Effect of UV-B Radiation on the Growth of Alien Myriophyllum aquaticum
Abstract
1. Introduction
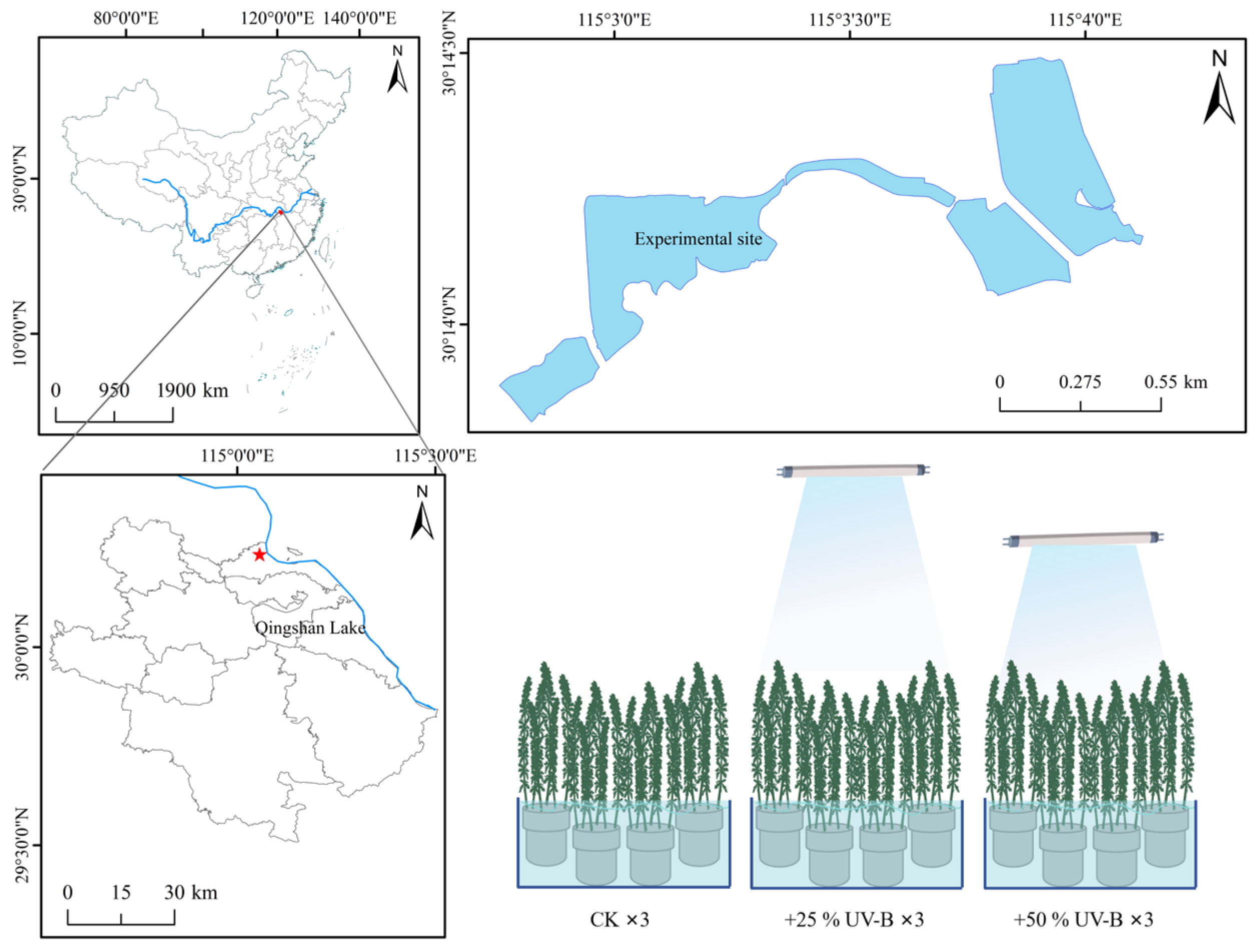
2. Materials and Methods
2.1. Experimental Design
2.2. Indicator Measurement
2.3. Data Analysis
3. Results
3.1. Effect of UV-B Radiation on the Growth of M. aquaticum
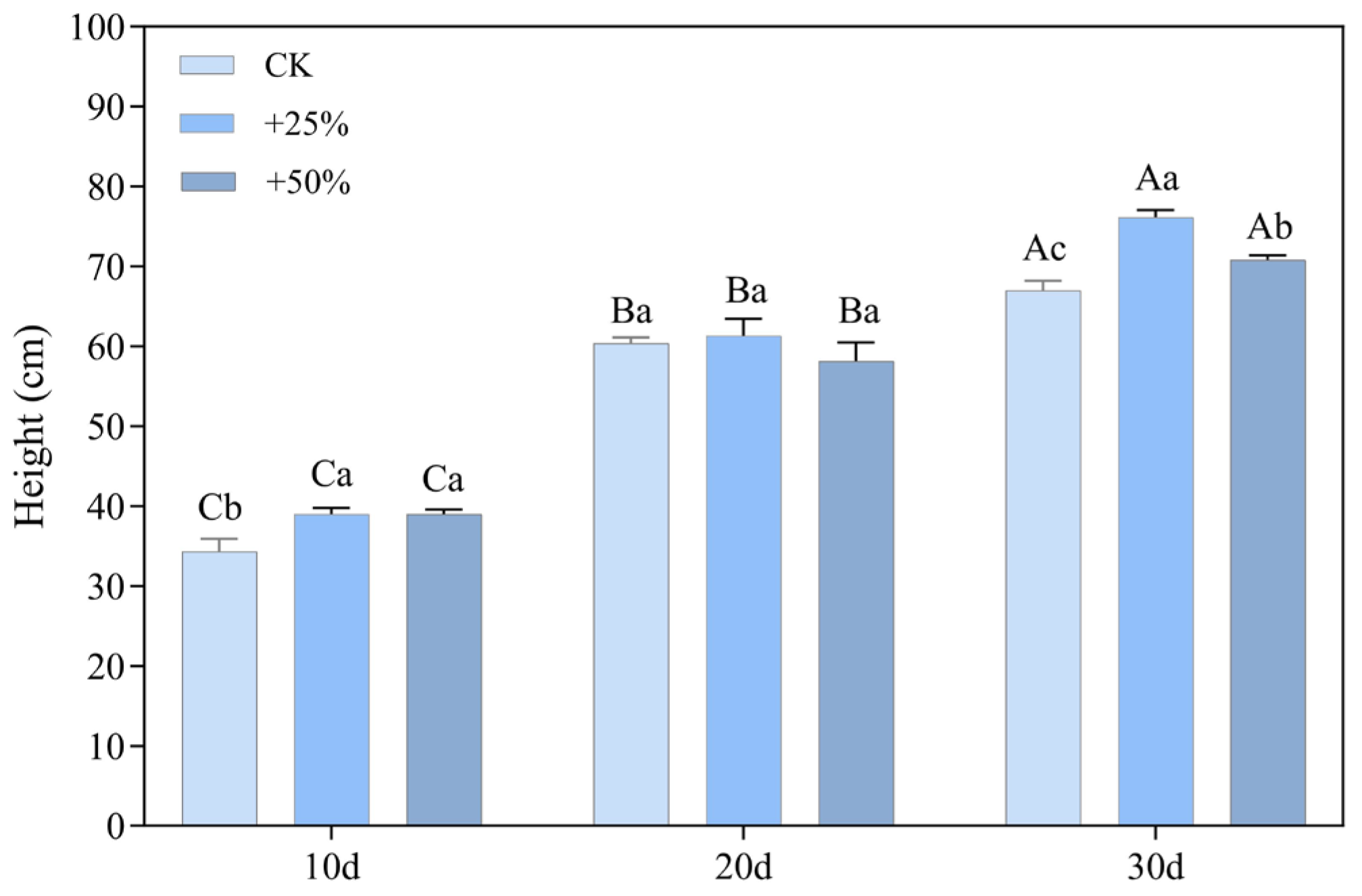
3.1.1. Plant Height
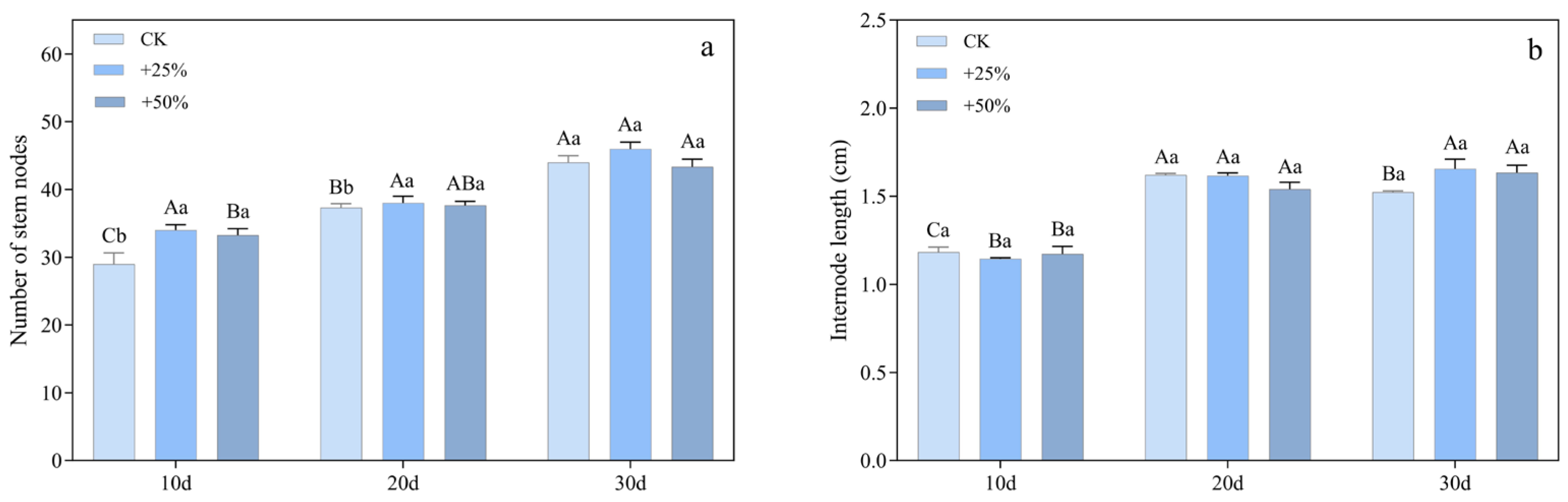
3.1.2. Number of Stem Nodes and Internode Spacing
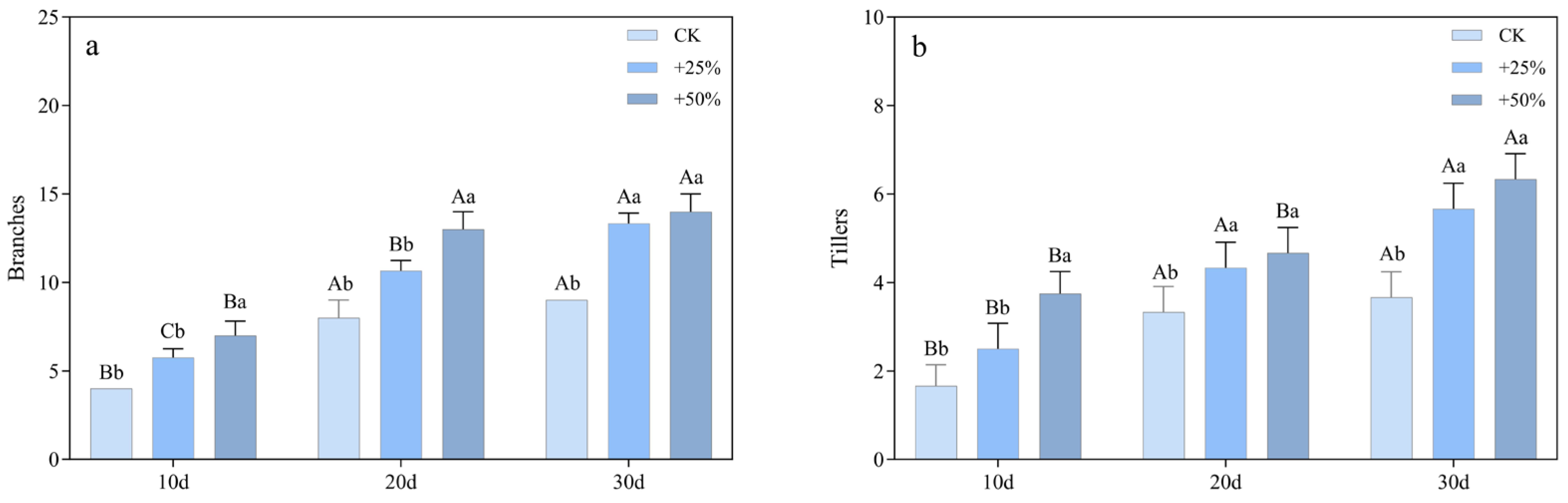
3.1.3. Branching and Tillering
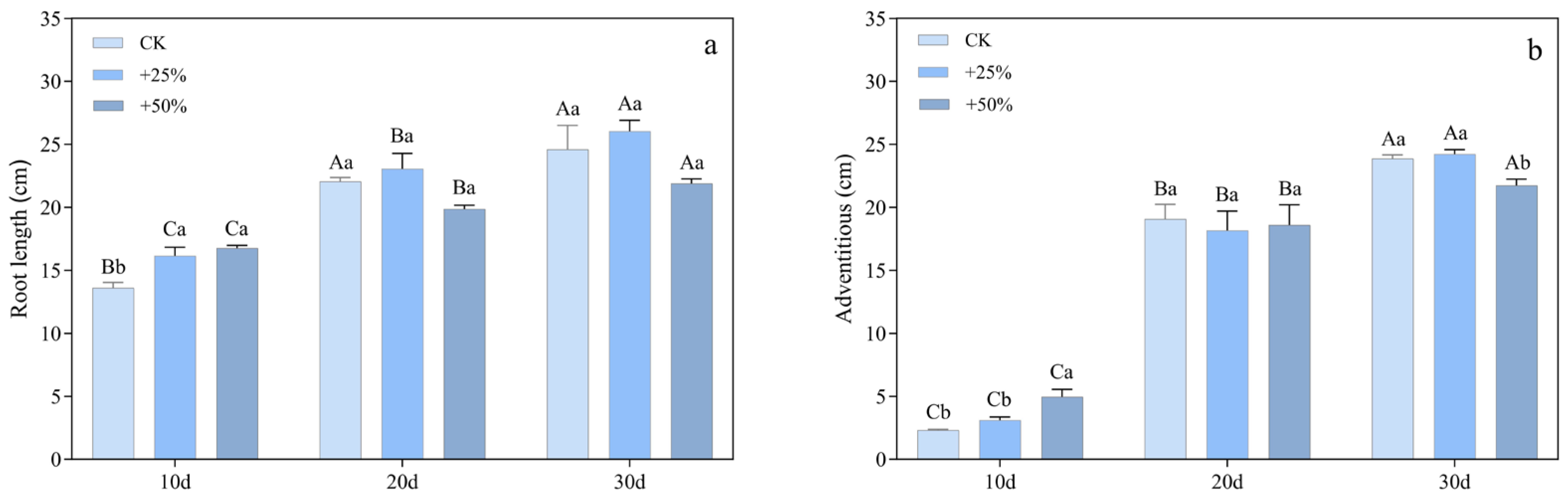
3.1.4. Root Length
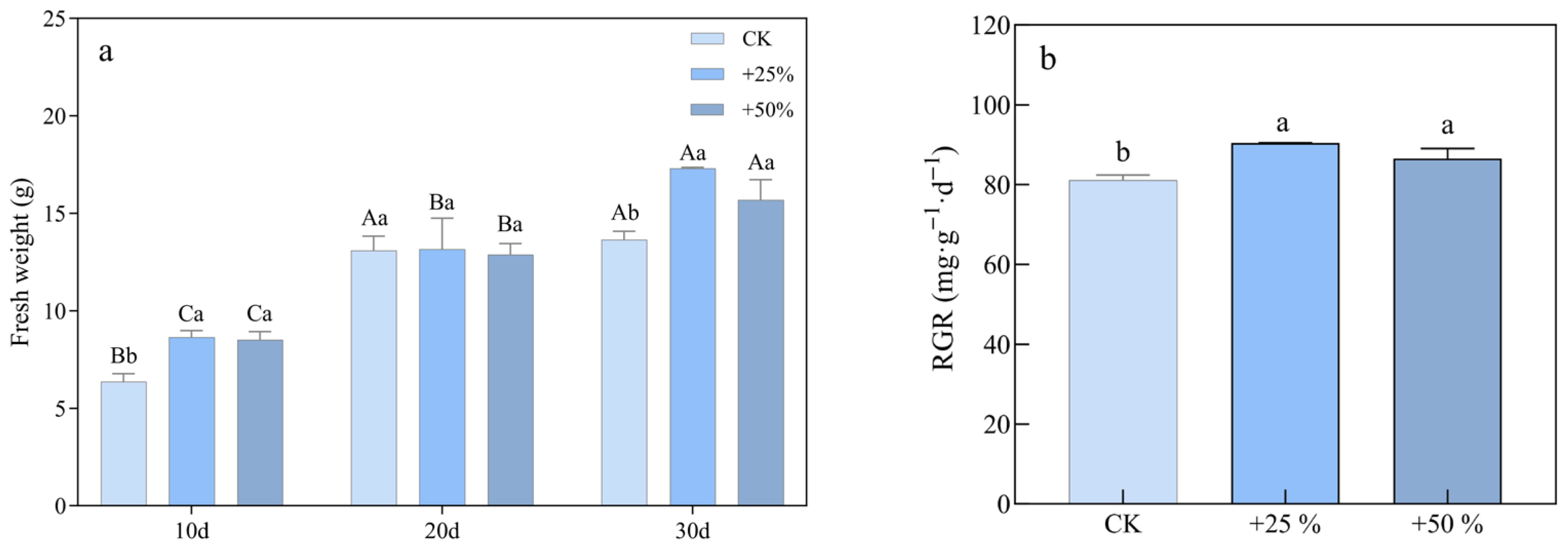
3.1.5. Fresh Weight and Relative Growth Rate
3.2. Effect of UV-B Radiation on the Physiological Indices of M. aquaticum
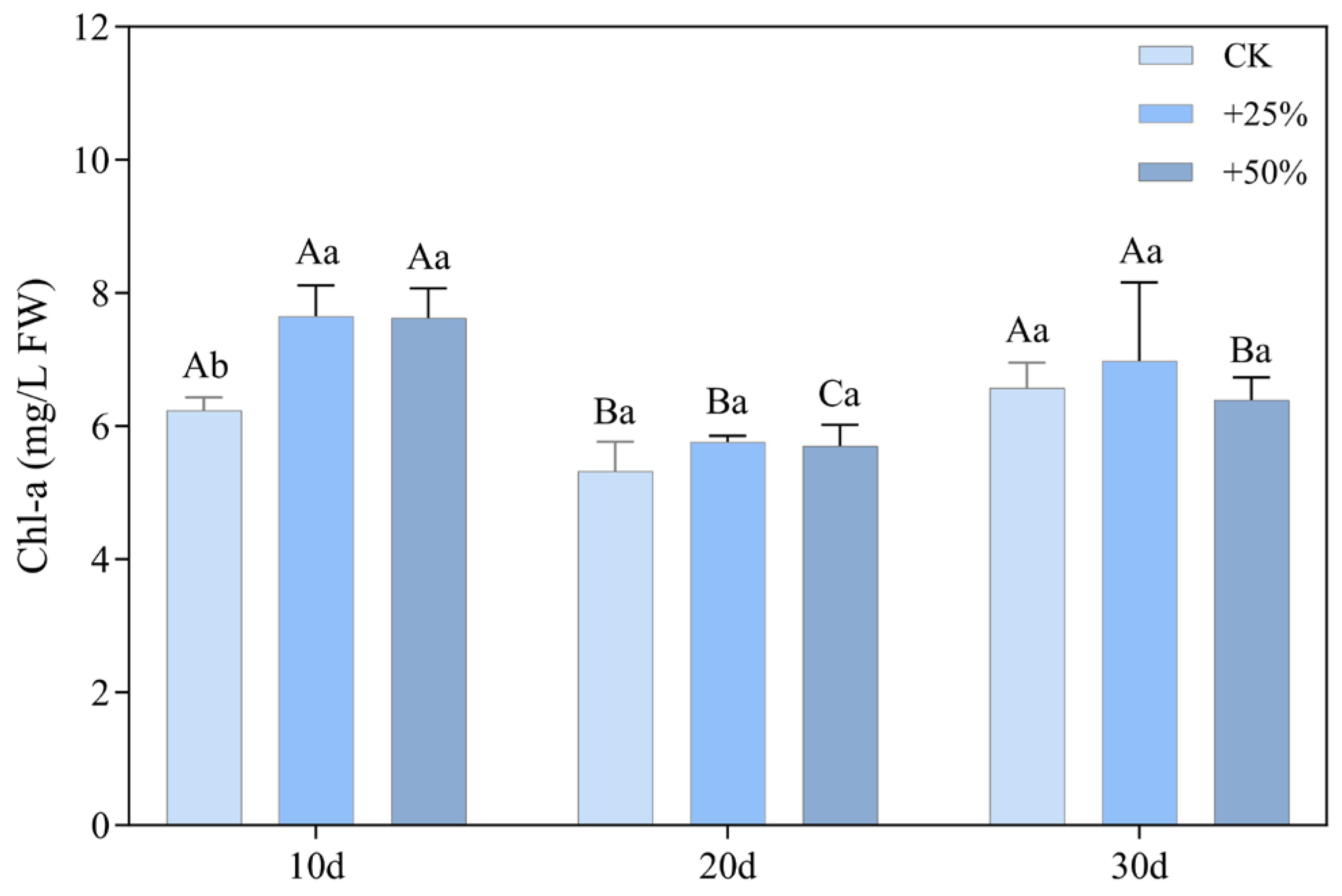
3.2.1. Chlorophyll a Content
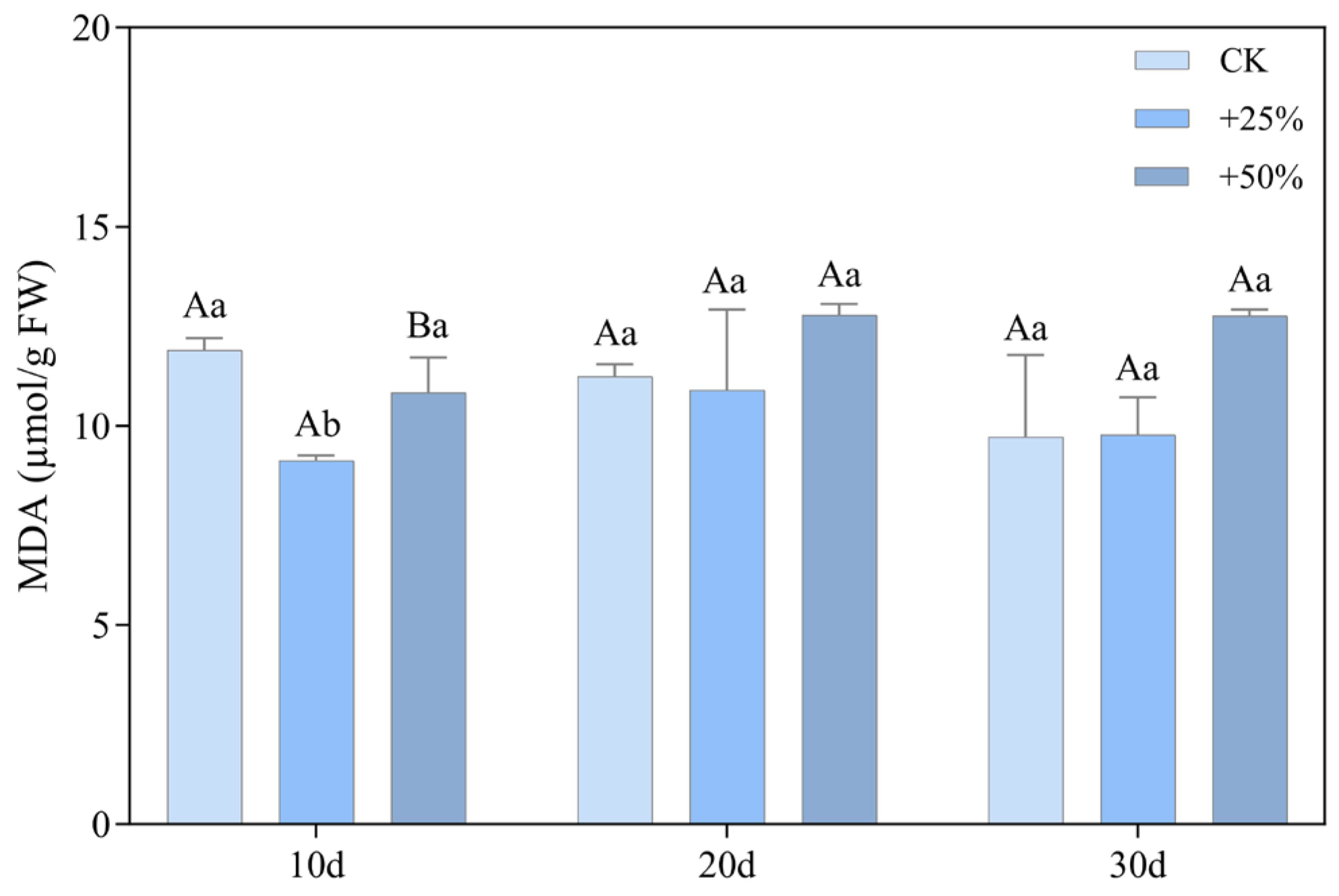
3.2.2. Malondialdehyde Content
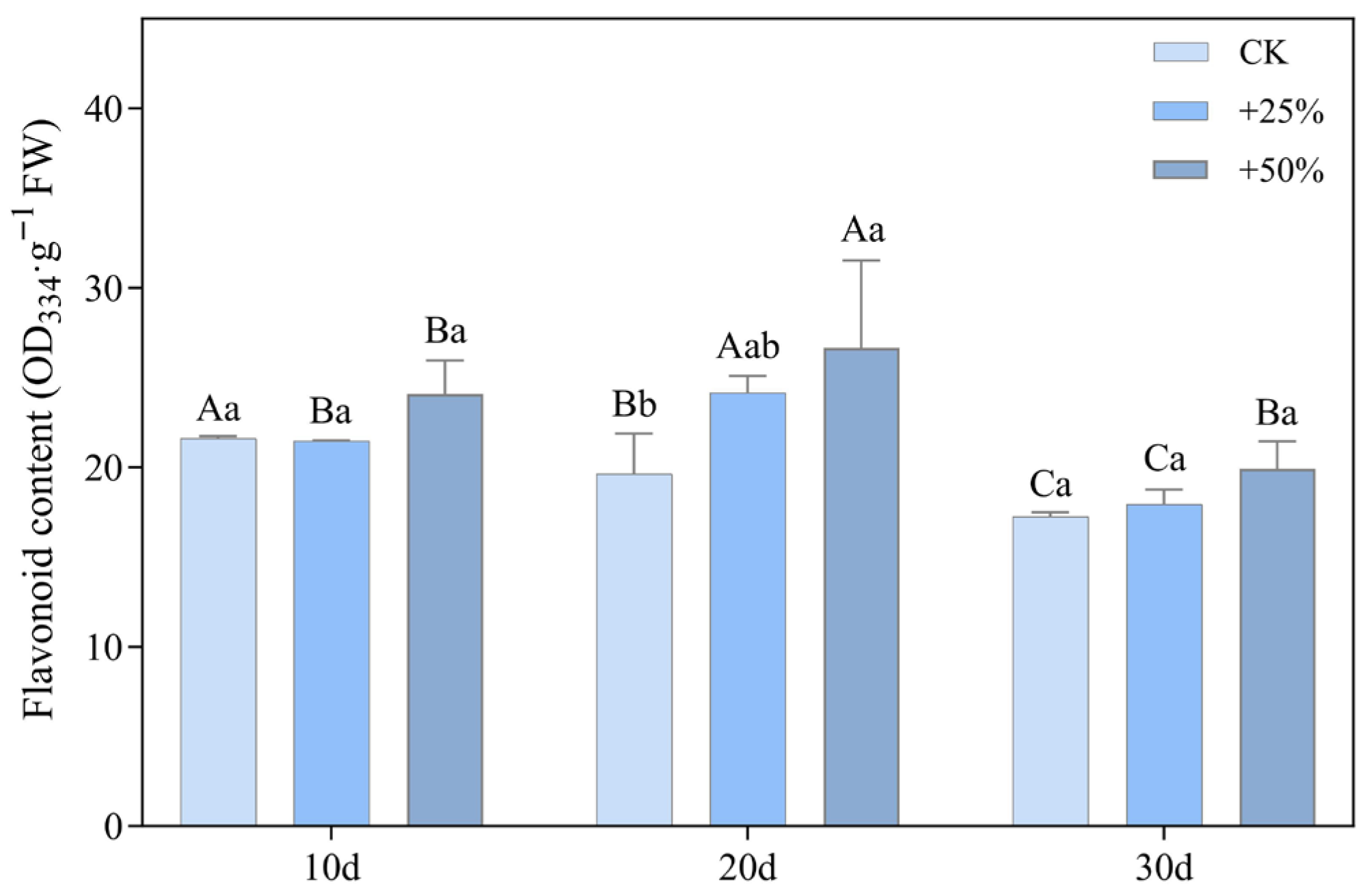
3.2.3. Flavonoid Content
4. Discussion
4.1. Effect of UV-B Radiation on the Morphological Characteristics and Biomass of M. aquaticum
4.2. Effects of UV-B Radiation on the Physiological Characteristics of M. aquaticum
4.3. Exploring the Invasiveness of M. aquaticum from the Perspective of UV-B Radiation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | Malondialdehyde |
| ROS | Reactive oxygen species |
| RGR | Relative growth rate |
| Chl-a | Chlorophyll a |
References
- Suominen, L.; Jussila, M.M.; Mäkeläinen, K. Evaluation of the Galega–Rhizobium galegae system for the bioremediation of oil-contaminated soil. Environ. Pollut. 2000, 107, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, J.; Chen, J.; Li, B. Spatial patterns of invasive alien plants in China and its relationship with environmental and anthropological factors. Chin. J. Plant Ecol. 2006, 30, 576. [Google Scholar] [CrossRef][Green Version]
