Tachinid Flies (Diptera), Caterpillar Hosts (Lepidoptera) and Their Food Plants, Reared in Área de Conservación Guanacaste (ACG), Northwestern Costa Rica: Documenting Community Structure with the Aid of DNA Barcodes
Abstract
1. Introduction
1.1. Tachinid Biology
1.2. Barcoding and Host Specialisation
2. Materials and Methods
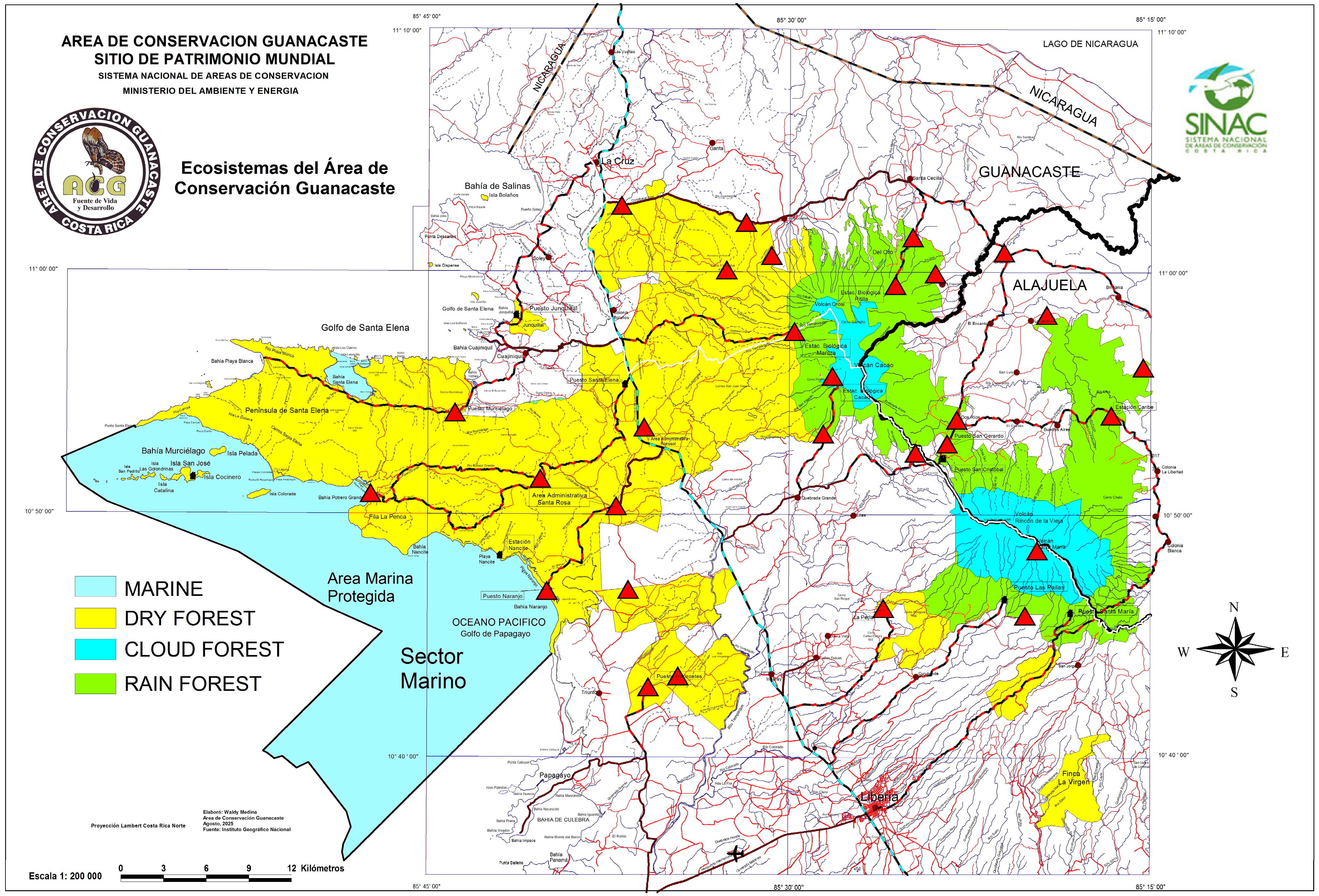
2.1. Study Area
2.2. The ACG Inventory
2.3. Tachinid Identification
2.4. DNA Barcoding
2.5. Data Analysis
2.6. Raw and Cleaned Data
3. Results
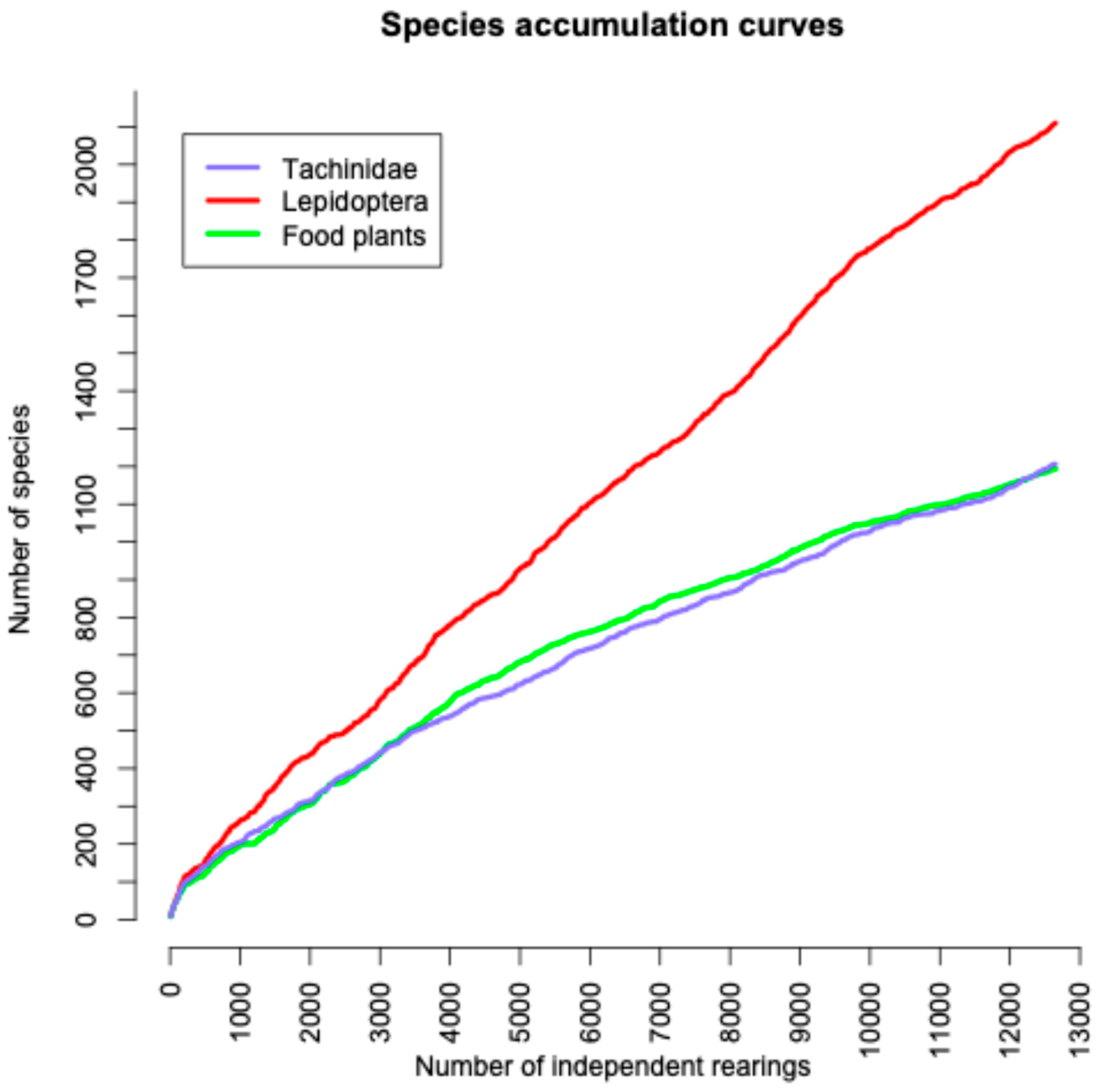
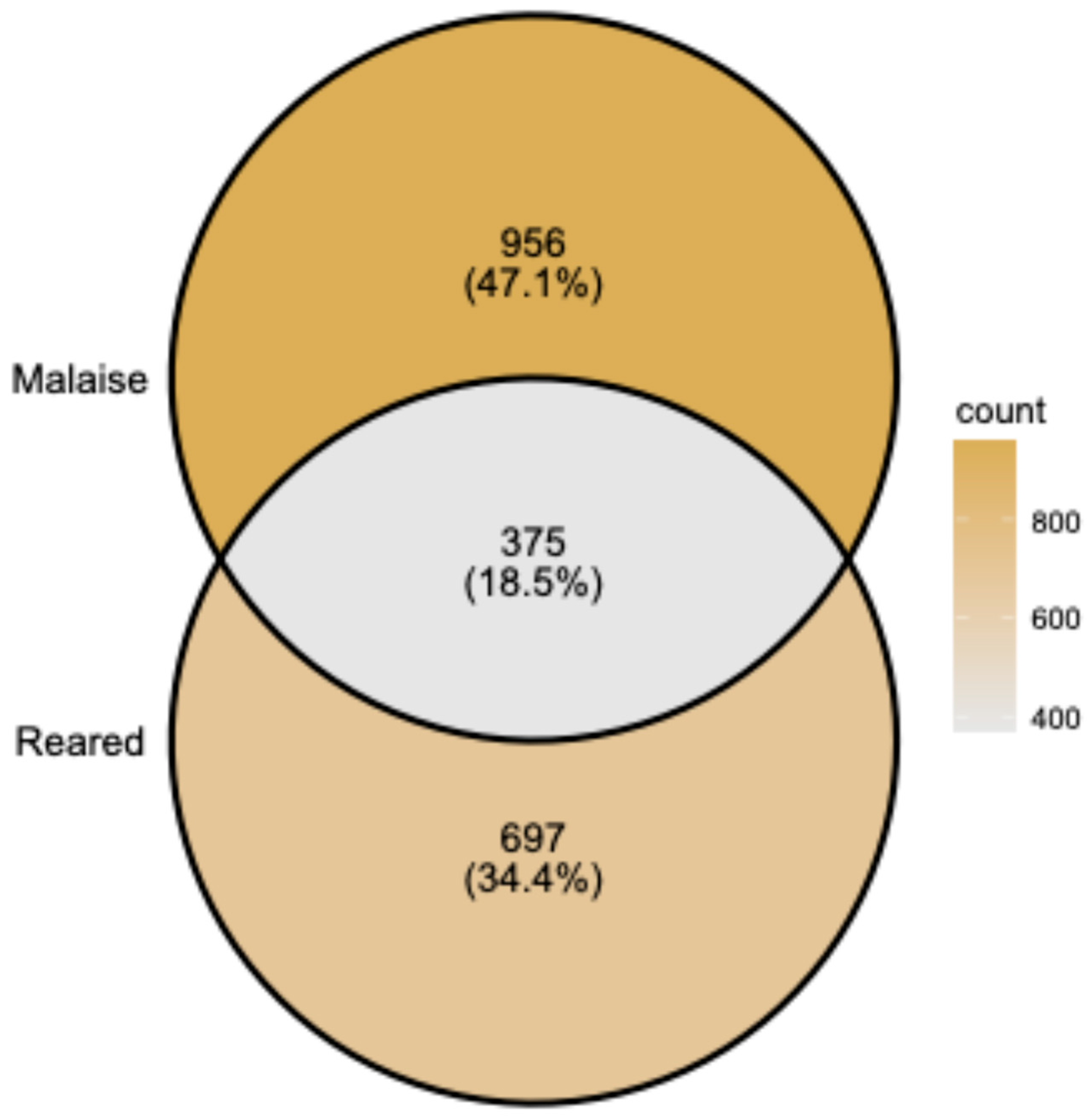
3.1. Overview of the ACG Tachinidae Rearing Data
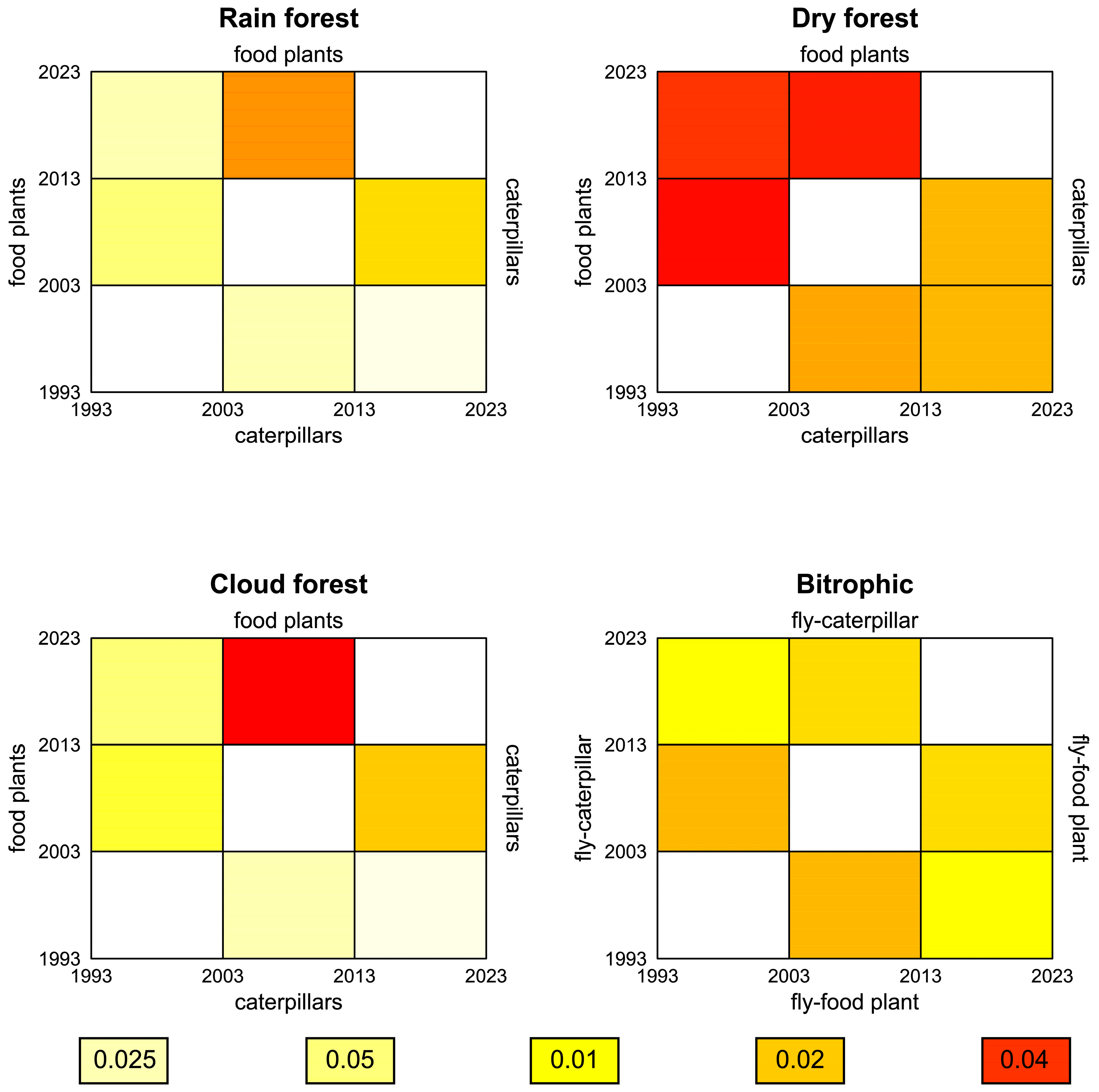
3.2. Changes in Abundance and Species Composition over Time
3.3. Relationships Between Rearing Effort/Successes and Species Growth by Taxon
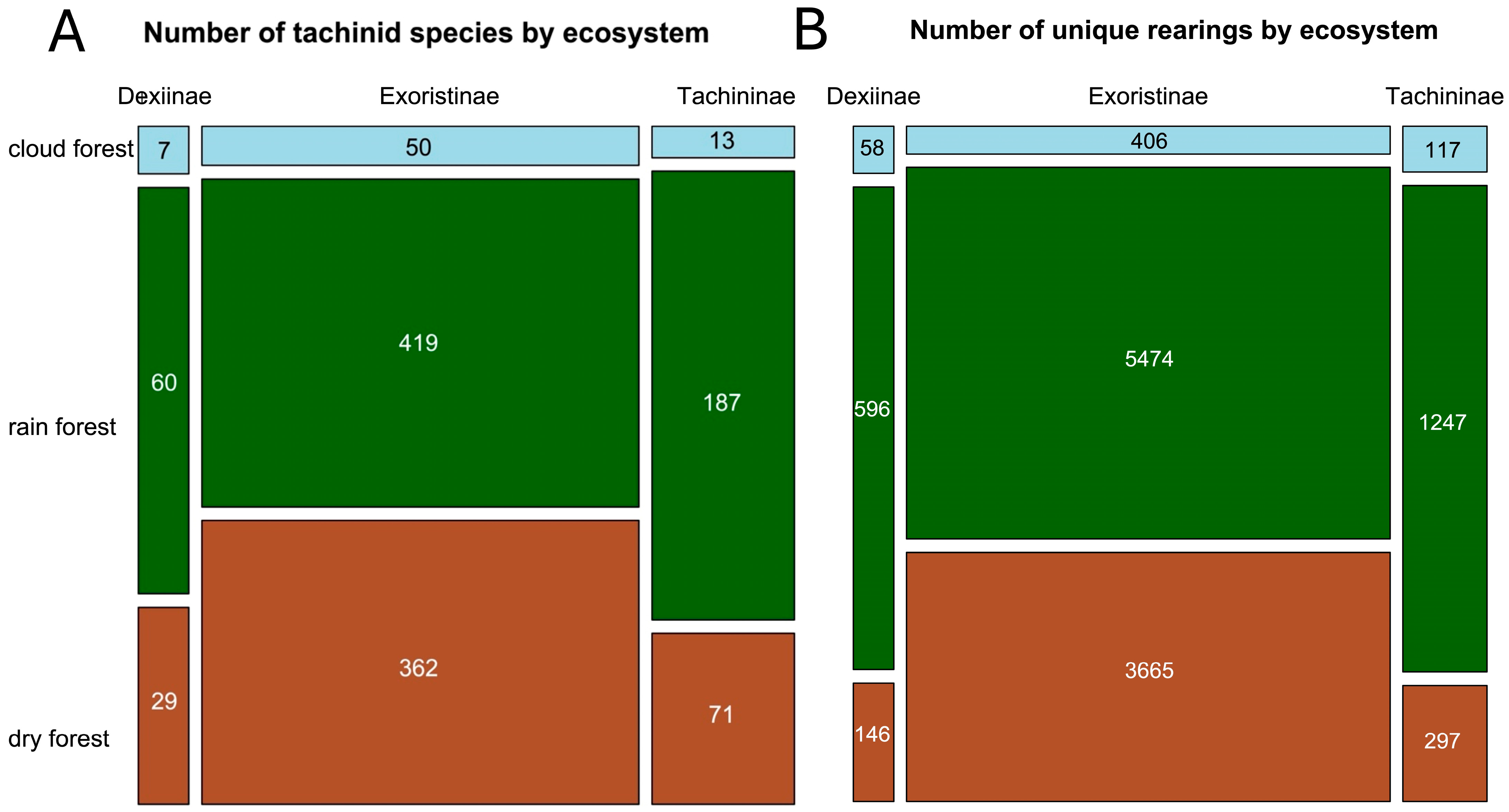
3.4. Subfamily Representation by Primary Ecosystem
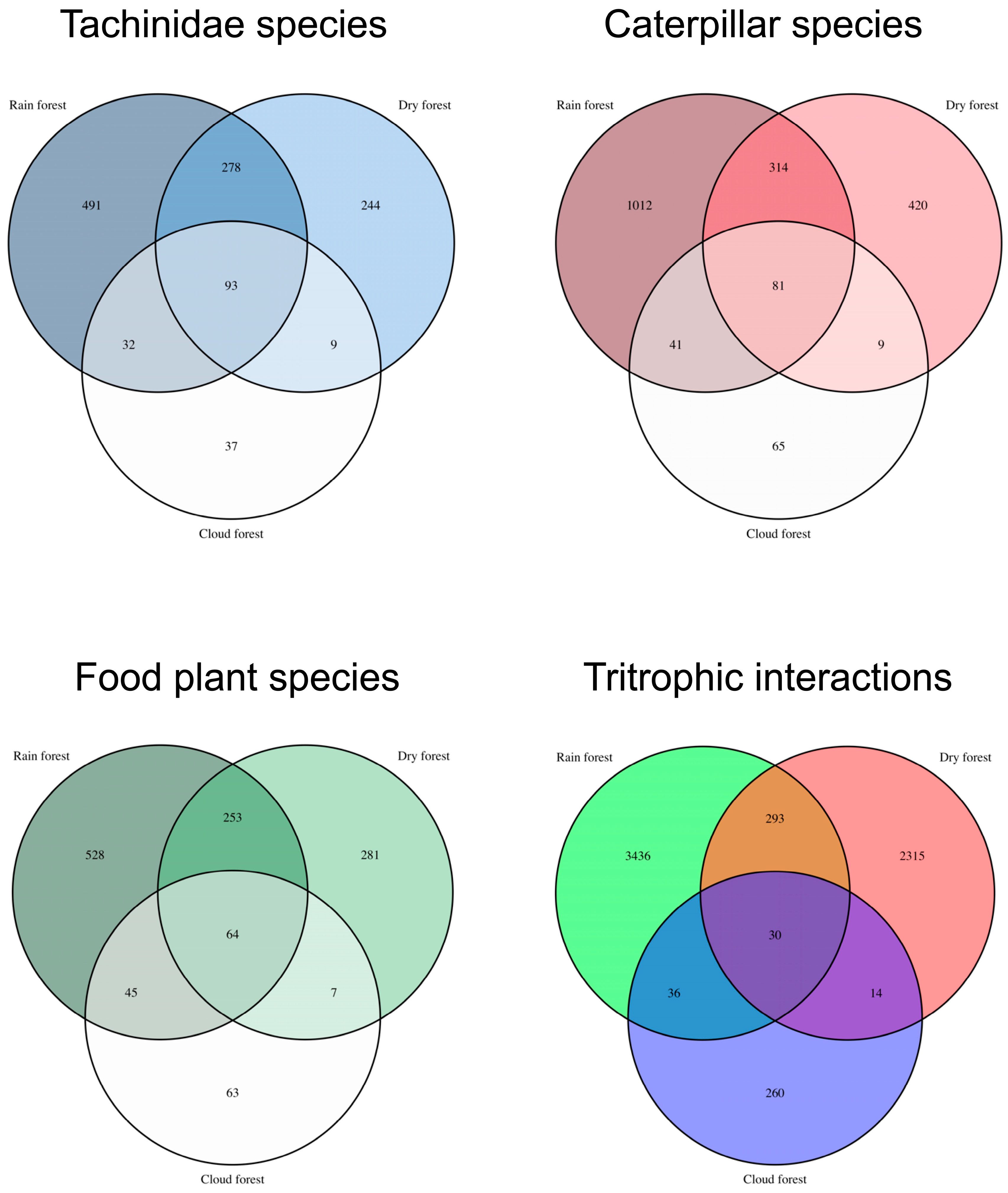
3.5. Species Overlap Between Primary Ecosystems
3.6. Chao Estimation of Likely Total Numbers of Species
3.7. Bi- and Tritrophic Interactions
3.8. Food Web Metrics
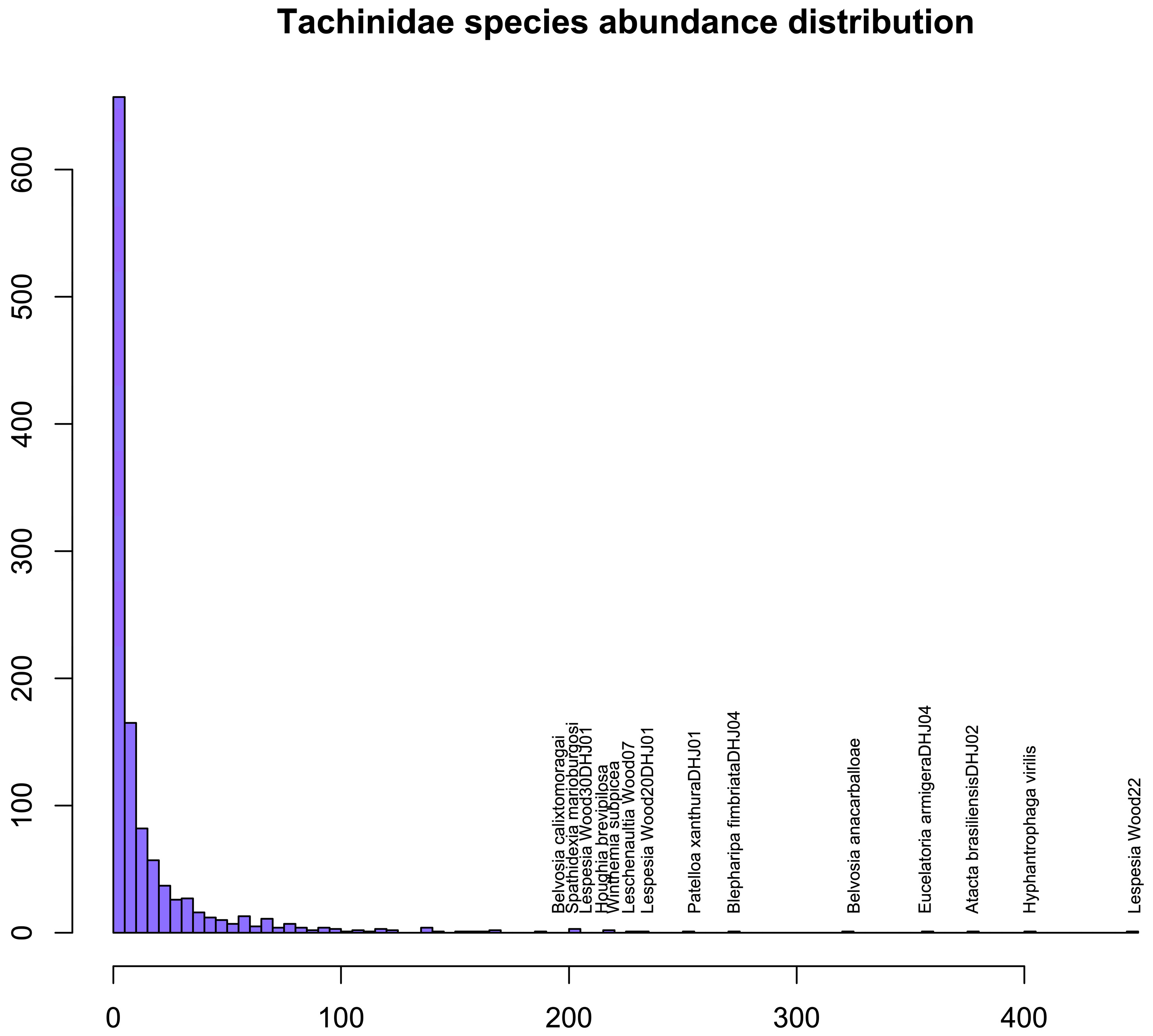
3.9. Species Abundance Distribution
3.10. Host Ranges
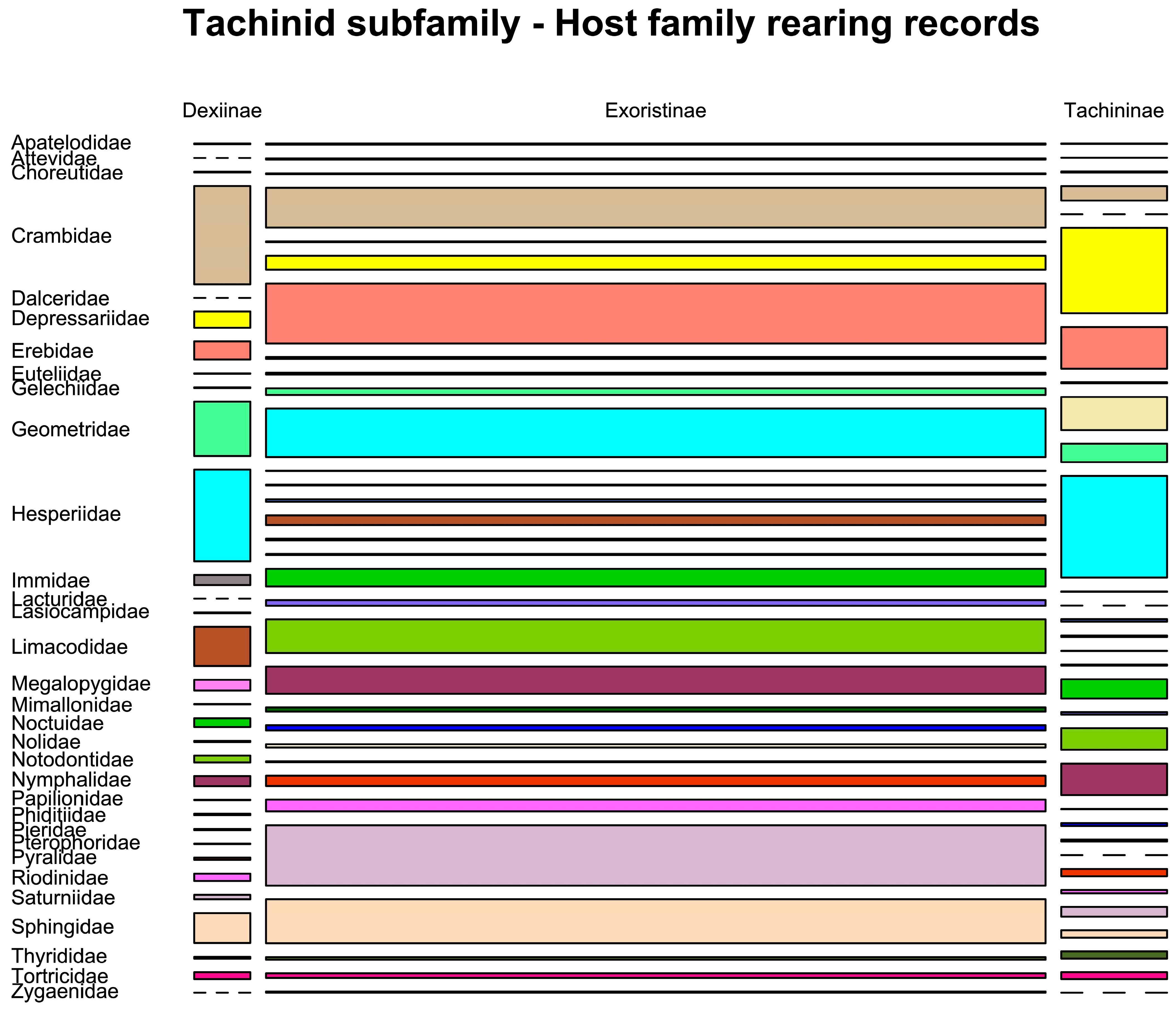
3.10.1. Utilisation of Host Families
3.10.2. Utilisation of Host Species—Generalists Versus Specialists
3.11. Relationship Between Host Range and Range of Host Food Plants
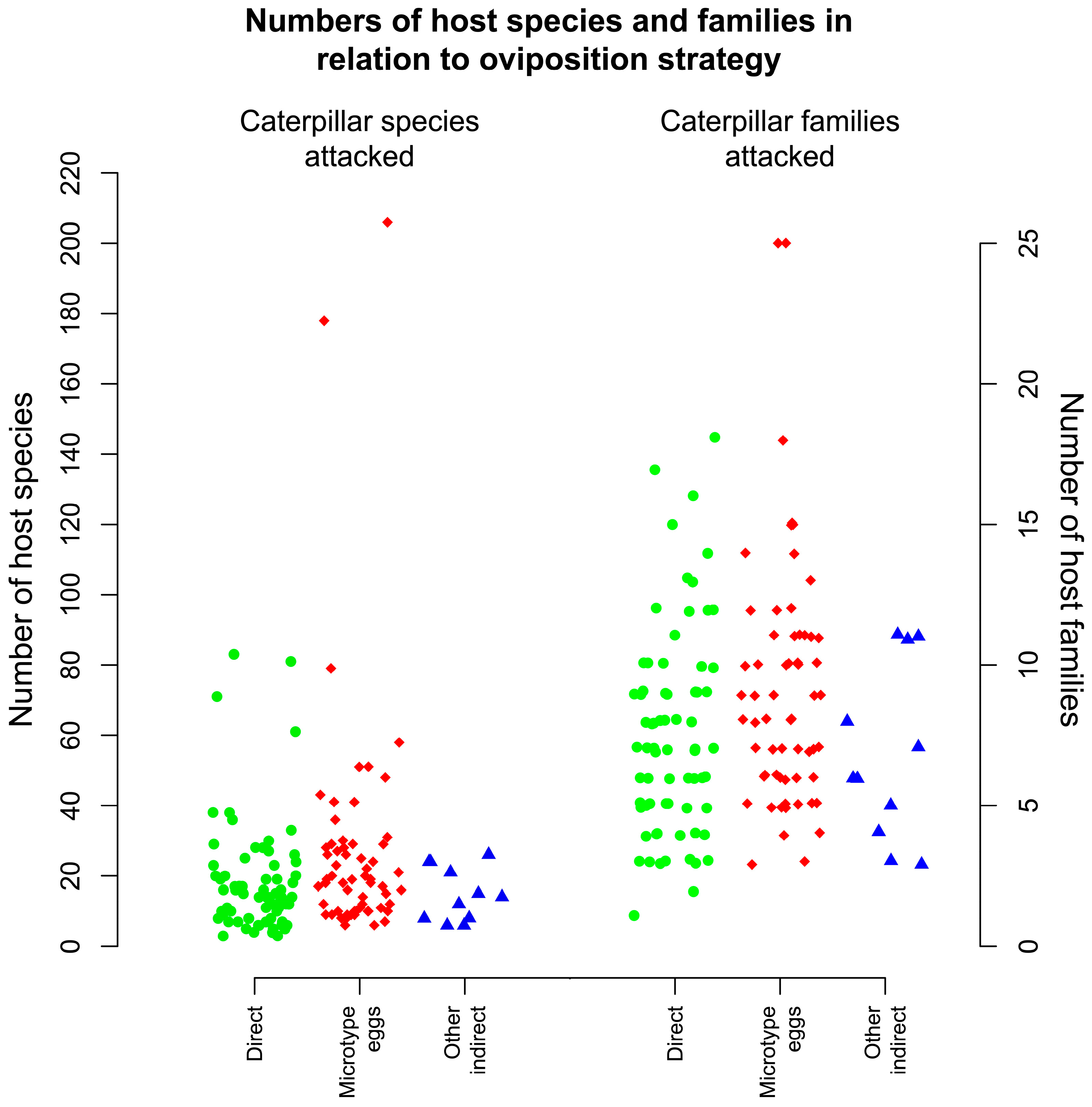
3.12. Oviposition Strategy
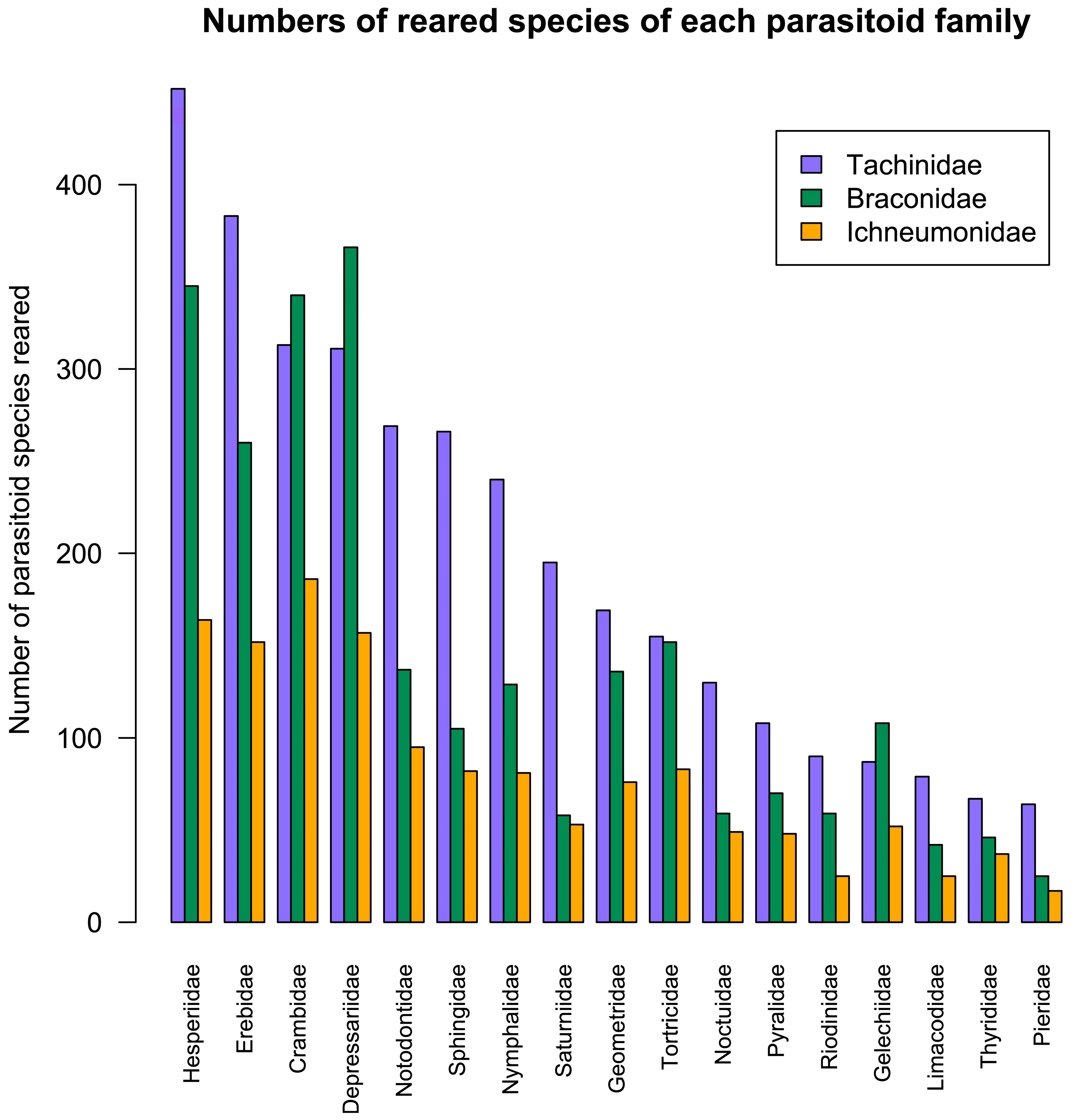
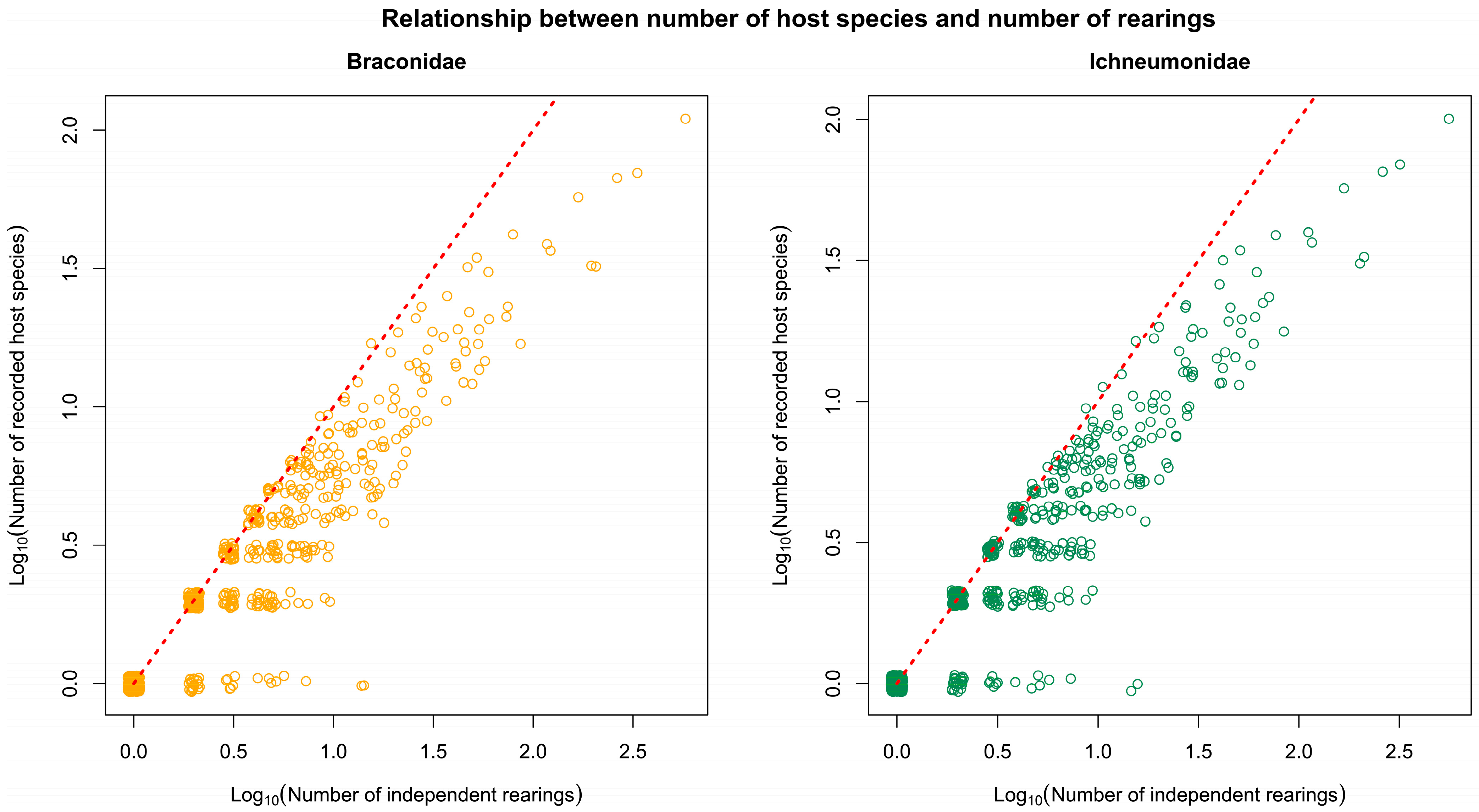
3.13. Comparison of Tachinidae and Ichneumonoidea Hosts
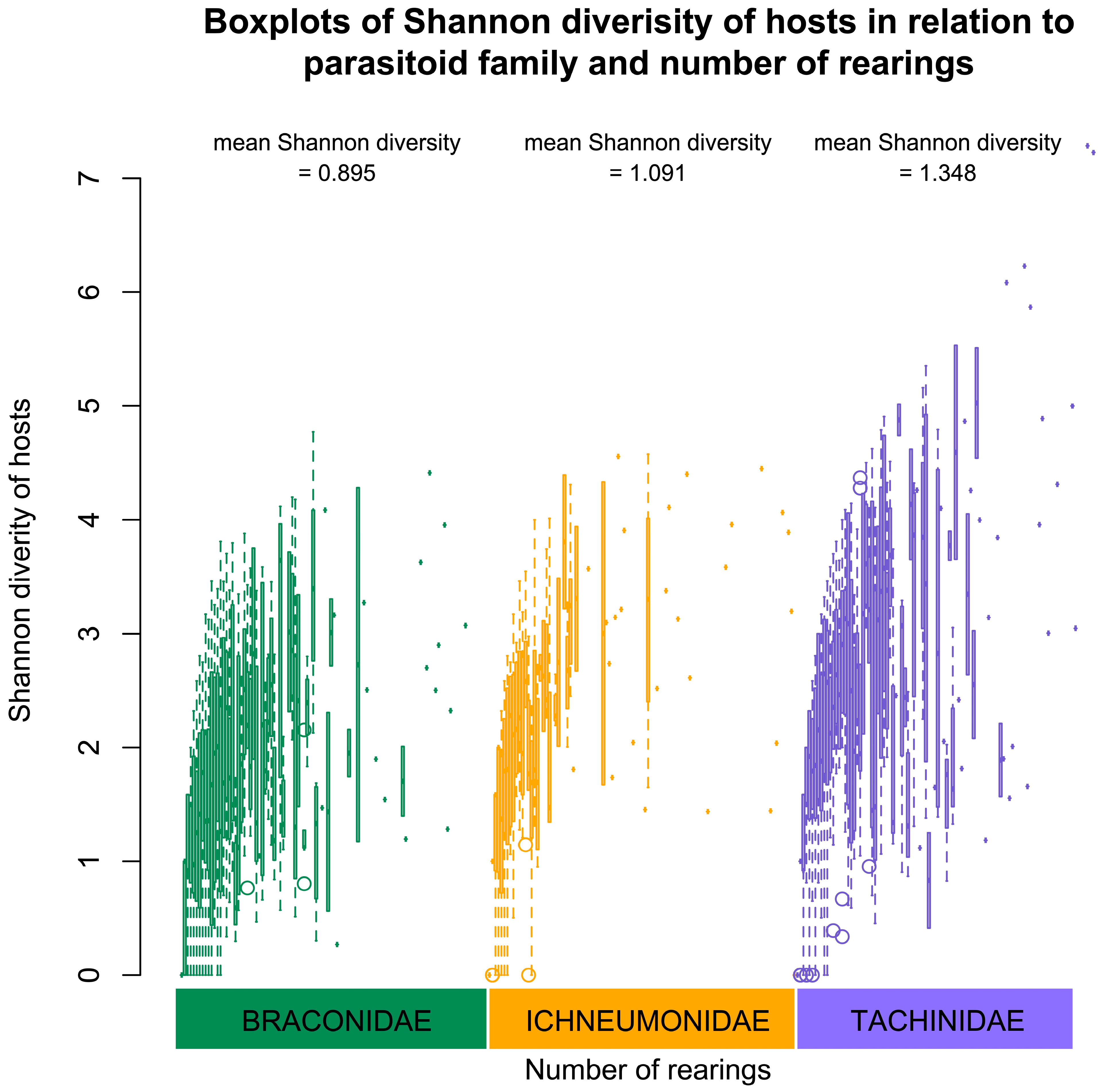
3.14. Ichneumonoidea Host Range and Diversity
4. Discussion
4.1. Completeness of the ACG Dataset
4.2. Are the Hosts of Generalists Also Generalists at ACG?
4.3. Oviposition Strategies
4.4. Comparison of Host Utilisation by Tachinidae, Braconidae and Ichneumonidae
4.5. Numbers and Diversity of Hosts by Tachinidae, Braconidae and Ichneumonidae
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burington, Z.L.; Inclán-Luna, D.J.; Pollet, M.; Stireman, J.O., III. Latitudinal patterns in tachinid parasitoid diversity (Diptera: Tachinidae): A review of the evidence. Insect Conserv. Diver. 2020, 13, 419–431. [Google Scholar] [CrossRef]
- Janzen, D.H. Setting up tropical biodiversity for conservation through non-damaging use: Participation by parataxonomists. J. Appl. Ecol. 2004, 1, 181–187. [Google Scholar] [CrossRef]
- Janzen, D.H. Ecology of dry-forest wildland insects in the Area de Conservación Guanacaste. In Biodiversity Conservation in Costa Rica: Learning the Lessons in a Seasonal Dry Forest; Frankie, G.W., Mata, A., Vinson, S.B., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 80–96. [Google Scholar]
