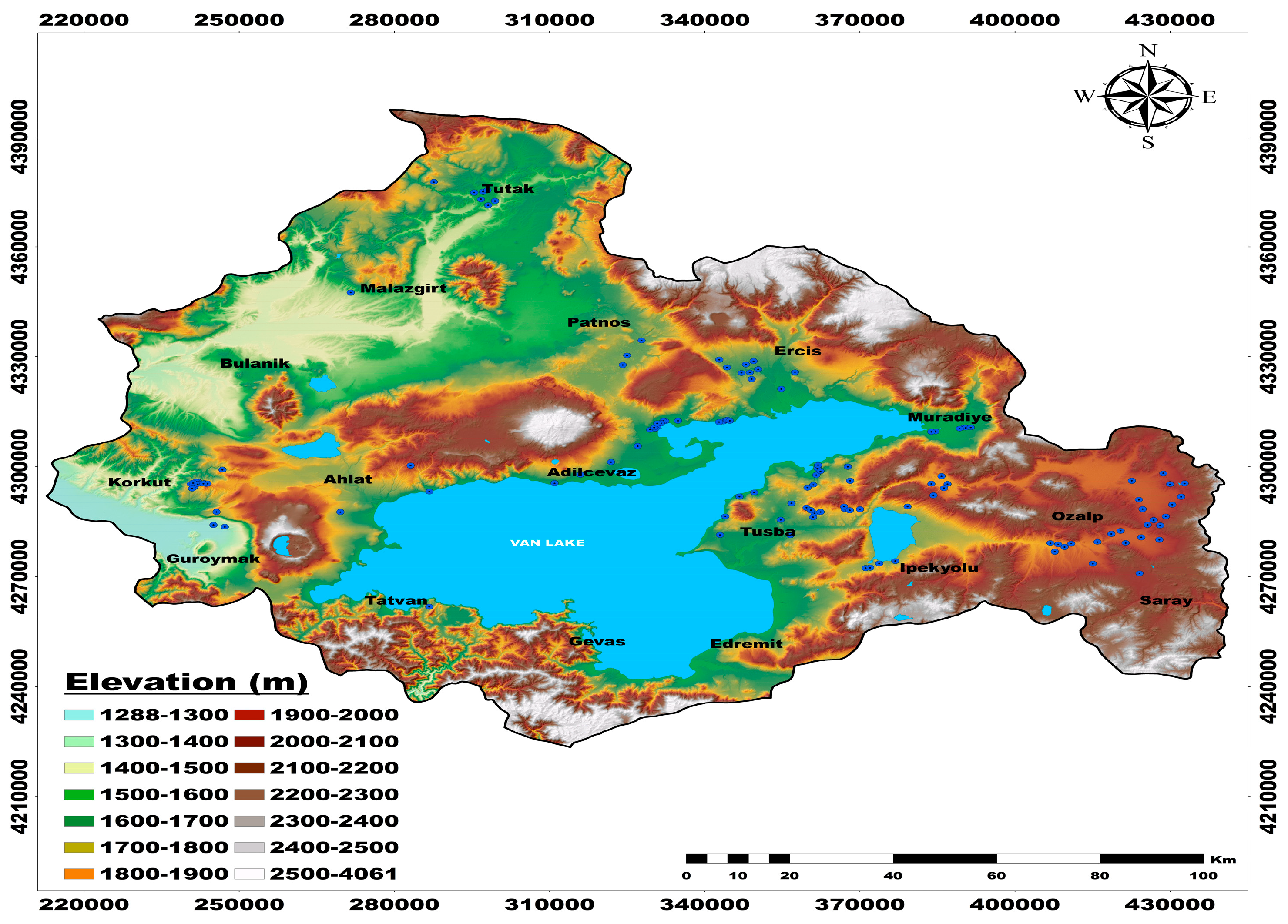
2.1. Plant Sampling and Measurements
2.2. Statistical Analysis
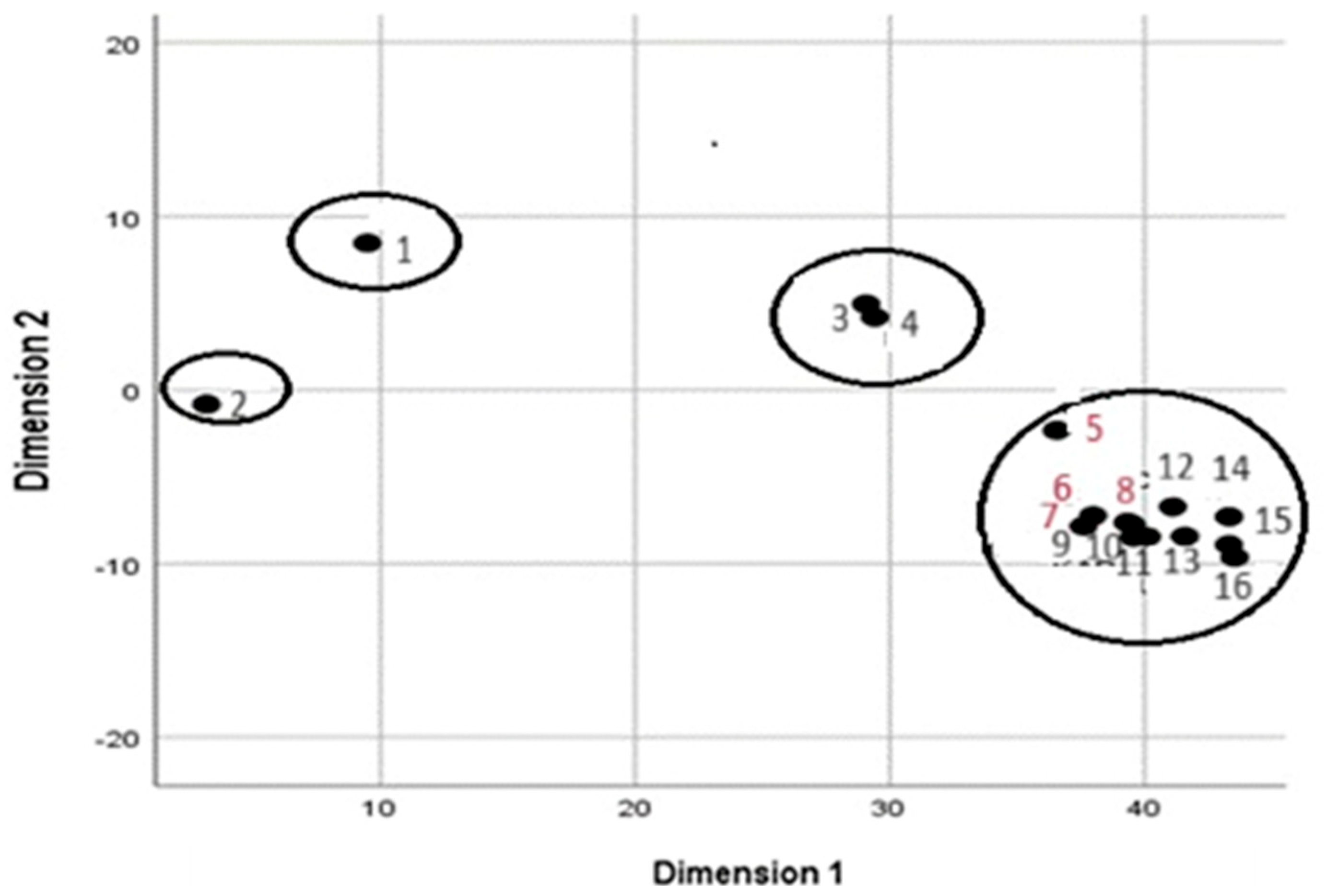
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- FAO FAOSTAT Statistical Database. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 7 May 2025).