- Rai, P.; Singh, J. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 106020. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Shou, H.; Ma, J. The Problem and Status of the Alien Invasive Plants in China. Plant Divers. 2012, 34, 287. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhao, J. Advances in the study of reproductive biology characteristics of invasive alien plants. J. Chang. Norm. Univ. 2023, 42, 120–124. [Google Scholar]
- Wang, C.; Cheng, H.; Wang, S. Plant community and the influence of plant taxonomic diversity on community stability and invasibility: A case study based on Solidago canadensis L. Sci. Total Environ. 2021, 768, 144518. [Google Scholar] [CrossRef]
- Chen, J.; Feng, H.; Su, H.; Luo, Y.; Zhou, L. Physiological response and drought resistance evaluation of invasive plants Cenchrus pauciflorus to drought. Grassl. Turf 2023, 43, 77–83. [Google Scholar] [CrossRef]
- Williamson, C.E.; Zepp, R.G.; Lucas, R.M. Solar ultraviolet radiation in a changing climate. Nat. Clim. Change 2014, 4, 434–441. [Google Scholar] [CrossRef]
- Li, X.; Yue, H.; Wang, S. Research of different effects on activity of plant antioxidant enzymes. Chin. J. Tradit. Chin. Med. 2013, 38, 973–978. [Google Scholar] [CrossRef]
- Lou, Y.; Huang, Y.; Li, Y. Effect of Enhanced Ultraviolet-B Radiation on Physiological Properties of Different Cultivars of Barley. J. Ecol. Rural Environ. Sci. 2011, 27, 51–55. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Flint, S.D. Stratospheric ozone reduction, solar UV-B radiation and terrestrial ecosystems. Clim. Change 1994, 28, 375–394. [Google Scholar] [CrossRef]
- Yuan, X.; Yin, K.; Zhou, W. Effects of ultraviolet radiation B (UV-B) on photosynthesis of natural phytoplankton assemblages in a marine bay in Southern China. Chin. Sci. Bull. 2007, 52, 545–552. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, L.; Deng, B. Effects of enhanced UV-B radiation and nitrogen deposition on chlorophyll fluorescence parameters of invasive plant Tradica sebifera. J. Zhejiang Univ. Agric. Life Sci. 2018, 30, 248–254. [Google Scholar] [CrossRef]