- Janzen, D.H.; Hallwachs, W. Biodiversity conservation history and future in Costa Rica: The case of Área de Conservación Guanacaste (ACG). In Costa Rican Ecosystems; Kappelle, M., Ed.; The University of Chicago Press: Chicago, IL, USA; London, UK, 2016; pp. 290–314. [Google Scholar]
- Janzen, D.H.; Hallwachs, W. Área de Conservación Guanacaste, northwestern Costa Rica: Converting a tropical national park to conservation via biodevelopment. Biotropica 2020, 52, 1017–1029. [Google Scholar] [CrossRef]
- Quicke, D.L.J.; Janzen, D.H.; Hallwachs, W.; Sharkey, M.J.; Hebert, P.D.N.; Butcher, B.A. Forty-five years of caterpillar rearing in Area de Conservación Guanacaste (ACG) Northwestern Costa Rica: DNA barcodes, BINs, and a first description of plant–caterpillar–ichneumonoid interactions detected. Diversity 2024, 16, 683. [Google Scholar] [CrossRef]
- McDade, L.A.; Hartshorn, G.S. Introduction to the La Selva Biological Station. In La Selva: Ecology and Natural History of a Neotropical Rain Forest; McDade, L.A., Bawa, K.S., Hespenheide, H., S Hartshorn, G.S., Eds.; University of Chicago Press: Chicago, IL, USA, 1994; pp. 6–14. [Google Scholar]
- Gentry, G.L.; Dyer, L.A. On the conditional nature of Neotropical caterpillar defenses against their natural enemies. Ecology 2002, 83, 3108–3119. [Google Scholar] [CrossRef]
- Hespenheide, H.A. Introduction to the natural history and interaction diversity of neotropical plant-caterpillar-parasitoid systems. Ann. Entomol. Soc. Am. 2011, 104, 1029–1032. [Google Scholar] [CrossRef]
- O’Hara, J.E. Tachinid flies (Diptera: Tachinidae). In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 3675–3686. [Google Scholar]
- O’Hara, J.E.; Henderson, S.J. World Genera of the Tachinidae (Diptera) and Their Regional Occurrence. Version 11.0. PDF Document, 90p. 2020. Available online: https://www.uoguelph.ca/nadsfly/Tach/WorldTachs/Genera/Worldgenera.htm (accessed on 12 August 2025).
- Pohjoismäki, J.L.O.; Kahanpää, J.; Mutanen, M. DNA barcodes for the Northern European tachinid flies (Diptera: Tachinidae). PLoS ONE 2016, 11, e0164933. [Google Scholar] [CrossRef]
- O’Hara, J.E. History of tachinid classification (Diptera, Tachinidae). ZooKeys 2013, 316, 1. [Google Scholar] [CrossRef] [PubMed]
- Stireman III, J.O.; Cerretti, P.; O’Hara, J.E.; Blaschke, J.D.; Moulton, J.K. Molecular phylogeny and evolution of world Tachinidae (Diptera). Mol. Phylog. Evol. 2019, 139, 106358. [Google Scholar] [CrossRef]
- Smith, M.A.; Woodley, N.E.; Janzen, D.; Hallwachs, W.; Hebert, P.D.N. DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proc. Natl Acad. Sci. USA 2006, 103, 3657–3662. [Google Scholar] [CrossRef]