- Shewry, P.R.; Hey, S.J. The Contribution of Wheat to Human Diet and Health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- de Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat! Foods 2021, 10, 2124. [Google Scholar] [CrossRef] [PubMed]
- Harlan, J.R. Our Vanishing Genetic Resources. Science (1979) 1975, 188, 618–621. [Google Scholar] [CrossRef]
- Marone, D.; Russo, M.A.; Mores, A.; Ficco, D.B.M.; Laidò, G.; Mastrangelo, A.M.; Borrelli, G.M. Importance of Landraces in Cereal Breeding for Stress Tolerance. Plants 2021, 10, 1267. [Google Scholar] [CrossRef]
- Karagöz, A. Wheat landraces of Turkey. Emir. J. Food Agric. 2014, 26, 149–156. [Google Scholar] [CrossRef]
- Harlan, J.R. Collection of Crop Plants in Turkey, 19484. Agron. J. 1950, 42, 258–259. [Google Scholar] [CrossRef]
- Miedaner, T.; Garbelotto, M.M. Human-Mediated Migration of Plants, Their Pathogens and Parasites. J. Plant Pathol. 2024, 106, 301–325. [Google Scholar] [CrossRef]
- Bonjean, A.P.; Angus, W.J.; Sági, F. The World Wheat Book: A History of Wheat Breeding. Cereal Res. Commun. 2001, 29, 459. [Google Scholar] [CrossRef]
- Heun, M.; Schäfer-Pregl, R.; Klawan, D.; Castagna, R.; Accerbi, M.; Borghi, B.; Salamini, F. Site of Einkorn Wheat Domestication Identified by DNA Fingerprinting. Science (1979) 1997, 278, 1312–1314. [Google Scholar] [CrossRef]
- Özkan, H.; Willcox, G.; Graner, A.; Salamini, F.; Kilian, B. Geographic Distribution and Domestication of Wild Emmer Wheat (Triticum dicoccoides). Genet. Resour. Crop Evol. 2011, 58, 11–53. [Google Scholar] [CrossRef]
- Salamini, F.; Özkan, H.; Brandolini, A.; Schäfer-Pregl, R.; Martin, W. Genetics and Geography of Wild Cereal Domestication in the near East. Nat. Rev. Genet. 2002, 3, 429–441. [Google Scholar] [CrossRef]
- Tanno, K.; Willcox, G. How Fast Was Wild Wheat Domesticated? Science (1979) 2006, 311, 1886. [Google Scholar] [CrossRef]
- Willcox, G. A Companion to the Archaeology of the Ancient Near East; Potts, D.T., Ed.; Wiley: Hoboken, NJ, USA, 2012; Volume 1, ISBN 9781405189880. [Google Scholar]
- Solmaz, T.; Dönmez, E.O. Archaeobotanical Studies at the Urartian Site of Ayanis in Van Province, Eastern Turkey. Turk. J. Bot. 2013, 37, 282–296. [Google Scholar] [CrossRef]
- Dönmez, E.O.; Belli, O. Urartian Plant Cultivation at Yoncatepe (Van), Eastern Turkey. Econ. Bot. 2007, 61, 290–298. [Google Scholar] [CrossRef]
