The Ecophysiological Role of Trees in Dryland Agroecosystems: Implications for Natural Resource Conservation and Sustainable Food Production in Sub-Saharan Africa
Abstract
1. Introduction
2. The Role of Trees in Water and Nutrient Cycling in Drylands
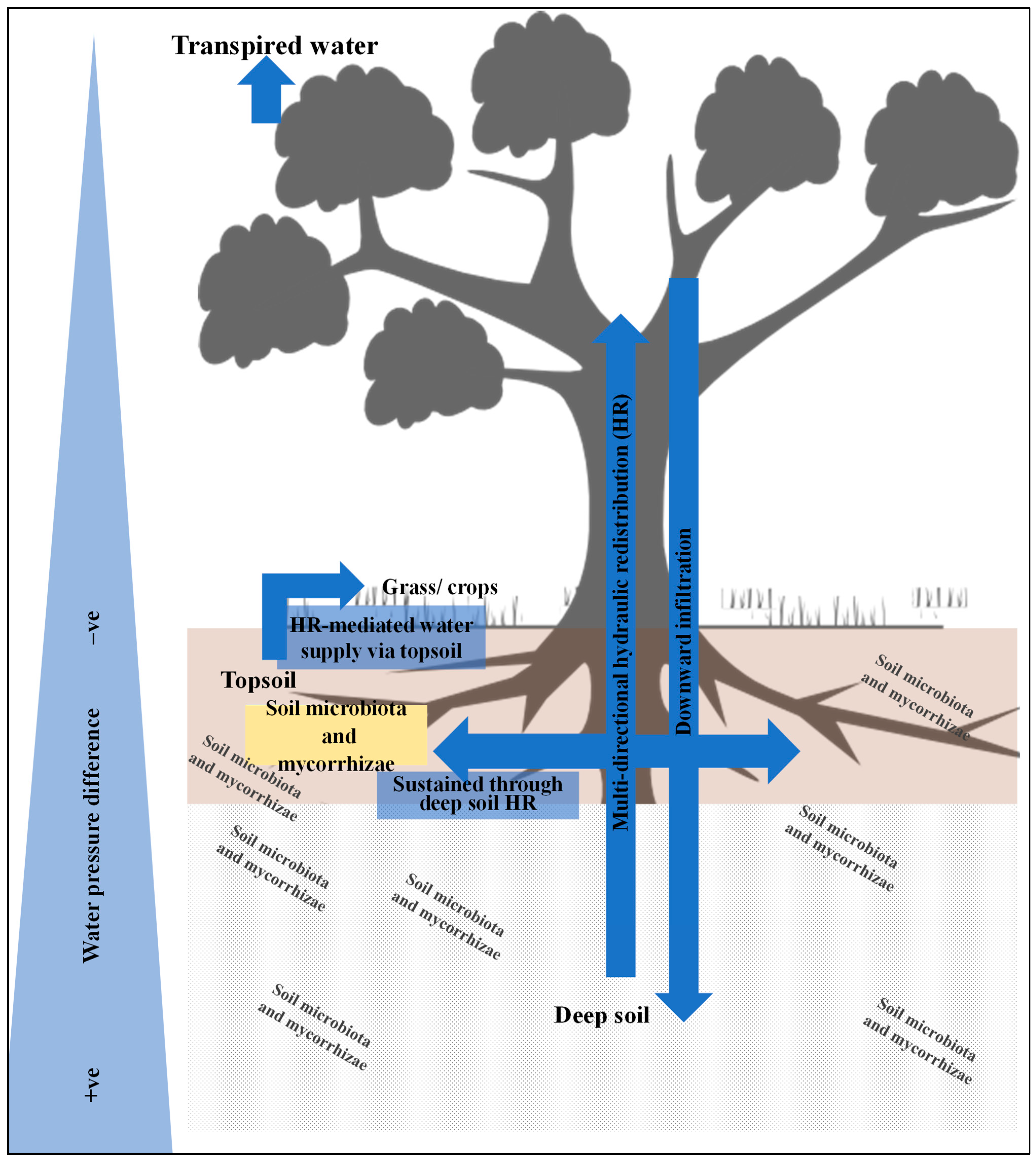
2.1. Water Redistribution by Trees
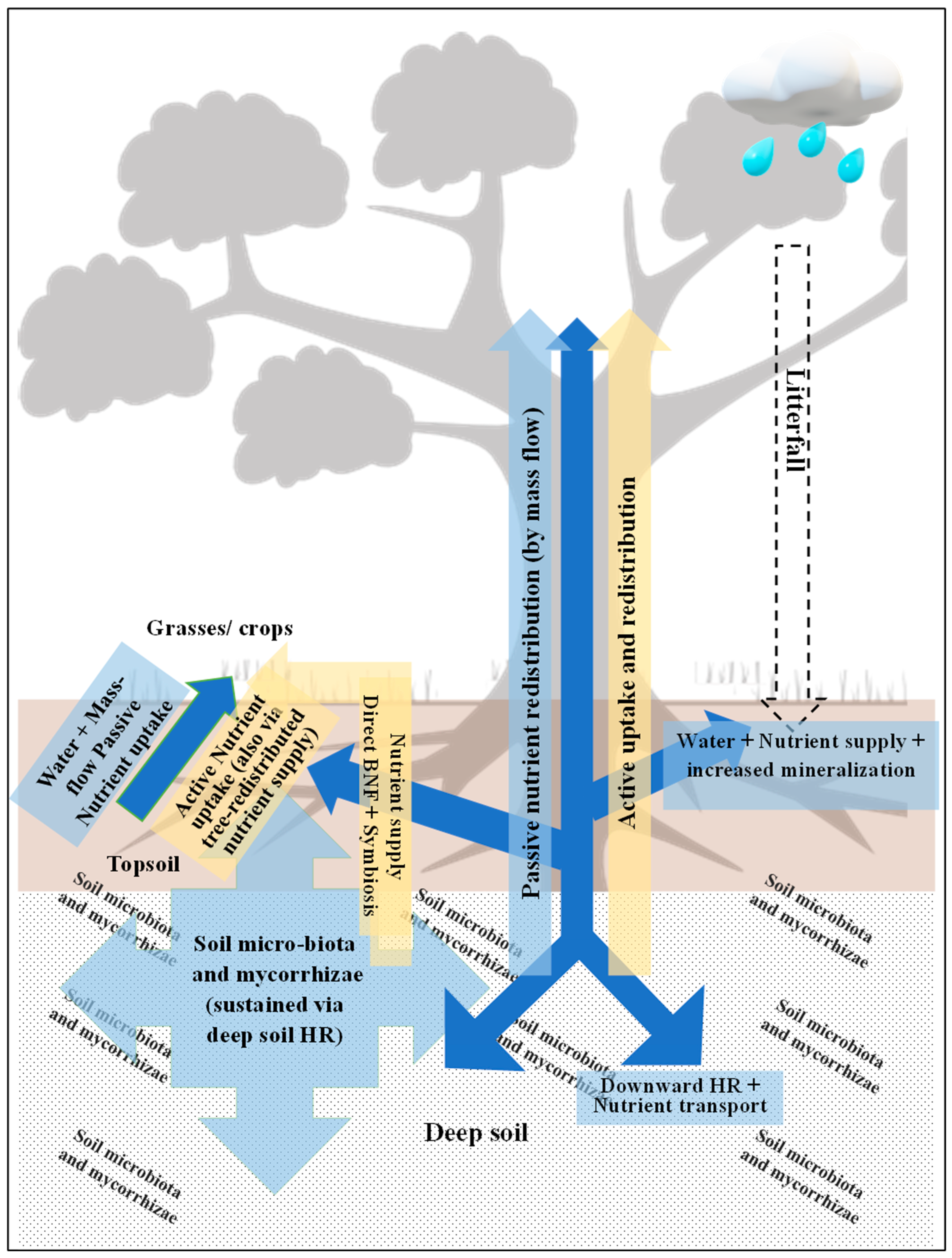
2.2. The Linkages Between Water Redistribution, Nutrient Transport, and Biotic Interactions
2.3. Nutrient Redistribution by Trees
3. Trees in Agroecosystems of Sub-Saharan Africa
3.1. Why Is the Focus on Sub-Saharan Africa Important?
3.2. The Role of Tree Ecophysiology in Agroforestry Systems of Sub-Saharan Africa
3.2.1. Influence of Tree Ecophysiology on Resource Use
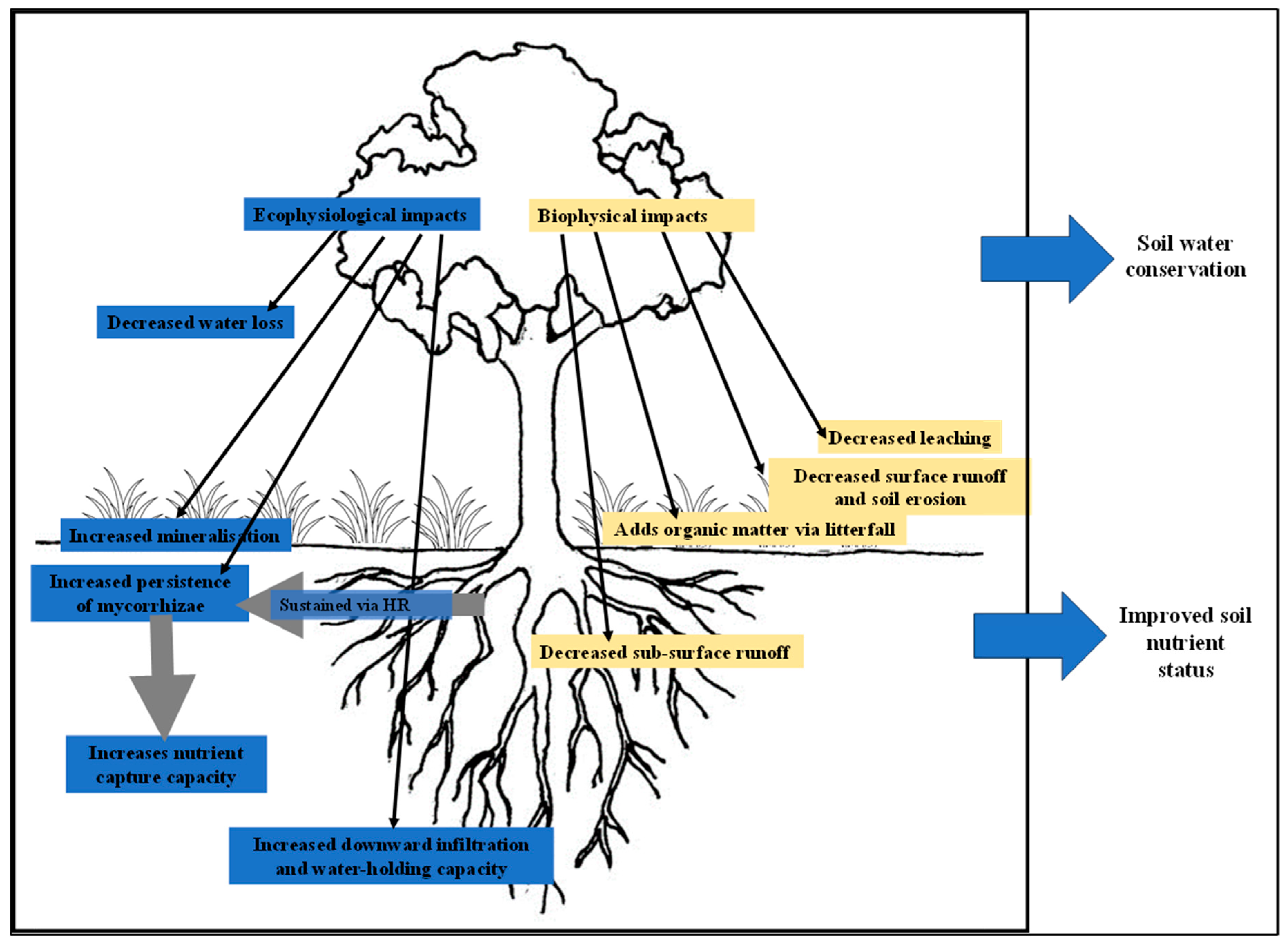
3.2.2. Influence of Tree Ecophysiology on Nutrient and Water Cycling
3.2.3. Influence of Tree Ecophysiology on Soil Quality
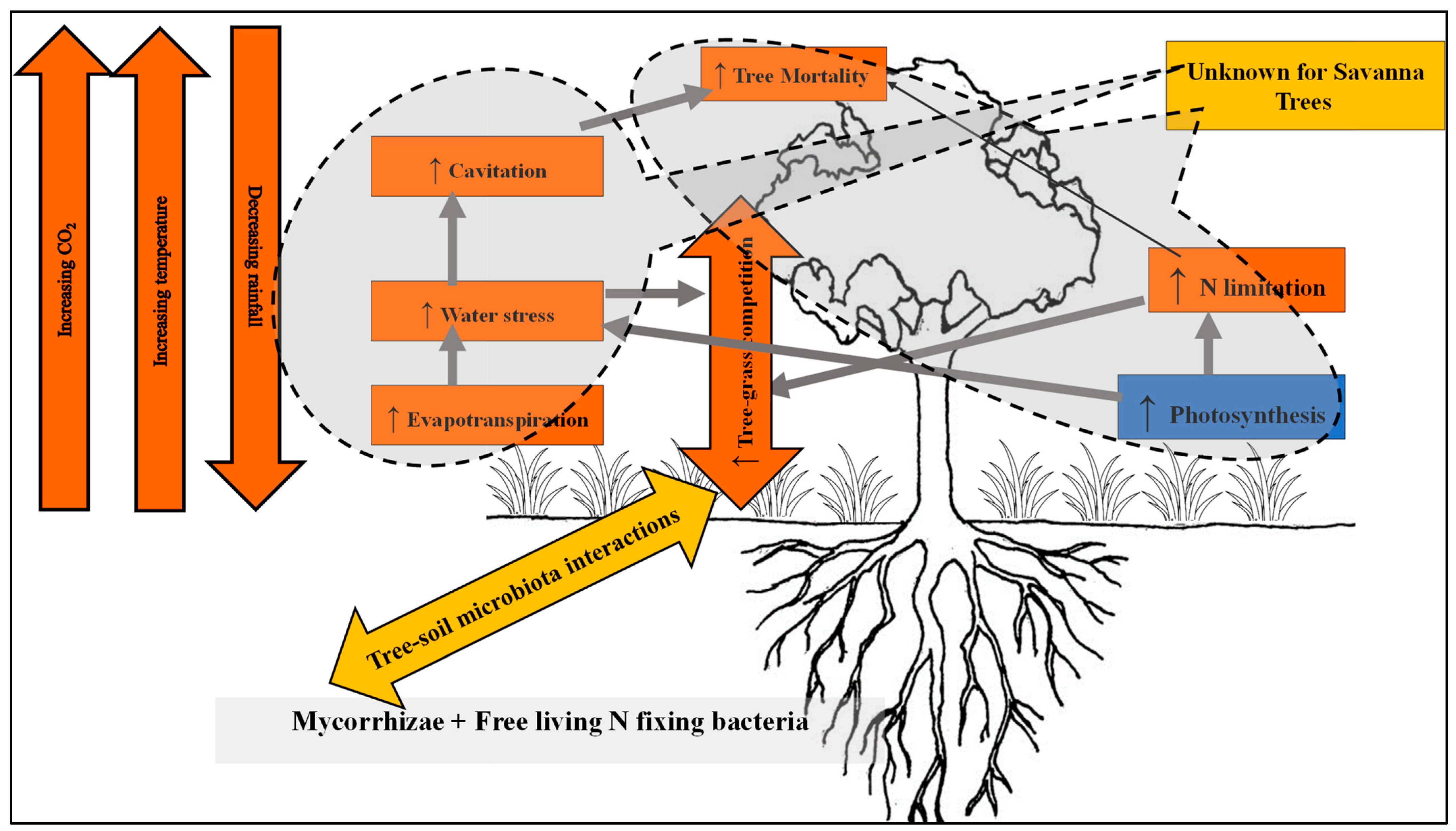
3.2.4. Influence of Tree Ecophysiology to Improve Climate Resilience
3.3. Tree Ecophysiological Function and Agroecological Sustainability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Noordwijk, M. (Ed.) Sustainable Development Through Trees on Farms: Agroforestry in Its Fifth Decade; World Agroforestry (ICRAF): Bogor, Indonesia, 2019; ISBN 978-602-5894-03-9. [Google Scholar]
- Tian, G.; Kang, B.T.; Kolawole, G.O.; Idinoba, P.; Salako, F.K. Long-Term Effects of Fallow Systems and Lengths on Crop Production and Soil Fertility Maintenance in West Africa. Nutr. Cycl. Agroecosyst. 2005, 71, 139–150. [Google Scholar] [CrossRef]
- Carsan, S.; Stroebel, A.; Dawson, I.; Kindt, R.; Mbow, C.; Mowo, J.; Jamnadass, R. Can Agroforestry Option Values Improve the Functioning of Drivers of Agricultural Intensification in Africa? Curr. Opin. Environ. Sustain. 2014, 6, 35–40. [Google Scholar] [CrossRef]
- Prinsley, R.T. The Role of Trees in Sustainable Agriculture—An Overview. Agrofor. Syst. 1992, 20, 87–115. [Google Scholar] [CrossRef]
- Buresh, R.J.; Tian, G. Soil Improvement by Trees in Sub-Saharan Africa. Agrofor. Syst. 1998, 38, 51–76. [Google Scholar] [CrossRef]
- Verchot, L.V.; Van Noordwijk, M.; Kandji, S.; Tomich, T.; Ong, C.; Albrecht, A.; Mackensen, J.; Bantilan, C.; Anupama, K.V.; Palm, C. Climate Change: Linking Adaptation and Mitigation through Agroforestry. Mitig. Adapt. Strateg. Glob. Change 2007, 12, 901–918. [Google Scholar] [CrossRef]
- Prieto, I.; Armas, C.; Pugnaire, F.I. Water Release through Plant Roots: New Insights into Its Consequences at the Plant and Ecosystem Level. New Phytol. 2012, 193, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, K.V.R. The Facilitative Role of Trees in Tree-Grass Interactions in Savannas. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar]
- Brantley, S.L.; Eissenstat, D.M.; Marshall, J.A.; Godsey, S.E.; Balogh-Brunstad, Z.; Karwan, D.L.; Papuga, S.A.; Roering, J.; Dawson, T.E.; Evaristo, J.; et al. Reviews and Syntheses: On the Roles Trees Play in Building and Plumbing the Critical Zone. Biogeosciences 2017, 14, 5115–5142. [Google Scholar] [CrossRef]
- Safriel, U.; Adeel, Z.; Niemeijer, D.; Puigdefabregas, J.; White, R.; Lal, R.; Winslow, M.; Ziedler, J.; Prince, S.; Archner, E.; et al. Dryland Systems. In Ecosystems Human Well-Being: Findings of the Conditions Trends Working Group of the Millennium Ecosystem Assessment; Hassan, R., Scholes, R.J., Ash, N., Eds.; Island Press: Washington DC, USA, 2005; Volume 1, pp. 1–58. [Google Scholar]
- Jones, P.G.; Thornton, P.K. The Potential Impacts of Climate Change on Maize Production in Africa and Latin America in 2055. Glob. Environ. Change 2003, 13, 51–59. [Google Scholar] [CrossRef]
- Challinor, A.; Wheeler, T.; Garforth, C.; Craufurd, P.; Kassam, A. Assessing the Vulnerability of Food Crop Systems in Africa to Climate Change. Clim. Change 2007, 83, 381–399. [Google Scholar] [CrossRef]
- Schlenker, W.; Lobell, D.B. Robust Negative Impacts of Climate Change on African Agriculture. Environ. Res. Lett. 2010, 5, 014010. [Google Scholar] [CrossRef]
- The Montpellier Panel. Sustainable Intensification: A New Paradigm for African Agriculture; The Montpellier Panel: London, UK, 2013. [Google Scholar]
- Jayne, T.S.; Sanchez, P.A. Agricultural Productivity Must Improve in Sub-Saharan Africa. Science 2021, 372, 1045–1047. [Google Scholar] [CrossRef]
- Sivakumar, M.V.K.; Das, H.P.; Brunini, O. Impacts of Present and Future Climate Variability and Change on Agriculture and Forestry in the Arid and Semi-Arid Tropics. Clim. Change 2005, 70, 31–72. [Google Scholar] [CrossRef]
- Rosegrant, M.W.; Cai, X. Global Water Demand and Supply Projections Part 2: Results and Prospects to 2025. Water Int. 2002, 27, 170–182. [Google Scholar] [CrossRef]
- Oldeman, L.R. Global Extent of Soil Degradation; ISRIC: Wageningen, The Netherlands, 1992. [Google Scholar]
- Huang, J.; Li, Y.; Fu, C.; Chen, F.; Fu, Q.; Dai, A.; Shinoda, M.; Ma, Z.; Guo, W.; Li, Z.; et al. Dryland climate change: Recent progress and challenges. Rev. Geophys. 2017, 55, 719–778. [Google Scholar] [CrossRef]
- Rosa, L.; Chiarelli, D.D.; Sangiorgio, M.; Beltran-Peña, A.A.; Rulli, M.C.; D’Odorico, P.; Fung, I. Potential for Sustainable Irrigation Expansion in a 3 °C Warmer Climate. Proc. Natl. Acad. Sci. USA 2020, 117, 29526–29534. [Google Scholar] [CrossRef]