- Smith, M.A.; Wood, D.M.; Janzen, D.H.; Hallwachs, W.; Hebert, P.D.N. DNA barcodes affirm that 16 species of apparently generalist tropical parasitoid flies (Diptera, Tachinidae) are not all generalists. Proc. Natl Acad. Sci. USA 2007, 104, 4967–4972. [Google Scholar] [CrossRef]
- Janzen, D.H.; Hallwachs, W. Joining inventory by parataxonomists with DNA barcoding of a large complex tropical conserved wildland in northwestern Costa Rica. PLoS ONE 2011, 6, e18123. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.J.; Wood, D.M.; Smith, M.A.; Hallwachs, W.; Janzen, D.H. Revision of the New World species of Houghia Coquillett (Diptera, Tachinidae) reared from caterpillars in Area de Conservación Guanacaste, Costa Rica. Zootaxa 2014, 3858, 1–90. [Google Scholar] [CrossRef]
- Fleming, A.J.; Wood, D.M.; Smith, M.A.; Dapkey, T.; Hallwachs, W.; Janzen, D.H. Five new species of Vibrissina Rondani, 1861 (Diptera: Tachinidae) from Area de Conservación Guanacaste in Northwestern Costa Rica. Biodiv. Data J. 2017, 5, e10967. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.J.; Wood, D.M.; Smith, M.A.; Hallwachs, W. and Janzen, D.H. A new species of Voria Robineau-Desvoidy (Diptera: Tachinidae) from Area de Conservación Guanacaste in Northwestern Costa Rica. Biodiv. Data J. 2017, 5, e20123. [Google Scholar]
- Fleming, A.J.; Wood, D.M.; Smith, M.A.; Hallwachs, W.; Janzen, D.H. Twenty-two new species in the genus Hyphantrophaga Townsend (Diptera: Tachinidae) from Area de Conservación Guanacaste, with a key to the species of Mesoamerica. Biodiv. Data J. 2019, 7, e29553. [Google Scholar] [CrossRef]
- Fleming, A.J.; Woodley, N.; Smith, M.A.; Hallwachs, W.; Janzen, D.H. Revision of Belvosia Robineau-Desvoidy (Diptera, Tachinidae) and 33 new species from Area de Conservación Guanacaste in northwestern Costa Rica with a key to known North and Mesoamerican species. Biodiver. Data J. 2023, 11, e103667. [Google Scholar]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the Neotropical skipper butterfly Astraptes fulgerator. Proc. Natl Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Burns, J.M.; Janzen, D.H. Pan-neotropical genus Venada (Hesperiidae: Pyrginae) is not monotypic: Four new species occur on one volcano in the Area de Conservación Guanacaste, Costa Rica. J. Lepid. Soc. 2005, 59, 19–34. [Google Scholar]
- Solis, M.A.; Davis, D.R.; Nishida, K. Biology and systematics of Albusambia elaphaglossumae, a new genus and species of Crambidae (Lepidoptera: Pyraloidea: Crambidae) mining the fronds of Elaphoglossum conspersum (Pteridophyta: Lomariopsidaceae) in Costa Rica. Int. J. Tropic. Biol. 2005, 53, 487–501. [Google Scholar]
- Burns, J.M.; Janzen, D.H.; Hallwachs, W. Of many similar species in the Neotropical genus Porphyrogenes (Lepidoptera: Hesperiidae), a new one, repeatedly reared in Costa Rica, is relatively distinct. Proc. Entomol. Soc. Wash. 2010, 112, 32–42. [Google Scholar] [CrossRef]