- Erdoğan, Z.; Selçuk, F.; Akgün, A. Wheat Self-Sufficiency in Turkey: Production and Climate Change in Focus. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Dergisi 2022, 32, 654–670. [Google Scholar] [CrossRef]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting Genetic Diversity from Landraces in Wheat Breeding for Adaptation to Climate Change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Robbana, C.; Kehel, Z.; Ben Naceur, M.; Sansaloni, C.; Bassi, F.; Amri, A. Genome-Wide Genetic Diversity and Population Structure of Tunisian Durum Wheat Landraces Based on DArTseq Technology. Int. J. Mol. Sci. 2019, 20, 1352. [Google Scholar] [CrossRef] [PubMed]
- Bernis-Fonteneau, A.; Aakairi, M.; Saadani-Hassani, O.; Castangia, G.; Ait Babahmad, R.; Colangelo, P.; D’Ambrosio, U.; Jarvis, D.I. Farmers’ Variety Naming and Crop Varietal Diversity of Two Cereal and Three Legume Species in the Moroccan High Atlas, Using DATAR. Sustainability 2023, 15, 10411. [Google Scholar] [CrossRef]
- Karagöz, A.; Zencirci, N. Variation in Wheat (Triticum Spp.) Landraces from Different Altitudes of Three Regions of Turkey. Genet. Resour. Crop Evol. 2005, 52, 775–785. [Google Scholar] [CrossRef]
- Morgounov, A.; Keser, M.; Kan, M.; Küçükçongar, M.; Özdemir, F.; Gummadov, N.; Muminjanov, H.; Zuev, E.; Qualset, C.O. Wheat Landraces Currently Grown in Turkey: Distribution, Diversity, and Use. Crop Sci. 2016, 56, 3112–3124. [Google Scholar] [CrossRef]
- UPOV. International Union for the Protection of New Varieties of Plants Wheat UPOV Code(s): TRITI_AES Triticum aestivum L. Emend. Fiori et Paol. Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability; UPOV: Geneve, Switzerland, 2017. [Google Scholar]
- Niane, A.A.; Madarati, A.; Abbas, A.; Turner, M. Manual of Morphological Variety Description for Wheat and Barley with Examples from Syria; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 1999; ISBN 92-9127-089-M. [Google Scholar]
- Yiğit, S.; Mendeş, M. Usage of Multidimensional Scaling Technique for Evaluating Performances of Multivariate Normality Tests. Br. J. Appl. Sci. Technol. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Kruskal, J.; Wish, M. Multidimensional Scaling; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 1978; ISBN 9780803909403. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows 2022, IBM Corp.: Armonk, NY, USA, 2022.