- Drechsel, P.; Heffer, P.; Magen, H.; Mikkelsen, R.; Singh, H.; Wichelns, D. Managing Water and Nutrients to Ensure Global Food Security, While Sustaining Ecosystem Services. In Managing Water and Fertilizer for Sustainable Agricultural Intensification; Drechsel, P., Heffer, P., Magen, H., Mikkelsen, R., Wichelns, D., Eds.; International Fertilizer Industry Association, International Water Management Institute, International Plant Nutrition Institute, and International Potash Institute: Paris, France, 2015; pp. 1–7. [Google Scholar]
- Neumann, R.B.; Cardon, Z.G. The Magnitude of Hydraulic Redistribution by Plant Roots: A Review and Synthesis of Empirical and Modeling Studies. New Phytol. 2012, 194, 337–352. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A Global Analysis of Root Distributions for Terrestrial Biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Bassirirad, H. (Ed.) Nutrient Acquisition by Plants: An Ecological Perspective; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Pierret, A.; Maeght, J.L.; Clément, C.; Montoroi, J.P.; Hartmann, C.; Gonkhamdee, S. Understanding Deep Roots and Their Functions in Ecosystems: An Advocacy for More Unconventional Research. Ann. Bot. 2016, 118, 621–635. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Dawson, T.E.; Richards, J.H. Hydraulic Lift: Consequences of Water Efflux from the Roots of Plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Ong, C.K. The Redistribution of Soil Water by Tree Root Systems. Oecologia 1998, 115, 306–311. [Google Scholar] [CrossRef]
- Nadezhdina, N.; David, T.S.; David, J.S.; Ferreira, M.I.; Dohnal, M.; Tesař, M.; Gartner, K.; Leitgeb, E.; Nadezhdin, V.; Cermak, J.; et al. Trees Never Rest: The Multiple Facets of Hydraulic Redistribution. Ecohydrology 2010, 3, 431–444. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Pate, J.S.; Adams, M.A.; Dawson, T.E. Seasonal Water Acquisition and Redistribution in the Australian Woody Phreatophyte, Banksia Prionotes. Ann. Bot. 2000, 85, 215–224. [Google Scholar] [CrossRef]
- Ludwig, F.; Dawson, T.E.; Kroon, H.; Berendse, F.; Prins, H.H.T. Hydraulic Lift in Acacia Tortilis Trees on an East African Savanna. Oecologia 2003, 134, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.-C.; King, J.S.; Noormets, A.; Treasure, E.; Gavazzi, M.J.; Sun, G.; McNulty, S.G. Hydraulic Redistribution of Soil Water by Roots Affects Whole-Stand Evapotranspiration and Net Ecosystem Carbon Exchange. New Phytol. 2010, 187, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, K.V.R.; Prins, H.H.T.; de Bie, S.; Heitkönig, I.M.A.; Woodborne, S.; Gort, G.; Kirkman, K.; Ludwig, F.; Dawson, T.E.; de Kroon, H. Seasonality of Hydraulic Redistribution by Trees to Grasses and Changes in Their Water-Source Use That Change Tree-Grass Interactions. Ecohydrology 2016, 9, 218–228. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Egerton-Warburton, L.M.; Allen, M.F. Hydraulic Lift May Buffer Rhizosphere Hyphae against the Negative Effects of Severe Soil Drying in a California Oak Savanna. Soil Biol. Biochem. 2007, 39, 409–417. [Google Scholar] [CrossRef]
- Brooks, J.R.; Barnard, H.R.; Coulombe, R.; McDonnell, J.J. Ecohydrologic Separation of Water between Trees and Streams in a Mediterranean Climate. Nat. Geosci. 2010, 3, 100–104. [Google Scholar] [CrossRef]
- D’Odorico, P.; Caylor, K.; Okin, G.S.; Scanlon, T.M. On Soil Moisture–Vegetation Feedbacks and Their Possible Effects on the Dynamics of Dryland Ecosystems. J. Geophys. Res. 2007, 112, 1–10. [Google Scholar] [CrossRef]
- Ryel, R.J.; Caldwell, M.M.; Leffler, A.J.; Yoder, C.K. Rapid Soil Moisture Recharge to Depth by Roots in a Stand of Artemisia Tridentata. Ecology 2003, 84, 757–764. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Freudenberger, D. Ecosystem Wicks: Woodland Trees Enhance Water Infiltration in a Fragmented Agricultural Landscape in Eastern Australia. Austral Ecol. 2005, 30, 336–347. [Google Scholar] [CrossRef]
- Scott, R.L.; Cable, W.L.; Hultine, K.R. The Ecohydrologic Significance of Hydraulic Redistribution in a Semiarid Savanna. Water Resour. Res. 2008, 44, 1–12. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Warren, J.M.; Brooks, J.R. Species-Specific Partitioning of Soil Water Resources in an Old-Growth Douglas-Fir-Western Hemlock Forest. Tree Physiol. 2007, 27, 871–880. [Google Scholar] [CrossRef]
- Treydte, A.C.; Heitkönig, I.M.A.; Prins, H.H.T.; Ludwig, F. Trees Improve Grass Quality for Herbivores in African Savannas. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 197–205. [Google Scholar] [CrossRef]
- Ludwig, F.; De Kroon, H.; Prins, H.H.T. Impacts of Savanna Trees on Forage Quality for a Large African Herbivore. Oecologia 2008, 155, 487–496. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Prieto, I.; Pugnaire, F.I.; Ryel, R.J. Water Uptake and Redistribution during Drought in a Semiarid Shrub Species. Funct. Plant Biol. 2014, 41, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Egerton-Warburton, L.M.; Querejeta, J.I.; Allen, M.F. Efflux of Hydraulically Lifted Water from Mycorrhizal Fungal Hyphae during Imposed Drought. Plant Signal Behav. 2008, 3, 68–71. [Google Scholar] [CrossRef]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, Nitrogen Transformations, and Ecosystem Services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Egerton-Warburton, L.M.; Allen, M.F. Direct Nocturnal Water Transfer from Oaks to Their Mycorrhizal Symbionts during Severe Soil Drying. Oecologia 2003, 134, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Armas, C.; Kim, J.H.; Bleby, T.M.; Jackson, R.B. The Effect of Hydraulic Lift on Organic Matter Decomposition, Soil Nitrogen Cycling, and Nitrogen Acquisition by a Grass Species. Oecologia 2012, 168, 11–22. [Google Scholar] [CrossRef]