- Chacón, I.A.; Janzen, D.H.; Hallwachs, W.; Sullivan, J.B.; Hajibabaei, M. Cryptic species within cryptic moths: New species of Dunama Schaus (Notodontidae, Nystaleinae) in Costa Rica. In: Schmidt BC, Lafontaine JD (Eds) Contributions to the systematics of New World macro-moths IV. ZooKeys 2013, 264, 11–45. [Google Scholar] [CrossRef]
- Grishin, N.V.; Burns, J.M.; Janzen, D.H.; Hallwachs, W.; Hajibabaei, M. Oxynetra: Facies and DNA barcodes point to a new species from Costa Rica (Hesperiidae: Pyrginae: Pyrrhopygini). J. Lepid. Soc. 2013, 67, 1–14. [Google Scholar] [CrossRef]
- Solis, M.A.; Phillips-Rodríguez, E.; Hallwachs, W.; Dapkey, T.; Janzen, D.H. Asturodes Amsel (Lepidoptera: Crambidae: Spilomelinae): Three new species from the Western Hemisphere and food plant records from Area de Conservación Guanacaste, Costa Rica. Proc. Entomol. Soc. Wash. 2020, 122, 147–171. [Google Scholar] [CrossRef]
- Phillips-Rodríguez, E.; Brown, J.W.; Hallwachs, W.; Janzen, D.H. Chlamydastis Meyrick of Costa Rica: Barcodes, biology, and descriptions of 36 new species (Lepidoptera: Depressariidae). Insecta Mundi 2021, 868, 1–96. [Google Scholar]
- Janzen, D.H. Lumpy integration of tropical wild biodiversity with its society. In A New Century of Biology; Kress, W.J., Barrett, G.W., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2001; pp. 133–148. [Google Scholar]
- Janzen, D.H.; Hallwachs, W. DNA barcoding the Lepidoptera inventory of a large complex tropical conserved wildland, Area de Conservacion Guanacaste, northwestern Costa Rica. Genome 2016, 59, 641–660. [Google Scholar] [CrossRef]
- Crosskey, R.W. The immature stages and affinities of the tachinid fly Glaurocara flava, a parasite of the African bush-cricket Homorocoryphus nitidulus Vicinus. In Proceedings of the Zoological Society of London; Blackwell Publishing Ltd.: Oxford, UK, 1965; Volume 144, pp. 203–218. [Google Scholar]
- Bratti, A.; Gardenghi, G.; Miglioli, G. Behavior and growth rate of Archytas marmoratus (Town.) (Diptera: Tachinidae) planidia in larvae of Galleria mellonella L. (Lepidoptera Galleriidae). Boll. Ist. Entomol. Univ. Bologna 1993, 47, 141–149. [Google Scholar]
- Dindo, M.L.; Nakamura, S. Oviposition strategies of tachinid parasitoids: Two Exorista species as case studies. Int. J. Insect Sci. 2018, 10, 1179543318757491. [Google Scholar] [CrossRef] [PubMed]
- Stireman, J.O., III; Dyer, L.A.; Greeney, H.F. Specialised generalists? Food web structure of a tropical tachinid-caterpillar community. Insect Conserv. Divers. 2017, 10, 367–384. [Google Scholar] [CrossRef]