- R Core Team. R: A Language and Environment for Statistical Computing, R Core Team: Vienna, Austria, 2023.
- Zhou, H.; Riche, A.B.; Hawkesford, M.J.; Whalley, W.R.; Atkinson, B.S.; Sturrock, C.J.; Mooney, S.J. Determination of Wheat Spike and Spikelet Architecture and Grain Traits Using X-Ray Computed Tomography Imaging. Plant Methods 2021, 17, 26. [Google Scholar] [CrossRef]
- Vermeesch, P.; Lipp, A.G.; Hatzenbühler, D.; Caracciolo, L.; Chew, D. Multidimensional Scaling of Varietal Data in Sedimentary Provenance Analysis. J. Geophys. Res. Earth Surf. 2023, 128, e2022JF006992. [Google Scholar] [CrossRef]
- Başpınar, E. İki Yönlü Tablolarda Uyum Analizi Tekniğinin Kullanımı. Tarım Bilim. Derg. 2000, 6, 98–106. [Google Scholar] [CrossRef]
- Kruskal, J.B. Nonmetric Multidimensional Scaling: A Numerical Method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Borg, I.; Groenen, P.J. Modern Multidimensional Scaling; Springer Series in Statistics; Springer: New York, NY, USA, 2005; ISBN 978-0-387-25150-9. [Google Scholar]
- Gökgöl, M. Turkish Wheats, Vol. II. Yeşilköy Seed Breeding Institute Publications No.14; Tan Press: Istanbul, Turkey, 1939; Volume 2. [Google Scholar]
- Gökgöl, M. Turkish Wheats, Vol. I. Yesilkoy Seed Breeding Institute Publications No. 7; Devlet Press: Instanbul, Turkey, 1935. [Google Scholar]
- Yılmaz, N.; Akyürek, A. The “Tir” Seeding Method and Its Application in the Van Region. Yuz. Yıl Univ. J. Agric. Sci. 2016, 1, 170–181. [Google Scholar]
- Kong, L.; Sun, M.; Xie, Y.; Wang, F.; Zhao, Z. Photochemical and Antioxidative Responses of the Glume and Flag Leaf to Seasonal Senescence in Wheat. Front. Plant Sci. 2015, 6, 358. [Google Scholar] [CrossRef]
- Duma, S.W.; Shimelis, H.; Tesfamariam, S.A.; Tsilo, T.J. Genetic Parameters and Trait Associations in Wheat Under Drought and Low Nitrogen Conditions. Nitrogen 2024, 5, 1196–1214. [Google Scholar] [CrossRef]
- Wang, J.; Luo, M.C.; Chen, Z.; You, F.M.; Wei, Y.; Zheng, Y.; Dvorak, J. Aegilops tauschii Single Nucleotide Polymorphisms Shed Light on the Origins of Wheat D-Genome Genetic Diversity and Pinpoint the Geographic Origin of Hexaploid Wheat. New Phytol. 2013, 198, 925–937. [Google Scholar] [CrossRef]