- Priyadarshini, K.V.R.; Prins, H.H.T.; de Bie, S.; Heitkönig, I.M.A.; Woodborne, S.; Gort, G.; Kirkman, K.; Fry, B.; de Kroon, H. Overlap in Nitrogen Sources and Redistribution of Nitrogen between Trees and Grasses in a Semi-Arid Savanna. Oecologia 2014, 174, 1107–1116. [Google Scholar] [CrossRef]
- Högberg, P. Soil Nutrient Availability, Root Symbioses and Tree Species Composition in Tropical Africa: A Review. J. Trop. Ecol. 1986, 2, 359–372. [Google Scholar] [CrossRef]
- Högberg, P. Root Symbioses of Trees in Savannas. In Mineral Nutrients in Tropical Forest and Savanna Ecosystems; Proctor, J., Ed.; Blackwell Science: Hoboken, NJ, USA, 1989; pp. 121–136. [Google Scholar]
- Chapman, S.K.; Langley, J.A.; Hart, S.C.; Koch, G.W. Plants Actively Control Nitrogen Cycling: Uncorking the Microbial Bottleneck. New Phytol. 2005, 169, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Willis, A.; Rodrigues, B.F.; Harris, P.J.C. The Ecology of Arbuscular Mycorrhizal Fungi. CRC Crit. Rev. Plant Sci. 2013, 32, 1–20. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal Ecology and Evolution: The Past, the Present, and the Future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Kuyper, T.W.; Cardoso, I.M.; Onguene, N.A.; Murniati, M.; Van Noordwijk, M. Managing Mycorrhiza in Tropical Multispecies Agroecosystems. In Belowground Interactions in Tropical Agroecosystems; Van Noordwijk, M., Cadisch, G., Ong, C.K., Eds.; CAB International: Oxfordshire, UK, 2004; pp. 243–261. [Google Scholar]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van der Putten, W.H.; Wall, D.H. Ecological Linkages Between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Finlay, R. Action and Interaction in the Mycorrhizal Hyphosphere—A Re-Evaluation of the Role of Mycorrhizas in Nutrient Acquisition and Plant Ecology. In Nutrient Acquisition by Plants; Bassirirad, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 221–276. ISBN 978-3-540-24186-7. [Google Scholar]
- Van der Heijden, M.G.A.; Horton, T.R. Socialism in Soil? The Importance of Mycorrhizal Fungal Networks for Facilitation in Natural Ecosystems. J. Ecol. 2009, 97, 1139–1150. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; Van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Buresh, R.J.; Mugendi, D.N.; Latt, C.R. Nutrient Cycling in Tropical Agroforestry Systems: Myths and Science. In Agroforestry in Sustainable Agricultural Systems; Buck, L.E., Lassoie, J.P., Fernandes, E.C.M., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 1999; pp. 15–45. [Google Scholar]
- Phillips, R.P.; Fahey, T.J. Tree Species and Mycorrhizal Associations Influence the Magnitude of Rhizosphere Effects. Ecology 2006, 87, 1302–1313. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial Nitrogen Acquisition by Arbuscular Mycorrhizal Fungi from Organic Material Has Implications for N Cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Wiemken, A.; Sanders, I.R. Different Arbuscular Mycorrhizal Fungi Alter Coexistence and Resource Distribution between Co-Occurring Plants. New Phytol. 2003, 158, 601. [Google Scholar] [CrossRef]
- Warren, J.M.; Brooks, J.R.; Meinzer, F.C.; Eberhart, J.L. Hydraulic Redistribution of Water from Pinus Ponderosa Trees to Seedlings: Evidence for an Ectomycorrhizal Pathway. New Phytol. 2008, 178, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Siegwolf, R.T.W.; Körner, C. Belowground Carbon Trade among Tall Trees in a Temperate Forest. Science 2016, 352, 342–344. [Google Scholar] [CrossRef]
- Coetsee, C.; February, E.C.; Bond, W.J. Nitrogen Availability Is Not Affected by Frequent Fire in a South African Savanna. J. Trop. Ecol. 2008, 24, 647–654. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Huygens, D.; Boeckx, P.; Kuyper, T.W.; Lubbers, I.M.; Rütting, T.; Groffman, P.M. The Soil N Cycle: New Insights and Key Challenges. Soil 2015, 1, 235–256. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen Mineralization: Challenges of a Changing Paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A Plant Perspective on Nitrogen Cycling in the Rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Belsky, A.J.; Mwonga, S.M.; Duxbury, J.M. Effects of Widely Spaced Trees and Livestock Grazing on Understory Environments in Tropical Savannas. Agrofor. Syst. 1993, 24, 1–20. [Google Scholar] [CrossRef]
- Ludwig, F.; de Kroon, H.; Berendse, F.; Prins, H.H.T. The Influence of Savanna Trees on Nutrient, Water and Light Availability and the Understorey Vegetation. Plant Ecol. 2004, 170, 93–105. [Google Scholar] [CrossRef]
- Augustine, D.J.; Veblen, K.E.; Goheen, J.R.; Riginos, C.; Young, T.P. Pathways for Positive Cattle–Wildlife Interactions in Semiarid Rangelands. Smithson. Contrib. Zool. 2011, 632, 55–71. [Google Scholar] [CrossRef]
- Tagliavini, M.; Millard, P. Fluxes of Nitrogen within Deciduous Fruit Trees. Acta Sci. Pol. Hortorum Cultus 2005, 4, 21–30. [Google Scholar]
- Millard, P.; Grelet, G.-A. Nitrogen Storage and Remobilization by Trees: Ecophysiological Relevance in a Changing World. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of Organic Nitrogen by Plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Muraoka, T.; Zech, W. Root Activity Patterns in an Amazonian Agroforest with Fruit Trees Determined by 32P, 33P and 15N Applications. Agrofor. Syst. 2001, 52, 185–197. [Google Scholar] [CrossRef]