- Václavík, T.; Beckmann, M.; Cord, A.F. Effects of UV-B radiation on leaf hair traits of invasive plants—Combining historical herbarium records with novel remote sensing data. PLoS ONE 2017, 12, e0175671. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.D.; Keller, W.; Scully, N.M. Increased UV-B penetration in a lake owing to drought-induced acidification. Nature 1996, 381, 141–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, B.; Ma, R. Attenuation of solar ultraviolet radiation and analysis of attenuators in typical macrophytic, algal lake zones of Lake Taihu. Acta Ecol. Sin. 2005, 9, 2354–2361. [Google Scholar]
- Williamson, C.E. What role does UV-B radiation play in freshwater ecosystems? Limnol. Oceanogr. 1995, 40, 386–392. [Google Scholar] [CrossRef]
- Sutton, D.L. Biology and ecology of Myriophyllum aquaticum. In Proceedings of the 1st International Symposium on Watermilfoil, Vancouver, BC, Canada, 23–24 July 1985; pp. 59–71. [Google Scholar]
- Moreira, I.; Monteiro, A.; Ferreira, M.T. Biology and control of parrotfeather (Myriophyllum aquaticum) in Portugal. Ecol. Environ. Conserv. 1999, 5, 171–179. [Google Scholar]
- Guillarmod, A.J. Water weeds in southern Africa. J. Aquat. Bot. 1979, 6, 377–391. [Google Scholar] [CrossRef]
- Beckmann, M.; Václavík, T.; Manceur, A.M. gl UV: A global UV-B radiation data set for macroecological studies. Methods Ecol. Evol. 2014, 5, 372–383. [Google Scholar] [CrossRef]
- Chu, R. Responses and the Physiological and Biochemical Mechanisms of Three Wetland Plants to UV-B Radiation. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2018. [Google Scholar]