- Börner, A.; Schäfer, M.; Schmidt, A.; Grau, M.; Vorwald, J. Associations between Geographical Origin and Morphological Characters in Bread Wheat (Triticum aestivum L.). Plant Genet. Resour. 2005, 3, 360–372. [Google Scholar] [CrossRef]
- Mao, W.; Wang, X.; Chen, Y.; Wang, Y.; Ma, L.; Xie, X.; Wu, X.; Xu, J.; Zhang, Y.; Zhao, Y.; et al. Map-Based Cloning and Characterization Reveal That an R2R3 MYB Gene Confers Red Glume in Wheat. Crop J. 2024, 13, 887–899. [Google Scholar] [CrossRef]
- Kaya, Y.; Akcura, M. Effects of Genotype and Environment on Grain Yield and Quality Traits in Bread Wheat (T. aestivum L.). Food Sci. Technol. (Camp.) 2014, 34, 386–393. [Google Scholar] [CrossRef]
- Lullien-Pellerin, V. Both Genetic and Environmental Conditions Affect Wheat Grain Texture: Consequences for Grain Fractionation and Flour Properties. J. Cereal Sci. 2020, 92, 102917. [Google Scholar] [CrossRef]
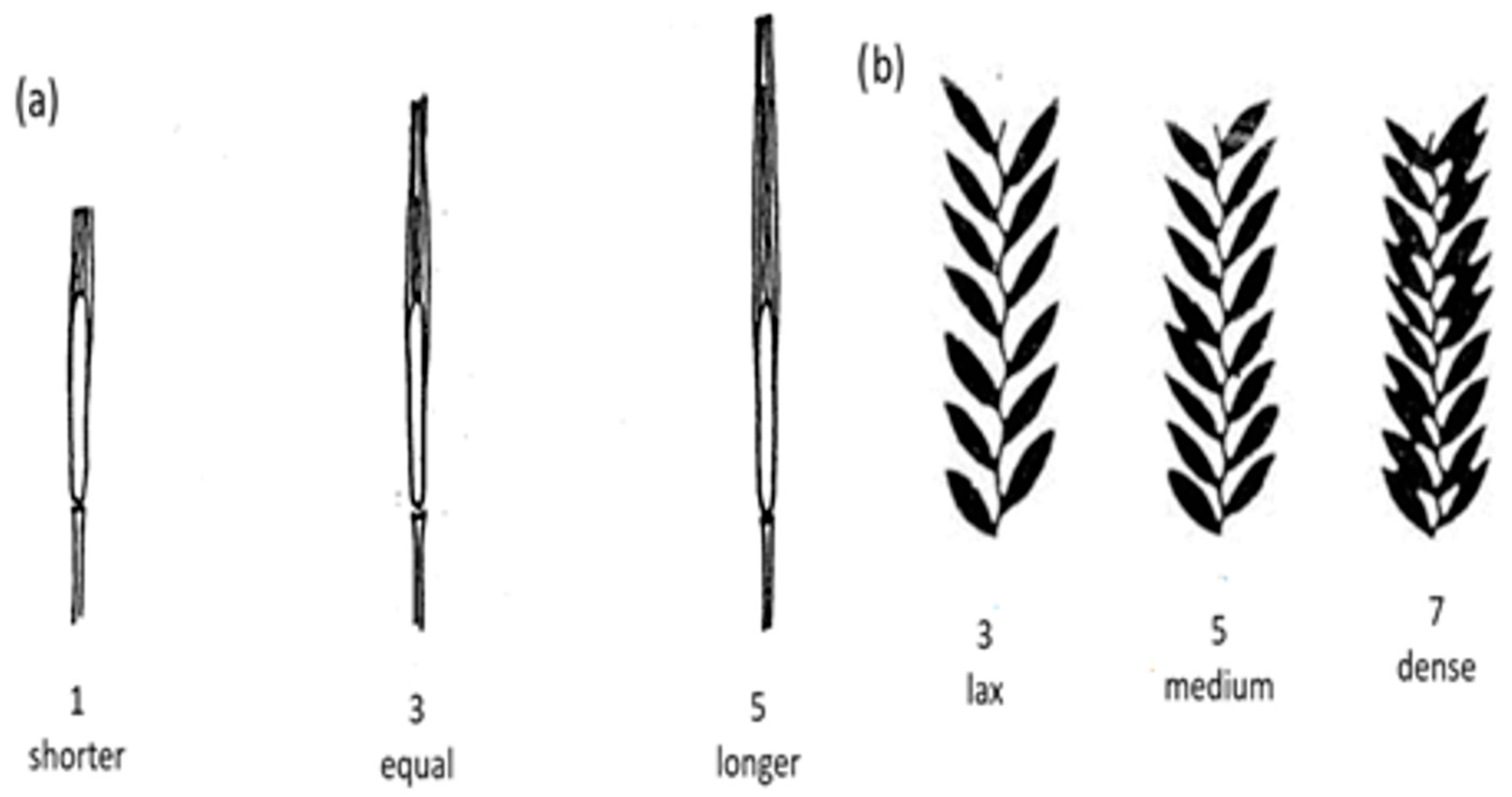



| Characteristics | Abbreviations | Classification and scoring |
|---|---|---|
| Ratio of awn to spike at the tip of the spike | ASR | 0: awn-less 1: shorter 2: equal 3: longer |
| Spike density | EAD | 1: very lax 3: lax 5: medium 7: dense 9: very dense |
| Awn color | AWC | 0: awn-less 1: white 2: light brown 3: brown 4: brown-white 5: white-black 6: brown-black 7: white-purple 8: purple-brown |
| Glume color | GLC | 1: white 2: red-brown 3: purple-black 4: white-purple 5: white-black 6: white-brown 7: red-brown 8: black 9: brown-black 10: brown 11: brown-purple 12: red-black 13: black-purple 14: red-purple 15: white-red |
| Glume hairiness in sterile spikelet | GLH | 0: smooth 3: low 7: high |
| Grain color | GRC | 1: white 2: red 3: amber |
| Grain shape | GRSH | 3: rounded 5: ovoid 7: elongated |
| Grain size | GRS | 3: small 5: medium 7: large 9: very large |
| Vitreousness of grain | GVI | 3: non-vitreous 5: partially vitreous 7: vitreous |
| Fullness of grain | GRF | 3: plump 5: medium 7: wrinkled |
| Spike length (cm) | SPL | |
| Number of spikelets | NOS | |
| Number of fertile spikelets spike-1 | NFS | |
| Number of seeds spike-1 | NSS | |
| Grain yield spike-1 (g) | GRY | |
| Thousand-grain weight (g) | TGW |
| Parameter | n | Mean | Std. Error of the Mean | Min | Max | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|
| Spike length (SPL) | 588 | 8.91 | 1.79 | 3.60 | 15.10 | −0.33 | 0.39 |
| Number of spikelets (NOS) | 588 | 16.02 | 3.03 | 6.00 | 23.00 | −0.27 | −0.34 |