- McCulley, R.L.; Jobbágy, E.G.; Pockman, W.T.; Jackson, R.B. Nutrient Uptake as a Contributing Explanation for Deep Rooting in Arid and Semi-Arid Ecosystems. Oecologia 2004, 141, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Tittonell, P.; Giller, K.E. When Yield Gaps Are Poverty Traps: The Paradigm of Ecological Intensification in African Smallholder Agriculture. Field Crops Res. 2013, 143, 76–90. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; Corbeels, M.; Tittonell, P. Conservation Agriculture and Smallholder Farming in Africa: The Heretics’ View. Field Crops Res. 2009, 114, 23–34. [Google Scholar] [CrossRef]
- Struik, P.C.; Kuyper, T.W. Sustainable Intensification in Agriculture: The Richer Shade of Green. A Review. Agron. Sustain. Dev. 2017, 37, 39. [Google Scholar] [CrossRef]
- Mwase, W.; Sefasi, A.; Njoloma, J.; Nyoka, B.I.; Manduwa, D.; Nyaika, J. Factors Affecting Adoption of Agroforestry and Evergreen Agriculture in Southern Africa. Environ. Nat. Resour. Res. 2015, 5, 148–157. [Google Scholar] [CrossRef]
- Kuyah, S.; Whitney, C.W.; Jonsson, M.; Sileshi, G.W.; Öborn, I.; Muthuri, C.W.; Luedeling, E. Agroforestry Delivers a Win-Win Solution for Ecosystem Services in Sub-Saharan Africa. A Meta-Analysis. Agron. Sustain. Dev. 2019, 39, 47. [Google Scholar] [CrossRef]
- Arnold, M.; Persson, R. Reassessing the Fuelwood Situation in Developing Countries. Int. For. Rev. 2003, 5, 379–383. [Google Scholar] [CrossRef]
- Raunikar, R.; Buongiorno, J.; Turner, J.A.; Zhu, S. Global Outlook for Wood and Forests with the Bioenergy Demand Implied by Scenarios of the Intergovernmental Panel on Climate Change. For. Policy Econ. 2010, 12, 48–56. [Google Scholar] [CrossRef]
- Jerneck, A.; Olsson, L. More than Trees! Understanding the Agroforestry Adoption Gap in Subsistence Agriculture: Insights from Narrative Walks in Kenya. J. Rural. Stud. 2013, 32, 114–125. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Giller, K.E. Popular Myths around Soil Fertility Management in Sub-Saharan Africa. Agric. Ecosyst. Environ. 2006, 116, 34–46. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Wendt, J.; Giller, K.E.; Corbeels, M.; Gerard, B.; Nolte, C. A Fourth Principle Is Required to Define Conservation Agriculture in Sub-Saharan Africa: The Appropriate Use of Fertilizer to Enhance Crop Productivity. Field Crops Res. 2014, 155, 10–13. [Google Scholar] [CrossRef]
- Mbow, C.; Smith, P.; Skole, D.; Duguma, L.; Bustamante, M. Achieving Mitigation and Adaptation to Climate Change through Sustainable Agroforestry Practices in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 8–14. [Google Scholar] [CrossRef]
- Sida, T.S.; Baudron, F.; Ndoli, A.; Tirfessa, D.; Giller, K.E. Should Fertilizer Recommendations Be Adapted to Parkland Agroforestry Systems? Case Studies from Ethiopia and Rwanda. Plant Soil. 2020, 453, 173–188. [Google Scholar] [CrossRef]
- Kuyah, S.; Öborn, I.; Jonsson, M.; Dahlin, A.S.; Barrios, E.; Muthuri, C.; Malmer, A.; Nyaga, J.; Magaju, C.; Namirembe, S.; et al. Trees in Agricultural Landscapes Enhance Provision of Ecosystem Services in Sub-Saharan Africa. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2016, 12, 255–273. [Google Scholar] [CrossRef]
- Bayala, J.; Sileshi, G.W.; Coe, R.; Kalinganire, A.; Tchoundjeu, Z.; Sinclair, F.; Garrity, D. Cereal Yield Response to Conservation Agriculture Practices in Drylands of West Africa: A Quantitative Synthesis. J. Arid. Environ. 2012, 78, 13–25. [Google Scholar] [CrossRef]
- Mbow, C.; Van Noordwijk, M.; Prabhu, R.; Simons, T. Knowledge Gaps and Research Needs Concerning Agroforestry’s Contribution to Sustainable Development Goals in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 162–170. [Google Scholar] [CrossRef]
- Mbow, C.; Van Noordwijk, M.; Luedeling, E.; Neufeldt, H.; Minang, P.A.; Kowero, G. Agroforestry Solutions to Address Food Security and Climate Change Challenges in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 61–67. [Google Scholar] [CrossRef]
- Chapin, F.S. Ecological Aspects of Plant Nutrition. Adv. Miner. Nutr. 1988, 3, 161–191. [Google Scholar]
- Brooker, R.W. Plant—Plant Interactions and Environmental Change. New Phytol. 2006, 171, 271–284. [Google Scholar] [CrossRef]
- Dybzinski, R.; Tilman, D. Resource Use Patterns Predict Long-Term Outcomes of Plant Competition for Nutrients and Light. Am. Nat. 2007, 170, 305–318. [Google Scholar] [CrossRef]
- Van der Putten, W.H. A Multitrophic Perspective on Functioning and Evolution of Facilitation in Plant Communities. J. Ecol. 2009, 97, 1131–1138. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving Intercropping: A Synthesis of Research in Agronomy, Plant Physiology and Ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lekberg, Y.; Bever, J.D.; Bunn, R.A.; Callaway, R.M.; Hart, M.M.; Kivlin, S.N.; Klironomos, J.; Larkin, B.G.; Maron, J.L.; Reinhart, K.O.; et al. Relative Importance of Competition and Plant–Soil Feedback, Their Synergy, Context Dependency and Implications for Coexistence. Ecol. Lett. 2018, 21, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Liniger, H.; Studer, R.M.; Hauert, C.; Gurtner, M. Sustainable Land Management in Practice: Guidelines and Best Management Practices for Sub-Saharan Africa. 2011. Available online: https://www.fao.org/4/i1861e/i1861e00.htm (accessed on 1 May 2025).