- Lois, R. Accumulation of UV-absorbing flavonoids induced by UV-B radiation in Arabidopsis thaliana L. I. Mechanisms of UV-resistance in Arabidopsis. Planta 1994, 194, 498–503. [Google Scholar] [CrossRef]
- Langhans, R.W.; Tibbitts, T.W. Plant Growth Chamber Handbook: North Central Regional Research Publication No. 340; lowa State University: Ames, IA, USA, 1997. [Google Scholar]
- Wang, X.; Huang, J.L. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Liu, P.; Li, M. Editorial Board of Plant Physiology Experimental Technology, 1st ed.; Science Press: Beijing, China, 2007; pp. 150–152. [Google Scholar]
- Besemer, K.; Singer, G.; Limberger, R. Biophysical controls on community succession in stream biofilms. Appl. Environ. Microbiol. 2007, 73, 4966–4974. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Principles and Techniques of Plant Physiological and Biochemica Experiments; Higher Education Press: Beijing, China, 2000; pp. 134–137. [Google Scholar]
- Liaqat, W.; Altaf, M.T.; Barutçular, C.; Nawaz, H.; Ullah, I.; Basit, A.; Mohamed, H.I. Ultraviolet-B radiation in relation to agriculture in the context of climate change: A review. Cereal Res. Commun. 2024, 52, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ulm, R.; Jenkins, G.I. Q&A: How do plants sense and respond to UV-B radiation? BMC Biol. 2015, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Cuadra, P.; Herrera, R.; Fajardo, V. Effects of UV-B radiation on the Patagonian Jaborosa magellanica Brisben. J. Photochem. Photobiol. B 2004, 76, 61–68. [Google Scholar] [CrossRef]
- Choudhary, K.K.; Agrawal, B.S. Effect of elevated ultraviolet-B on four tropical soybean cultivars: Quantitative and qualitative aspects with special emphasis on gas exchange, chlorophyll fluorescence, biomass and yield. Acta Physiol. Plant. 2015, 37, 1–12. [Google Scholar] [CrossRef]