| Number of fertile spikelets per spike (NFS) | 588 | 14.30 | 3.44 | 5.00 | 23.00 | −0.16 | −0.30 |
| Number of seeds per spike (NSS) | 588 | 31.97 | 10.26 | 8.00 | 64.00 | 0.30 | −0.10 |
| Grain yield per spike (GRY) | 588 | 1.47 | 0.61 | 0.30 | 3.55 | 0.58 | 0.04 |
| Thousand-grain weight (TGW) | 588 | 45.29 | 8.58 | 22.14 | 68.08 | −0.15 | −0.54 |
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratio of awn to spike at the tip (ASR) | 8.8 | 85.7 | 1.6 | 3.9 | ||||||||||||
| Spike density (EAD) | 0.0 | 2.4 | 33.0 | 48.8 | 15.8 | |||||||||||
| Awn color (AWC) | 9.0 | 15.8 | 17.3 | 1.9 | 7.0 | 10.2 | 16.7 | 19.0 | 3.1 | |||||||
| Glume color (GLC) | 16.2 | 18.6 | 0.2 | 32.8 | 7.7 | 7.7 | 2.6 | 2.7 | 3.3 | 0.5 | 6.8 | 0.2 | 0.3 | 0.2 | 0.2 | |
| Glume hairiness (GLH) | 81.3 | 18.2 | 0.5 | |||||||||||||
| Glume shape (GRC) | 11.7 | 5.0 | 83.3 | |||||||||||||
| Glume shoulder shape (GRSH) | 17.0 | 81.0 | 2.0 | |||||||||||||
| Glume shoulder form (GRS) | 13.3 | 78.1 | 8.3 | 0.3 | ||||||||||||
| Glume beak shape (GVI) | 31.3 | 47.1 | 21.6 | |||||||||||||
| Grain form (GRF) | 9.2 | 83.8 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altuner, F.; Jamal-Salih, S.; Özdemir, B.; Oral, E.; Mendes, M.; Ulker, M.; Najafi, S.; Farda, B.; Pace, L. Multidimensional Scaling Analysis of Morphological Spike Traits in Local Wheat Genotypes from the Van Lake Basin. Diversity 2025, 17, 663. https://doi.org/10.3390/d17090663
Altuner F, Jamal-Salih S, Özdemir B, Oral E, Mendes M, Ulker M, Najafi S, Farda B, Pace L. Multidimensional Scaling Analysis of Morphological Spike Traits in Local Wheat Genotypes from the Van Lake Basin. Diversity. 2025; 17(9):663. https://doi.org/10.3390/d17090663
Chicago/Turabian StyleAltuner, Fevzi, Sana Jamal-Salih, Burak Özdemir, Erol Oral, Mehmet Mendes, Mehmet Ulker, Solmaz Najafi, Beatrice Farda, and Loretta Pace. 2025. "Multidimensional Scaling Analysis of Morphological Spike Traits in Local Wheat Genotypes from the Van Lake Basin" Diversity 17, no. 9: 663. https://doi.org/10.3390/d17090663
APA StyleAltuner, F., Jamal-Salih, S., Özdemir, B., Oral, E., Mendes, M., Ulker, M., Najafi, S., Farda, B., & Pace, L. (2025). Multidimensional Scaling Analysis of Morphological Spike Traits in Local Wheat Genotypes from the Van Lake Basin. Diversity, 17(9), 663. https://doi.org/10.3390/d17090663