- Barrett, C.B.; Place, F.; Aboud, A.; Brown, D.R. The Challenge of Stimulating Adoption of Improved Natural Resource Management Practices in African Agriculture. In Natural Resources Management in African Agriculture; Barrett, C.B., Place, F., Aboud, A.A., Eds.; CAB International: Oxfordshire, UK, 2002; pp. 1–21. [Google Scholar]
- Rass, N. Policies and Strategies to Address the Vulnerability of Pastoralists in Sub-Saharan Africa; Pro-poor Livestock Policy Initiative (PPLPI) Working Paper Series 37; FAO: Rome, Italy, 2006. [Google Scholar]
- De Leeuw, J.; Njenga, M.; Wagner, B.; Iiyama, M.; Liyama, M. Treesilience: An Assessment of the Resilience Provided by Trees in the Drylands of Eastern Africa; De Leeuw, J., Njenga, M., Wagner, B., Liyama, M., Eds.; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2014; ISBN 978-92-9059-352-2. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well Being: Desertification Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- UNFCC. Climate Change in the African Drylands: Options and Opportunities for Adaptation and Mitigation; UNFCC: Bonn, Germany, 2009. [Google Scholar]
- The Montpellier Panel. No Ordinary Matter: Conserving, Restoring and Enhancing Africa’s Soils; The Montpellier Panel: London, UK, 2014. [Google Scholar]
- The Montpellier Panel the Farms of Change: African Small Holders Responding to an Uncertain Climate Future; The Montpellier Panel: London, UK, 2015.
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural Expansion and Its Impacts on Tropical Nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Pfeifer, M.; Platts, P.J.; Burgess, N.D.; Swetnam, R.D.; Willcock, S.; Lewis, S.L.; Marchant, R. Land Use Change and Carbon Fluxes in East Africa Quantified Using Earth Observation Data and Field Measurements. Environ. Conserv. 2013, 40, 241–252. [Google Scholar] [CrossRef]
- Tully, K.; Sullivan, C.; Weil, R.; Sanchez, P. The State of Soil Degradation in Sub-Saharan Africa: Baselines, Trajectories, and Solutions. Sustainability 2015, 7, 6523–6552. [Google Scholar] [CrossRef]
- Tappan, G.; McGahuey, M. Tracking Environmental Dynamics and Agricultural Intensification in Southern Mali. Agric. Syst. 2007, 94, 38–51. [Google Scholar] [CrossRef]
- Binswanger-Mkhize, H.P.; Savastano, S. Agricultural Intensification: The Status in Six African Countries; World Bank Policy Paper 7116; World Bank: Washington, DC, USA, 2014. [Google Scholar]
- Luoga, E.J.; Witkowski, E.T.F.; Balkwill, K. Land Cover and Use Changes in Relation to the Institutional Framework and Tenure of Land and Resources in Eastern Tanzania Miombo Woodlands. Environ. Dev. Sustain. 2005, 7, 71–93. [Google Scholar] [CrossRef]
- Jew, E.K.K.; Dougill, A.J.; Sallu, S.M.; O’Connell, J.; Benton, T.G. Miombo Woodland under Threat: Consequences for Tree Diversity and Carbon Storage. For. Ecol. Manag. 2016, 361, 144–153. [Google Scholar] [CrossRef]
- Zomer, R.J.; Trabucco, A.; Coe, R.; Place, F.; Van Noordwijk, M.; Xu, J.C. Trees on Farms: An Update and Reanalysis of Agroforestry’s Global Extent and Socio-Ecological Characteristics; World Agroforestry Centre: Bogor, Indonesia, 2014. [Google Scholar]
- Bai, Z.G.; Dent, D.L.; Olsson, L.; Schaepman, M.E. Global Assessment of Land Degradation and Improvement: 1. Identification by Remote Sensing; ISRIC: Wageningen, The Netherlands, 2008. [Google Scholar]
- Bai, Z.G.; de Jong, R.; Van Lynden, G.W.J. An Update of GLADA—Global Assessment of Land Degradation and Improvement; ISRIC: Wageningen, The Netherlands, 2010. [Google Scholar]
- Zomer, R.J.; Trabucco, A.; Coe, R.; Place, F. Trees on Farm: Analysis of Global Extent and Geographical Patterns of Agroforestry; World Agroforestry Centre: Nairobi, Kenya, 2009. [Google Scholar]
- Wessels, K.J.; Mathieu, R.; Erasmus, B.F.N.; Asner, G.P.; Smit, I.P.J.; van Aardt, J.A.N.; Main, R.; Fisher, J.; Marais, W.; Kennedy-Bowdoin, T.; et al. Impact of Communal Land Use and Conservation on Woody Vegetation Structure in the Lowveld Savannas of South Africa. For. Ecol. Manag. 2011, 261, 19–29. [Google Scholar] [CrossRef]
- Wessels, K.J.; Colgan, M.S.; Erasmus, B.F.N.N.; Asner, G.P.; Twine, W.C.; Mathieu, R.; Van Aardt, J.A.N.N.; Fisher, J.T.; Smit, I.P.J.J. Unsustainable Fuelwood Extraction from South African Savannas. Environ. Res. Lett. 2013, 8, 014007. [Google Scholar] [CrossRef]
- Matsika, R.; Erasmus, B.F.N.; Twine, W.C. A Tale of Two Villages: Assessing the Dynamics of Fuelwood Supply in Communal Landscapes in South Africa. Environ. Conserv. 2012, 40, 1–13. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2016; International Energy Agency: Paris, France, 2016. [Google Scholar]
- Santos, M.J.; Dekker, S.C.; Daioglou, V.; Braakhekke, M.C.; van Vuuren, D.P. Modeling the Effects of Future Growing Demand for Charcoal in the Tropics. Front. Environ. Sci. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Droppelmann, K.J.; Lehmann, J.; Ephrath, J.E.; Berliner, P.R. Water Use Efficiency and Uptake Patterns in a Runoff Agroforestry System in an Arid Environment. Agrofor. Syst. 2000, 49, 223–243. [Google Scholar] [CrossRef]
- Ong, C.K.; Black, C.R.; Muthuri, C.W. Modifying Forestry and Agroforestry to Increase Water Productivity in the Semi-Arid Tropics. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006, 1, 1–20. [Google Scholar] [CrossRef]
- Ong, C.K.; Anyango, S.; Muthuri, C.W.; Black, C.R. Water Use and Water Productivity of Agroforestry Systems in Semi-Arid Tropics. Ann. Arid. Zone 2007, 46, 255–284. [Google Scholar]
- Dile, Y.T.; Karlberg, L.; Temesgen, M.; Rockström, J. The Role of Water Harvesting to Achieve Sustainable Agricultural Intensification and Resilience against Water Related Shocks in Sub-Saharan Africa. Agric. Ecosyst. Environ. 2013, 181, 69–79. [Google Scholar] [CrossRef]
- Place, F.; Barrett, C.B.; Freeman, H.A.; Ramisch, J.J.; Vanlauwe, B. Prospects for Integrated Soil Fertility Management Using Organic and Inorganic Inputs: Evidence from Smallholder African Agricultural Systems. Food Policy 2003, 28, 365–378. [Google Scholar] [CrossRef]
- Rufino, M.C.; Dury, J.; Tittonell, P.; van Wijk, M.T.; Herrero, M.; Zingore, S.; Mapfumo, P.; Giller, K.E. Competing Use of Organic Resources, Village-Level Interactions between Farm Types and Climate Variability in a Communal Area of NE Zimbabwe. Agric. Syst. 2011, 104, 175–190. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Descheemaeker, K.; Giller, K.E.; Huising, J.; Merckx, R.; Nziguheba, G.; Wendt, J.; Zingore, S. Integrated Soil Fertility Management in Sub-Saharan Africa: Unravelling Local Adaptation. Soil 2015, 1, 491–508. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Sanginga, N. Impact of the Tree Component on N Cycling in Agroforestry Systems under Subhumid Tropical Conditions. West. Afr. J. Appl. Ecol. 2004, 6, 75–84. [Google Scholar]
- Barrios, E.; Sileshi, G.W.; Shepherd, K.; Sinclair, F. Agroforestry and Soil Health: Linking Trees, Soil Biota, and Ecosystem Services. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Behan-Pelletier, V., Herrick, J.E., Hefin Jones, T., Ritz, K., Six, J., Strong, D.R., van der Putten, W.H., Eds.; Oxford University Press: Oxford, UK, 2012; Volume 15, pp. 583–605. ISBN 9780199682676. [Google Scholar]