- Feyza, A.I.; Abdulkadir, C. The impact of ultraviolet B (UV-B) radiation in combination with different temperatures in the early life stage of zebrafish (Danio rerio). Photochem. Photobiol. Sci. 2018, 17, 35–41. [Google Scholar] [CrossRef]
- Wang, S.; Duan, L.; Eneji, A.E. Variations in growth, photosynthesis and defense system among four weed species under increased UV-B radiation. J. Integr. Plant Biol. 2007, 49, 621–627. [Google Scholar] [CrossRef]
- Ji, M.; Feng, H.; An, L. Present status and prospects in research on effect of enhanced UV-B radiation on plants. J. Appl. Ecol. 2002, 3, 359–364. [Google Scholar]
- Xian, Y.; Zhang, Y.; Li, B. Responses of photosynthetic pigments composition, nitrogen and phosphorus stoichiometric characteristics of Myriophyllum aquaticum to exogenous ammonium. Acta Bot. Sin. 2022, 46, 451–460. [Google Scholar] [CrossRef]
- Wang, Y.L.; Gao, Y.Y.; Yu, D. Physiological response of three submerged macrophytes to the high temperature and light intensity of summer. Hydroecology 2015, 5, 95–101. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. J. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Lv, Z.; Feng, Q.; Lv, Y. Response of 140 Winter Wheat Varieties to UV-B Radiation. J. Cereal Crop. 2017, 37, 841–845. [Google Scholar] [CrossRef]
- Dai, Q.; Shaobing, P.; Chavez, A.Q. Intraspecific responses of 188 rice cultivars to enhanced UVB radiation. Environ. Exp. Bot. 1994, 34, 433–442. [Google Scholar] [CrossRef]
- Qian, M.; Rosenqvist, E.; Prinsen, E. Downsizing in plants—UV light induces pronounced morphological changes in the absence of stress. Plant Physiol. 2021, 187, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; Liu, C. Adaptive plasticity in response to light and nutrient availability in the clonal plant Duchesnea indica. J. Plant Ecol. 2022, 15, 795–807. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Qian, C. Nuclear accumulation of rice UV-B photoreceptors is UV-B-and OsCOP1-independent for UV-B responses. Nat. Commun. 