- Mellini, E. Sinossi di biologia dei ditteri larvevoridi (studi sui ditteri larvevoridi. L Contributo). Boll. Ist. Ent. ‘G. Grandi’ Univ. Bologna 1991, 45, 1–38. [Google Scholar]
- Wood, D.M. Tachinidae. In Manual of Nearctic Diptera Monograph No. 28; McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., Eds.; Research–branch, Agriculture Canada: Ottawa, ON, Canada, 1987; pp. 1193–1269. [Google Scholar]
- Belshaw, R. Life history characteristics of Tachinidae (Diptera) and their effect on polyphagy. In Parasitoid Community Ecology; Hawkins, B.A., Sheehan, W., Eds.; Oxford University Press: Oxford, UK, 1994; pp. 145–162. [Google Scholar]
- Stireman, J.O., III; Singer, M.S. Determinants of parasitoid-host associations: Insights from a natural tachinid-lepidopteran community. Ecology 2003, 84, 296–310. [Google Scholar] [CrossRef]
- Stireman, J.O., III; Singer, M.S. What determines host range in parasitoids? An analysis of a tachinid parasitoid community. Oecologia 2003, 135, 629–638. [Google Scholar] [CrossRef]
- Stireman, J.O., III; Dyer, L.A.; Janzen, D.H.; Singer, M.S.; Lill, J.T.; Marquis, R.J.; Ricklefs, R.E.; Gentry, G.L.; Hallwachs, W.; Coley, P.D.; et al. Climatic unpredictability and parasitism of caterpillars: Implications of global warming. Proc. Natl. Acad. Sci. USA 2005, 102, 17384–17387. [Google Scholar] [CrossRef]
- Stireman, J.O.; O’Hara, J.E.; Wood, D.M. Tachinidae: Evolution, behavior, and ecology. Annu. Rev. Entomol. 2006, 51, 525–555. [Google Scholar] [CrossRef]
- Askew, R.R. Parasitic Insects; Heinemann: London, UK, 1971; 316p. [Google Scholar]
- Gauthier, J.; Boulain, H.; van Vugt, J.J.F.A.; Baudry, L.; Persyn, E.; Aury, J.M.; Noel, B.; Bretaudeau, A.; Legeai, F.; Warris, S.; et al. Chromosomal scale assembly of parasitic wasp genome reveals symbiotic virus colonization. Commun. Biol. 2021, 4, 104. [Google Scholar] [CrossRef]
- Santos, B.F.; Klopfstein, S.; Whitfield, J.B.; Sharanowski, B.J. Many evolutionary roads led to virus domestication in ichneumonoid parasitoid wasps. Curr. Opin. Insect Sci. 2022, 50, 100861. [Google Scholar] [CrossRef] [PubMed]
- Serbielle, C.; Dupas, S.; Perdereau, E.; Héricourt, F.; Dupuy, C.; Huguet, E.; Drezen, J.M. Evolutionary mechanisms driving the evolution of a large polydnavirus gene family coding for protein tyrosine phosphatases. BMC Evol. Biol. 2012, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Coffman, K.; Kauwe, A.; Gillette, N.; Burke, G.; Geib, S. Host range of a parasitoid wasp is linked to host susceptibility to its mutualistic viral symbiont. Mol. Ecol. 2024, 33, e17485. [Google Scholar] [CrossRef] [PubMed]
- Moreau, S.J.; Marchal, L.; Boulain, H.; Musset, K.; Labas, V.; Tomas, D.; Gauthier, J.; Drezen, J.-M. Multi-omic approach to characterize the venom of the parasitic wasp Cotesia congregata (Hymenoptera: Braconidae). BMC Genom. 2025, 26, 431. [Google Scholar] [CrossRef]