- Bazié, H.R.; Bayala, J.; Zombré, G.; Sanou, J.; Ilstedt, U. Separating Competition-Related Factors Limiting Crop Performance in an Agroforestry Parkland System in Burkina Faso. Agrofor. Syst. 2012, 84, 377–388. [Google Scholar] [CrossRef]
- Sanou, J.; Bayala, J.; Teklehaimanot, Z.; Bazié, P. Effect of Shading by Baobab (Adansonia Digitata) and Néré (Parkia Biglobosa) on Yields of Millet (Pennisetum Glaucum) and Taro (Colocasia Esculenta) in Parkland Systems in Burkina Faso, West Africa. Agrofor. Syst. 2012, 85, 431–441. [Google Scholar] [CrossRef]
- Schroth, G.; Lehmann, J. Contrasting Effects of Roots and Mulch from Three Agroforestry Tree Species on Yields of Alley Cropped Maize. Agric. Ecosyst. Environ. 1995, 54, 89–101. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Kihara, J.; Chivenge, P.; Pypers, P.; Coe, R.; Six, J. Agronomic Use Efficiency of N Fertilizer in Maize-Based Systems in Sub-Saharan Africa within the Context of Integrated Soil Fertility Management. Plant Soil. 2011, 339, 35–50. [Google Scholar] [CrossRef]
- Droppelmann, K.J.; Ephrath, J.E.; Berliner, P.R. Tree/Crop Complementarity in an Arid Zone Runoff Agroforestry System in Northern Kenya. Agrofor. Syst. 2000, 50, 1–16. [Google Scholar] [CrossRef]
- Lehmann, J.; Gebauer, G.; Zech, W. Nitrogen Cycling Assessment in a Hedgerow Intercropping System Using 15N Enrichment. Nutr. Cycl. Agroecosyst. 2002, 62, 1–9. [Google Scholar] [CrossRef]
- Ong, C.K.; Wilson, J.; Deans, J.D.; Mulayta, J.; Raussen, T.; Wajja-Musukwe, N. Tree–Crop Interactions: Manipulation of Water Use and Root Function. Agric. Water Manag. 2002, 53, 171–186. [Google Scholar] [CrossRef]
- Ilstedt, U.; Bargués Tobella, A.; Bazié, H.R.; Bayala, J.; Verbeeten, E.; Nyberg, G.; Sanou, J.; Benegas, L.; Murdiyarso, D.; Laudon, H.; et al. Intermediate Tree Cover Can Maximize Groundwater Recharge in the Seasonally Dry Tropics. Sci. Rep. 2016, 6, 21930. [Google Scholar] [CrossRef]
- Bayala, J.; Heng, L.K.; Van Noordwijk, M.; Ouedraogo, S.J. Hydraulic Redistribution Study in Two Native Tree Species of Agroforestry Parklands of West African Dry Savanna. Acta Oecol. 2008, 34, 370–378. [Google Scholar] [CrossRef]
- Sanchez, P.A. Science in Agroforestry. Agrofor. Syst. 1995, 30, 5–55. [Google Scholar] [CrossRef]
- Kwesiga, F.R.; Franzel, S.; Place, F.; Phiri, D.; Simwanza, C.P. Sesbania Sesban Improved Fallows in Eastern Zambia: Their Inception, Development and Farmer Enthusiasm. Agrofor. Syst. 1999, 47, 49–66. [Google Scholar] [CrossRef]
- Valbuena, D.; Erenstein, O.; Homann-Kee Tui, S.; Abdoulaye, T.; Claessens, L.; Duncan, A.J.; Gérard, B.; Rufino, M.C.; Teufel, N.; Van Rooyen, A.; et al. Conservation Agriculture in Mixed Crop-Livestock Systems: Scoping Crop Residue Trade-Offs in Sub-Saharan Africa and South Asia. Field Crops Res. 2012, 132, 175–184. [Google Scholar] [CrossRef]
- Brussaard, L. Biodiversity and Ecosystem Functioning in Soil: The Dark Side of Nature and the Bright Side of Life. Ambio 2021, 50, 1286–1288. [Google Scholar] [CrossRef]
- Chikowo, R.; Corbeels, M.; Mapfumo, P.; Tittonell, P.; Vanlauwe, B.; Giller, K.E. Nitrogen and Phosphorus Capture and Recovery Efficiencies, and Crop Responses to a Range of Soil Fertility Management Strategies in Sub-Saharan Africa. Nutr. Cycl. Agroecosyst. 2010, 88, 59–77. [Google Scholar] [CrossRef]
- Gaiser, T.; Judex, M.; Igué, A.M.; Paeth, H.; Hiepe, C. Future Productivity of Fallow Systems in Sub-Saharan Africa: Is the Effect of Demographic Pressure and Fallow Reduction More Significant than Climate Change? Agric. For. Meteorol. 2011, 151, 1120–1130. [Google Scholar] [CrossRef]
- Bayala, J.; Sanou, J.; Teklehaimanot, Z.; Ouedraogo, S.J.; Kalinganire, A.; Coe, R.; van Noordwijk, M. Advances in Knowledge of Processes in Soil-Tree-Crop Interactions in Parkland Systems in the West African Sahel: A Review. Agric. Ecosyst. Environ. 2015, 205, 25–35. [Google Scholar] [CrossRef]
- Gathumbi, S.M.; Cadisch, G.; Buresh, R.J.; Giller, K.E. Subsoil Nitrogen Capture in Mixed Legume Stands as Assessed by Deep Nitrogen-15 Placement. Soil. Sci. Soc. Am. J. 2003, 67, 573–582. [Google Scholar]
- de Carvalho, A.M.X.; de Castro Tavares, R.; Cardoso, I.M.; Kuyper, T.W. Mycorrhizal Associations in Agroforestry Systems. In Soil Biology and Agriculture in the Tropics; Dion, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 21, pp. 185–208. ISBN 978-3-642-05075-6. [Google Scholar]
- Isaac, M.E.; Borden, K.A. Nutrient Acquisition Strategies in Agroforestry Systems. Plant Soil. 2019, 444, 1–19. [Google Scholar] [CrossRef]
- Bender, S.F.; Van der Heijden, M.G.A. Soil Biota Enhance Agricultural Sustainability by Improving Crop Yield, Nutrient Uptake and Reducing Nitrogen Leaching Losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef]
- Haystead, A.; Malajczuk, N.; Grove, T.S. Underground Transfer of Nitrogen between Pasture Plants Infected with Vesicular-arbuscular Mycorrhizal Fungi. New Phytol. 1988, 108, 417–423. [Google Scholar] [CrossRef]
- He, X.; Xu, M.; Qiu, G.Y.; Zhou, J. Use of 15N Stable Isotope to Quantify Nitrogen Transfer between Mycorrhizal Plants. J. Plant Ecol. 2009, 2, 107–118. [Google Scholar] [CrossRef]
- Cardoso, I.M. Phosphorus in Agroforestry Systems: A Contribution to Sustainable Agriculture in the Zona Da Mata of Mina Gerais, Brazil; Wageningen University: Wageningen, The Netherlands, 2002. [Google Scholar]
- Cardoso, I.M.; Kuyper, T.W. Mycorrhizas and Tropical Soil Fertility. Agric. Ecosyst. Environ. 2006, 116, 72–84. [Google Scholar] [CrossRef]
- Hailemariam, M.; Birhane, E.; Asfaw, Z.; Zewdie, S. Arbuscular Mycorrhizal Association of Indigenous Agroforestry Tree Species and Their Infective Potential with Maize in the Rift Valley, Ethiopia. Agrofor. Syst. 2013, 87, 1261–1272. [Google Scholar] [CrossRef]
- Cramer, M.D.; Chimphango, S.B.M.; Van Cauter, A.; Waldram, M.S.; Bond, W.J. Grass Competition Induces N2 Fixation in Some Species of African Acacia. J. Ecol. 2007, 95, 1123–1133. [Google Scholar] [CrossRef]
- Sprent, J.I. Nodulation in Legumes; Royal Botanic Gardens: Kew, UK, 2001. [Google Scholar]
- Bogie, N.A.; Bayala, R.; Diedhiou, I.; Conklin, M.H.; Fogel, M.L.; Dick, R.P.; Ghezzehei, T.A. Hydraulic Redistribution by Native Sahelian Shrubs: Bioirrigation to Resist in-Season Drought. Front. Environ. Sci. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Belsky, A.J.; Amundson, R.G.; Duxbury, J.M.; Riha, S.J.; Ali, A.R.; Mwonga, S.M. The Effects of Trees on Their Physical, Chemical and Biological Environments in a Semi-Arid Savanna in Kenya. J. Appl. Ecol. 1989, 26, 1005–1024. [Google Scholar] [CrossRef]
- Belsky, A.J. Influences of Trees on Savanna Productivity: Tests of Shade, Nutrients and Tree-Grass Competition. Ecology 1994, 75, 922–932. [Google Scholar] [CrossRef]
- Ludwig, F.; De Kroon, H.; Prins, H.H.T.; Berendse, F. Effects of Nutrients and Shade on Tree-Grass Interactions in an East African Savanna. J. Veg. Sci. 2001, 12, 579–588. [Google Scholar] [CrossRef]