2024, 15, 6396. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Campbell, B.D.; Bloor, S.J. Responses to UV-B radiation in Trifolium repens L.–physiological links to plant productivity and water availability. Plant Cell Environ. 2003, 26, 603–612. [Google Scholar] [CrossRef]
- Huang, J.; Qin, F.; Zang, G. Mutation of OsDET1 increases chlorophyll content in rice. Plant Sci. 2013, 210, 241–249. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Guo, Q. Short-term UV-B radiation effects on morphology, physiological traits and accumulation of bioactive compounds in Prunella vulgaris L. Plant Interact. 2017, 12, 348–354. [Google Scholar] [CrossRef]
- Yokawa, K.; Kagenishi, T.; Baluška, F. UV-B induced generation of reactive oxygen species promotes formation of BFA-induced compartments in cells of Arabidopsis root apices. Front. Plant Sci. 2016, 6, 1162. [Google Scholar] [CrossRef]
- Goodman, L.D.; Ralhan, L.; Li, X. Tau is required for glial lipid droplet formation and resistance to neuronal oxidative stress. Nat. Neurosci. 2024, 27, 1918–1933. [Google Scholar] [CrossRef]
- Ke, S.; Jin, Z. Effects of Drought Stress on Lipid Peroxidation and Anfioxidant Systemin Leaves of Calycanthus chinens. Sci. Silvae Sin. 2007, 43, 28–33. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Weng, Y. Effects of UV-B Radiation on Phenolic Accumulation, Antioxidant Activity, and Physiological Changes in Wheat (Triticum aestivum L.) Seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Valentovic, P.; Luxova, M.; Kolarovic, L. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ. 2006, 52, 184. [Google Scholar] [CrossRef]
- Solovchenko, A.; Schmitz-Eiberger, M. Significance of skin flavonoids for UV-B-protection in apple fruits. J. Exp. Bot. 2003, 54, 1977–1984. [Google Scholar] [CrossRef]