- Stireman, J.O., III. Learning in the generalist tachinid parasitoid Exorista mella Walker (Diptera: Tachinidae). J. Insect Behav. 2002, 15, 689–706. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Kainoh, Y. Visual recognition of the host in the parasitoid fly Exorista japonica. Zool. Sci. 2005, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, R.T.; Kainoh, Y.; Yamawaki, Y.; Nakamura, S. The parasitoid fly Exorista japonica uses visual and olfactory cues to locate herbivore-infested plants. Entomol. Exp. Applic. 2011, 138, 175–183. [Google Scholar] [CrossRef]
- Nettles, W.C.; Burks, M.L. A substance from Heliothis virescens larvae stimulating larviposition by females of the tachinid Archytas marmoratus. J. Insect Physiol. 1975, 21, 965–978. [Google Scholar] [CrossRef]
- Loxdale, H.D.; Lushai, G.; Harvey, J.A. The evolutionary improbability of “generalism” in nature, with special reference to insects. Biol. J. Linn. Soc. 2011, 103, 1–18. [Google Scholar] [CrossRef]
- Sharkey, M.J.; Baker, A.; McCluskey, K.; Smith, A.; Naik, S.; Ratnasingham, S.; Manjunath, R.; Perez, K.; Sones, J.; D’souza, M.; et al. Minimalist revision of Mesochorus Gravenhorst, 1829 (Hymenoptera: Ichneumonidae: Mesochorinae) from Área de Conservación Guanacaste, Costa Rica, with 158 new species and host records for 129 species. Rev. Biol. Trop. 2023, 71, e53316. [Google Scholar] [CrossRef]
- Zuñiga, R.; Valerio, A.A.; Hanson, P.; Smith, M.A.; Hallwachs, W.; Janzen, D.H. Cryptic diversity of Leurus wasps (Hymenoptera: Ichneumonidae: Metopiinae), parasitoids of caterpillars in Area de Conservación Guanacaste, Costa Rica. Zootaxa 2024, 5529, 511–531. [Google Scholar] [CrossRef] [PubMed]
- Kärrnäs, E.; Hansson, C.; Wahlberg, N. Large-scale DNA barcoding reveals cryptic diversity in eulophid wasps (Hymenoptera, Chalcidoidea, Eulophidae). Arthrop. Syst. Phylog. 2025, 83, 415–425. [Google Scholar] [CrossRef]
- Armstrong, A.L.; Sones, J.E.; Lohrmann, V.; Hebert, P.D.N.; Janzen, D.H.; Hallwachs, W.; Blaschke, J.D. Six in one: Cryptic species and a new host record for Olixon Cameron (Rhopalosomatidae, Hymenoptera) revealed by DNA barcoding. J. Hym. Res. 2024, 97, 363–378. [Google Scholar] [CrossRef]
- Burns, J.M.; Janzen, D.H.; Hajibabaei, M.; Hallwachs, W.; Hebert, P.D.N. DNA barcodes and cryptic species of skipper butterflies in the genus Perichares in Area de Conservacion Guanacaste, Costa Rica. Proc. Natl. Acad. Sci. USA 2008, 105, 6350–6355. [Google Scholar] [CrossRef]
- Brown, J.W.; Janzen, D.H.; Hallwachs, W. A food plant specialist in Sparganothini: A new genus and species from Costa Rica (Lepidoptera, Tortricidae). ZooKeys 2013, 303, 53–63. [Google Scholar] [CrossRef]