- De Leeuw, J.; Njenga, M.; Jamnadass, R. Benefits from the Ecosystem Services Provided by Trees. In Treesilience: An Assessment of the Resilience Provided by Trees in the Drylands of Eastern Africa; De Leeuw, J., Njenga, M., Wagener, B., Liyama, M., Eds.; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2014; pp. 35–99. ISBN 978-92-9059-352-2. [Google Scholar]
- Dollinger, J.; Jose, S. Agroforestry for Soil Health. Agrofor. Syst. 2018, 92, 213–219. [Google Scholar] [CrossRef]
- Bayala, J.; Prieto, I. Water Acquisition, Sharing and Redistribution by Roots: Applications to Agroforestry Systems. Plant Soil. 2020, 453, 17–28. [Google Scholar] [CrossRef]
- Stephen, E.A.; Evans, K.D.; Akwasi, A.A. Effects of Faidherbia Albida on Some Important Soil Fertility Indicators on Agroforestry Parklands in the Semi-Arid Zone of Ghana. Afr. J. Agric. Res. 2020, 15, 256–268. [Google Scholar] [CrossRef][Green Version]
- Yengwe, J.; Gebremikael, M.T.; Buchan, D.; Lungu, O.; De Neve, S. Effects of Faidherbia Albida Canopy and Leaf Litter on Soil Microbial Communities and Nitrogen Mineralization in Selected Zambian Soils. Agrofor. Syst. 2018, 92, 349–363. [Google Scholar] [CrossRef]
- Kaur, K.; Jalota, R.K.; Midmore, D.J.; Rolfe, J. Pasture Production in Cleared and Uncleared Grazing Systems of Central Queensland, Australia. Rangel. J. 2005, 27, 143–149. [Google Scholar] [CrossRef][Green Version]
- Jackson, L.E.; Pulleman, M.M.; Brussaard, L.; Bawa, K.S.; Brown, G.G.; Cardoso, I.M.; de Ruiter, P.C.; García-Barrios, L.; Hollander, A.D.; Lavelle, P.; et al. Social-Ecological and Regional Adaptation of Agrobiodiversity Management across a Global Set of Research Regions. Glob. Environ. Change 2012, 22, 623–639. [Google Scholar] [CrossRef]
- Bayala, J.; Balesdent, J.; Marol, C.; Zapata, F.; Teklehaimanot, Z.; Ouedraogo, S.J. Relative Contribution of Trees and Crops to Soil Carbon Content in a Parkland System in Burkina Faso Using Variations in Natural 13C Abundance. Nutr. Cycl. Agroecosyst. 2006, 76, 193–201. [Google Scholar] [CrossRef]
- Bayala, J.; Sanou, J.; Bazié, H.R.; Coe, R.; Kalinganire, A.; Sinclair, F.L. Regenerated Trees in Farmers’ Fields Increase Soil Carbon across the Sahel. Agrofor. Syst. 2020, 94, 401–415. [Google Scholar] [CrossRef]
- Gebrewahid, Y.; Teka, K.; Gebre-Egziabhier, T.-B.; Tewolde-Berhan, S.; Birhane, E.; Eyasu, G.; Meresa, E. Dispersed Trees on Smallholder Farms Enhance Soil Fertility in Semi-Arid Ethiopia. Ecol. Process 2019, 8, 38. [Google Scholar] [CrossRef]
- Zomer, R.J.; Neufeldt, H.; Xu, J.; Ahrends, A.; Bossio, D.; Trabucco, A.; Van Noordwijk, M.; Wang, M. Global Tree Cover and Biomass Carbon on Agricultural Land: The Contribution of Agroforestry to Global and National Carbon Budgets. Sci. Rep. 2016, 6, 29987. [Google Scholar] [CrossRef]
- Tian, F.; Brandt, M.; Liu, Y.Y.; Rasmussen, K.; Fensholt, R. Mapping Gains and Losses in Woody Vegetation across Global Tropical Drylands. Glob. Change Biol. 2017, 23, 1748–1760. [Google Scholar] [CrossRef]
- Glover, E.K.; Ahmed, H.B.; Glover, M.K. Analysis of Socio-Economic Conditions Influencing Adoption of Agroforestry Practices. Int. J. Agric. For. 2013, 3, 178–184. [Google Scholar] [CrossRef]
- Bjornlund, V.; Bjornlund, H.; Van Rooyen, A.F. Why Agricultural Production in Sub-Saharan Africa Remains Low Compared to the Rest of the World–a Historical Perspective. Int. J. Water Resour. Dev. 2020, 36, 1–34. [Google Scholar] [CrossRef]
- Ndlovu, N.P.; Borrass, L. Promises and Potentials Do Not Grow Trees and Crops. A Review of Institutional and Policy Research in Agroforestry for the Southern African Region. Land. Use Policy 2021, 103, 105298. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated Dryland Expansion under Climate Change. Nat. Clim. Change 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Herrero, M.; Thornton, P.K.; Gerber, P.; Van der Zipp, A.; Van de Steeg, J.; Notenbaert, A.M.; Lecomte, P.; Tarawali, S.; Grace, D. The Way Forward for Livestock and Environment. In The Role of Livestock in Developing Communities: Enhancing Multifunctionality; Swanepoel, F., Stroebel, A., Moyo, S., Eds.; The Technical Centre for Agricultural and Rural Cooperation (CTA), University of Free State (South Africa), and International Livestock Research Institute: Bloemfontein, South Africa, 2010; pp. 51–76. ISBN 978-0-86886-798-4. [Google Scholar]
- Maestre, F.T.; Salguero-Gomez, R.; Quero, J.L. It Is Getting Hotter in Here: Determining and Projecting the Impacts of Global Environmental Change on Drylands. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3062–3075. [Google Scholar] [CrossRef]
- Thornton, P.K.; Herrero, M. Climate Change Adaptation in Mixed Crop-Livestock Systems in Developing Countries. Glob. Food Sec. 2014, 3, 99–107. [Google Scholar] [CrossRef]
- Herrero, M.; Thornton, P.K.; Notenbaert, A.; Msangi, S.; Wood, S.; Kruska, R.; Dixon, J.; Bossio, D.; van de Steeg, J.; Freeman, H.A.; et al. Drivers of Change in Crop-Livestock Systems and Their Potential Impacts on Agro-Ecosystems Services and Human Wellbeing to 2030: A Study Commissioned by the CGIAR Systemwide Livestock Programme; ILRI: Nairobi, Kenya, 2012. [Google Scholar]
- Lasco, R.D.; Delfino, R.J.P.; Catacutan, D.C.; Simelton, E.S.; Wilson, D.M. Climate Risk Adaptation by Smallholder Farmers: The Roles of Trees and Agroforestry. Curr. Opin. Environ. Sustain. 2014, 6, 83–88. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Iglesius, A.; Yang, X.B.; Epstein, P.R.; Chivian, E. Climate Change and Extreme Weather Events—Implications for Food Production, Plant Diseases, and Pests. Glob. Change Hum. Health 2001, 2, 90–104. [Google Scholar] [CrossRef]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging Infectious Diseases of Plants: Pathogen Pollution, Climate Change and Agrotechnology Drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate Change Effects on Plant Disease: Genomes to Ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef]
- Craufurd, P.Q.; Wheeler, T.R. Climate Change and the Flowering Time of Annual Crops. J. Exp. Bot. 2009, 60, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Rockström, J.; Karlberg, L.; Wani, S.P.; Barron, J.; Hatibu, N.; Oweis, T.; Bruggeman, A.; Farahani, J.; Qiang, Z. Managing Water in Rainfed Agriculture-The Need for a Paradigm Shift. Agric. Water Manag. 2010, 97, 543–550. [Google Scholar] [CrossRef]
- Rosa, L.; Chiarelli, D.D.; Rulli, M.C.; Dell’Angelo, J.; D’Odorico, P. Global Agricultural Economic Water Scarcity. Sci. Adv. 2020, 6, aaz6031. [Google Scholar] [CrossRef] [PubMed]
- Kandji, S.T.; Verchot, L.; Mackensen, J.; Palm, C. Opportunities for linking climate change adaptation and mitigation through agroforestry systems. In World Agroforestry into the Future; Garrity, D., Okono, A., Grayson, M., Parrott, S., Eds.; World Agroforestry Centre: Nairobi, Kenya, 2006; Volume 92, pp. 113–121. Available online: https://www.cifor-icraf.org/publications/downloads/Publications/PDFS/BC96091.pdf (accessed on 1 May 2025).