- Liang, B. Effect of Lanthanum on Flavonoids contents in Soybean Seedlings Exposed to Supplementary Ultraviolet-B Radiation. Master’s Thesis, Jiangnan University, Wuxi, China, 2006. [Google Scholar]
- Schenke, D.; Utami, H.P.; Zhou, Z. Suppression of UV-B stress induced flavonoids by biotic stress: Is there reciprocal crosstalk? Plant Physiol. Biochem. 2019, 134, 53–63. [Google Scholar] [CrossRef]
- He, Y.; Zhan, F.; Zu, Y. Effectsof UV-B Radiationon the Contentsof Silicon, Flavonoidsand Total Phenolicof Two Local Rice Varieties in Yuanyang Terrace Under Field Conditions. Am. Eurasian J. Agric. Environ. Sci. 2013, 32, 1500–1506. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Yin, Y. Differential epigenetic regulation by blue and UV-A light reveals the key role of CsSDG36-mediated H3K4 methylation in leaf development and secondary metabolism in Camellia sinensis. Genome Biol. 2025, 26, 1–27. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Wang, Q.; Chu, G.; Gao, C. Effect of light intensity on performance, microbial community and metabolic pathway of algal-bacterial symbiosis in sequencing batch biofilm reactor treating mariculture wastewater. Bioresour. Technol. 2025, 433, 132726. [Google Scholar] [CrossRef]
- Tuo, C.; Xunling, W. The effect of enhanced UV-B radiation on the activities of SOD, CAT and POX in wheat leaves. Wuhan Zhi Wu Xue Yan Jiu Wuhan Bot. Res. 1999, 17, 101–104. [Google Scholar]
- Bhattarai, S.; Jha, D.K.; Balyan, S. UV-B and Blue Light Supplementation Improves Tomato Quality and Antioxidant Dynamics: A Novel Approach for Sustainable Greenhouse Production. J. Agric. Food Res. 2025, 22, 102054. [Google Scholar] [CrossRef]
- Sytsma, M.D.; Anderson, L.W.J. Biomass, nitrogen, and phosphorus allocation in parrotfeather (Myriophyllum aquaticum). J. Aquat. Plant Manag. 1993, 31, 244. [Google Scholar]