- Heikkilä, M.; Metz, M.A.; Hallwachs, W.; Janzen, D.H. Three new species of Rectiostoma Becker, 1982 (Lepidoptera: Gelechioidea: Depressariidae) from Area De Conservación Guanacaste, Northwestern Costa Rica. Proc. Entomol. Soc. Wash 2017, 119, 47–62. [Google Scholar] [CrossRef]
- Janzen, D.H.; Burns, J.M.; Cong, Q.; Hallwachs, W.; Dapkey, T.; Manjunath, R.; Hajibabaei, M.; Hebert, P.D.; Grishin, N.V. Nuclear genomes distinguish cryptic species suggested by their DNA barcodes and ecology. Proc. Natl. Acad. Sci. USA 2017, 114, 8313–8318. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.A.; Hallwachs, W.; Janzen, D.H. Four new gelechioid species to honor Costa Rica’s conservation of wild biodiversity (Lepidoptera). Zootaxa 2020, 4810, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Zeegers, T.; Mutanen, M.; Pohjoismäki, J. The thin red line between species–genomic differentiation of Gymnosoma Meigen, a taxonomically challenging genus of parasitoid flies (Diptera: Tachinidae). Syst. Ent. 2021, 46, 96–110. [Google Scholar] [CrossRef]
- Janzen, D.H.; Hallwachs, W. Caterpillar Rearing Voucher Databases for the Area de Conservación in Northwestern Costa Rica. Available online: http://janzen.sas.upenn.edu/caterpillars/database.htm (accessed on 28 June 2024).
- Janzen, D.H.; Hallwachs, W. To us insectometers, it is clear that insect decline in our Costa Rican tropics is real, so let’s be kind to the survivors. Proc. Natl. Acad. Sci. USA 2021, 118, e2002546117. [Google Scholar] [CrossRef]
- Salcido, D.M.; Forister, M.L.; Garcia Lopez, H.; Dyer, L.A. Loss of dominant caterpillar genera in a protected tropical forest. Sci. Rep. 2020, 10, 422. [Google Scholar] [CrossRef]
- Herrera, W. Climate of Costa Rica. In Costa Rican Ecosystems; Kappelle, M., Ed.; The University of Chicago Press: Chicago, IL, USA, 2016; pp. 19–29. [Google Scholar]
- Smith, M.A. Temperature, Precipitation and Soil Characteristics of Volcan Cacao—Area de Conservacion Guanacaste (ACG), Costa Rica. 2023. Available online: https://dataverse.org/best-practices/data-citation (accessed on 14 August 2025).
- Basset, Y.; Novotny, V.; Miller, S.E.; Pyle, R. Quantifying biodiversity: Experience with parataxonomists and digital photography in Papua New Guinea and Guyana. BioScience 2000, 50, 899–908. [Google Scholar] [CrossRef]
- Basset, Y.; Novotny, V.; Miller, S.E.; Weiblen, G.D.; Missa, O.; Stewart, A.J. Conservation and biological monitoring of tropical forests: The role of parataxonomists. J. Appl. Ecol. 2004, 41, 163–174. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Ivanova, N.V.; Dewaard, J.R.; Hebert, P.D.N. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molec. Ecol. Notes 2006, 6, 998–1002. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Smith, M.A.; Eveleigh, E.S.; McCann, K.S.; Merilo, M.T.; McCarthy, P.C.; Van Rooyen, K.I.; Romanuk, T.N. Barcoding a quantified food web: Crypsis, concepts, ecology and hypotheses. PLoS ONE 2011, 6, e14424. [Google Scholar] [CrossRef]