- Garrity, D.P.; Akinnifesi, F.K.; Ajayi, O.C.; Weldesemayat, S.G.; Mowo, J.G.; Kalinganire, A.; Larwanou, M.; Bayala, J. Evergreen Agriculture: A Robust Approach to Sustainable Food Security in Africa. Food Secur. 2010, 2, 197–214. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for Ecosystem Services and Environmental Benefits: An Overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential Responses to Changes in Growth Temperature between Trees from Different Functional Groups and Biomes: A Review and Synthesis of Data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Jevon, F.V.; Gewirtzman, J.; Lang, A.K.; Ayres, M.P.; Matthes, J.H. Tree Species Effects on Soil CO2 and CH4 Fluxes in a Mixed Temperate Forest. Ecosystems 2023, 26, 1587–1602. [Google Scholar] [CrossRef]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E. Soil Fertility Limits Carbon Sequestration by Forest Ecosystems in a CO2- Enriched Atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [PubMed]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef]
- Sigurdsson, B.D.; Medhurst, J.L.; Wallin, G.; Eggertsson, O.; Linder, S. Growth of Mature Boreal Norway Spruce Was Not Affected by Elevated [CO 2] and/or Air Temperature Unless Nutrient Availability Was Improved. Tree Physiol. 2013, 33, 1192–1205. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Gessler, A. Global Climate Change and Tree Nutrition: Influence of Water Availability. Tree Physiol. 2010, 30, 1221–1234. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What Have We Learned from 15 Years of Free-Air CO2 Enrichment (FACE)? A Meta-Analytic Review of the Responses of Photosynthesis, Canopy Properties and Plant Production to Rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, C.R.; Hanan, N.P. Patterns in woody vegetation structure across African savannas. Biogeosciences 2017, 14, 3239–3252. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising Atmospheric Carbon Dioxide: Plants FACE the Future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Kgope, B.S.; Bond, W.J.; Midgley, G.F. Growth Responses of African Savanna Trees Implicate Atmospheric [CO2] as a Driver of Past and Current Changes in Savanna Tree Cover. Austral Ecol. 2010, 35, 451–463. [Google Scholar] [CrossRef]
- Maranz, S. Tree Mortality in the African Sahel Indicates an Anthropogenic Ecosystem Displaced by Climate Change. J. Biogeogr. 2009, 36, 1181–1193. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global Field Observations of Tree Die-off Reveal Hotter-Drought Fingerprint for Earth’s Forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef]
- Shantz, A.A.; Lemoine, N.P.; Burkepile, D.E. Nutrient Loading Alters the Performance of Key Nutrient Exchange Mutualisms. Ecol. Lett. 2016, 19, 20–28. [Google Scholar] [CrossRef]
- Tang, B.; Man, J.; Lehmann, A.; Rillig, M.C. Arbuscular Mycorrhizal Fungi Benefit Plants in Response to Major Global Change Factors. Ecol. Lett. 2023, 26, 2087–2097. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource Partitioning in Ecological Communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Van Noordwijk, M.; Ong, C.K. The Central Agroforestry Hypothesis: The Trees Must Acquire Resources That the Crop Would Not Otherwise Acquire. Agrofor. Syst. 1996, 34, 27–31. [Google Scholar] [CrossRef]
- Sanchez, P.A.; Buresh, R.J.; Leakey, R.R.B. Trees, Soils, and Food Security. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 949–961. [Google Scholar] [CrossRef]
- Ong, C.K.; Leakey, R.R.B. Why Tree-Crop Interactions in Agroforestry Appear at Odds with Tree-Grass Interactions in Tropical Savannahs. Agrofor. Syst. 1999, 45, 109–129. [Google Scholar]
- Van Noordwijk, M.; Ong, C.K. Can the Ecosystem Mimic Hypotheses Be Applied to Farms in African Savannahs? Agrofor. Syst. 1999, 45, 131–158. [Google Scholar] [CrossRef]
- Lehmann, J. Subsoil Root Activity in Tree-Based Cropping Systems. Plant Soil. 2003, 255, 319–331. [Google Scholar] [CrossRef]
- Callaway, R.M.; Pennings, S.C.; Richards, C.L. Phenotypic Plasticity and Interactions among Plants. Ecology 2003, 84, 1115–1128. [Google Scholar] [CrossRef]
- Hipondoka, M.H.T.; Versfeld, W.D. Root System of Terminalia Sericea Shrubs across Rainfall Gradient in a Semi-Arid Environment of Etosha National Park, Namibia. Ecol. Indic. 2006, 6, 516–524. [Google Scholar] [CrossRef]
- Gning, F.; Jourdan, C.; Marone, D.; Ngom, D.; Ræbild, A. Root Growth and Biomass Partitioning of Nine Juvenile Sahelian Agroforestry Tree Species under Drought and Irrigation Treatments. Plant Soil. 2025, 512, 1509–1527. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H. Root Niche Partitioning among Grasses, Saplings, and Trees Measured Using a Tracer Technique. Oecologia 2013, 171, 25–37. [Google Scholar] [CrossRef]
- Brooks, J.R.; Meinzer, F.C.; Coulombe, R.; Gregg, J. Hydraulic Redistribution of Soil Water during Summer Drought in Two Contrasting Pacific Northwest Coniferous Forests. Tree Physiol. 2002, 22, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C. Hydraulic Redistribution of Soil Water by Neotropical Savanna Trees. Tree Physiol. 2002, 22, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.Z.; Scholz, F.G.; Bucci, S.J.; Sternberg, L.S.; Goldstein, G.; Meinzer, F.C.; Franco, A.C. Hydraulic Lift in a Neotropical Savanna. Funct. Ecol. 2003, 17, 573–581. [Google Scholar] [CrossRef]
- Domec, J.C.; Scholz, F.G.; Bucci, S.J.; Meinzer, F.C.; Goldstein, G.; Villalobos-Vega, R. Diurnal and Seasonal Variation in Root Xylem Embolism in Neotropical Savanna Woody Species: Impact on Stomatal Control of Plant Water Status. Plant Cell Environ. 2006, 29, 26–35. [Google Scholar] [CrossRef]
- Brooksbank, K.; Veneklaas, E.J.; White, D.A.; Carter, J.L. Water Availability Determines Hydrological Impact of Tree Belts in Dryland Cropping Systems. Agric. Water Manag. 2011, 100, 76–83. [Google Scholar] [CrossRef]
- Brooksbank, K.; Veneklaas, E.J.; White, D.A.; Carter, J.L. The Fate of Hydraulically Redistributed Water in a Semi-Arid Zone Eucalyptus Species. Tree Physiol. 2011, 31, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, R.L.E.; Ehleringer, J.R. Water and Nitrogen Uptake Patterns Following Moisture Pulses in a Cold Desert Community. Ecology 2000, 81, 1415–1424. [Google Scholar] [CrossRef]
- Glass, A.D. Homeostatic Processes for the Optimization of Nutrient Absorption: Physiology and Molecular Biology. In Nutrient Acquisition by Plants; Bassirirad, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 181, pp. 117–145. [Google Scholar]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Mariotte, P.; Mehrabi, Z.; Bezemer, T.M.; De Deyn, G.B.; Kulmatiski, A.; Drigo, B.; Veen, G.F.; van der Heijden, M.G.A.; Kardol, P. Plant-Soil Feedback: Bridging Natural and Agricultural Sciences. Trends Ecol. Evol. 2017, 33, 129–142. [Google Scholar] [CrossRef]
- Giller, K.E.; Tittonell, P.; Rufino, M.C.; van Wijk, M.T.; Zingore, S.; Mapfumo, P.; Adjei-Nsiah, S.; Herrero, M.; Chikowo, R.; Corbeels, M.; et al. Communicating Complexity: Integrated Assessment of Trade-Offs Concerning Soil Fertility Management within African Farming Systems to Support Innovation and Development. Agric. Syst. 2011, 104, 191–203. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priyadarshini, K.V.R.; Prins, H.H.T.; de Bie, S. The Ecophysiological Role of Trees in Dryland Agroecosystems: Implications for Natural Resource Conservation and Sustainable Food Production in Sub-Saharan Africa. Diversity 2025, 17, 662. https://doi.org/10.3390/d17090662
Priyadarshini KVR, Prins HHT, de Bie S. The Ecophysiological Role of Trees in Dryland Agroecosystems: Implications for Natural Resource Conservation and Sustainable Food Production in Sub-Saharan Africa. Diversity. 2025; 17(9):662. https://doi.org/10.3390/d17090662
Chicago/Turabian StylePriyadarshini, K. V. R., Herbert H. T. Prins, and Steven de Bie. 2025. "The Ecophysiological Role of Trees in Dryland Agroecosystems: Implications for Natural Resource Conservation and Sustainable Food Production in Sub-Saharan Africa" Diversity 17, no. 9: 662. https://doi.org/10.3390/d17090662
APA StylePriyadarshini, K. V. R., Prins, H. H. T., & de Bie, S. (2025). The Ecophysiological Role of Trees in Dryland Agroecosystems: Implications for Natural Resource Conservation and Sustainable Food Production in Sub-Saharan Africa. Diversity, 17(9), 662. https://doi.org/10.3390/d17090662