- Li, B.; Zhang, Y.; Xian, Y.; Pei, L.; Run, L. Physiological response and tolerance of Myriophyllum aquaticum to a wide range of ammonium concentrations. J. Environ. Manag. 2022, 317, 115368. [Google Scholar] [CrossRef]
- Beckmann, M.; Hock, M.; Bruelheide, H. The role of UV-B radiation in the invasion of Hieracium pilosella—A comparison of German and New Zealand plants. Environ. Exp. Bot. 2012, 75, 173–180. [Google Scholar] [CrossRef]
- Hock, M.; Hofmann, R.; Essl, F. Native distribution characteristics rather than functional traits explain preadaptation of invasive species to high-UV-B environments. Diver. Distrib. 2020, 26, 1421–1438. [Google Scholar] [CrossRef]
- McKenzie, R.L.; Aucamp, P.J.; Bais, A.F. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2011, 10, 182–198. [Google Scholar] [CrossRef]
- Zhao, H.; Zuo, Z.; Yang, L. Similarities and differences in the physiological adaptation to water salinity between two life forms of aquatic plants in alpine and arid wetlands. Sci. Total Environ. 2024, 908, 168449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Leng, M.; Wu, X.; Ge, X.; Gong, M.; Liu, H.; Wang, X.; Li, H.; Mou, X. Effect of UV-B Radiation on the Growth of Alien Myriophyllum aquaticum. Diversity 2025, 17, 661. https://doi.org/10.3390/d17090661
Huang Z, Leng M, Wu X, Ge X, Gong M, Liu H, Wang X, Li H, Mou X. Effect of UV-B Radiation on the Growth of Alien Myriophyllum aquaticum. Diversity. 2025; 17(9):661. https://doi.org/10.3390/d17090661
Chicago/Turabian StyleHuang, Zhi, Mingkai Leng, Xiaodong Wu, Xuguang Ge, Mengting Gong, Haoran Liu, Xing Wang, Haoyue Li, and Xin Mou. 2025. "Effect of UV-B Radiation on the Growth of Alien Myriophyllum aquaticum" Diversity 17, no. 9: 661. https://doi.org/10.3390/d17090661
APA StyleHuang, Z., Leng, M., Wu, X., Ge, X., Gong, M., Liu, H., Wang, X., Li, H., & Mou, X. (2025). Effect of UV-B Radiation on the Growth of Alien Myriophyllum aquaticum. Diversity, 17(9), 661. https://doi.org/10.3390/d17090661