- Prieto, C.; Faynel, C.; Robbins, R.; Hausmann, A. Congruence between morphology-based species and Barcode Index Numbers (BINs) in Neotropical Eumaeini (Lycaenidae). PeerJ 2021, 9, e11843. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna. 2016. Available online: https://cran.r-project.org (accessed on 30 July 2024).
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Wright, K. _pals: Color Palettes, Colormaps, and Tools to Evaluate Them_. R Package Version 1.9. 2024. Available online: https://CRAN.R-project.org/package=pals (accessed on 4 September 2025).
- Ligges, U.; Mächler, M. Scatterplot3d—an R package for visualizing multivariate data. J. Statist. Softw. 2003, 8, 1–20. [Google Scholar] [CrossRef]
- Bersier, L.F.; Banasĕk-Richter, C.; Cattin, M.F. Quantitative descriptors of food-web matrices. Ecology 2002, 83, 2394–2407. [Google Scholar] [CrossRef]
- Lewis, O.T.; Memmott, J.; Lasalle, J.; Lyal, C.H.C.; Whitefoord, C.; Godfray, H.C.J. Structure of a diverse tropical forest insect–parasitoid community. J. Anim. Ecol. 2002, 71, 855–873. [Google Scholar] [CrossRef]
- Gilbert, A.J. Connectance indicates the robustness of food webs when subjected to species loss. Ecol. Indic. 2009, 9, 72–80. [Google Scholar] [CrossRef]
- Chao, A. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-7. 2020. Available online: http://cran.r-project.org/package=vegan (accessed on 15 August 2025).
- Quicke, D.L.J.; Brown, A.; Hajibabaei, M.; Manjunath, R.; Naik, S.; Ratnasingham, S.; Sones, J.; Jacques, B.S.; Smith, M.A.; Zamora, N.; et al. Caterpillar diet breadth in Area de Conservación Guanacaste (ACG), a large and diverse Neotropical wildland in northwestern Costa Rica: Toxins, silica, aluminium and sclerophylly. Front. Ecol. Evol. 2025, 13, 1647436. [Google Scholar]
- Shaw, M.R. Parasitoid host ranges. In Parasitoid Community Ecology; Hawkins, B.A., Sheehan, W., Eds.; Oxford University Press: Oxford, UK, 1994; pp. 111–144. [Google Scholar]
- Marohasy, J. The design and interpretation of host-specificity tests for weed biological control with particular reference to insect behaviour. Biocont. News Inform. 1998, 19, 13N–20N. [Google Scholar]
- Novotny, V.; Basset, Y.; Miller, S.E.; Drozd, P.; Cizek, L. Host specialisation of leaf chewing insects in a New Guinea rainforest. J. Anim. Ecol. 2002, 71, 400–412. [Google Scholar] [CrossRef]
- Janzen, D.H. An abandoned field is not a tree fall gap. Vida Silv. Neotrop. 1990, 2, 64–67. [Google Scholar]
- Smith, M.A.; Rodriguez, J.J.; Whitfield, J.B.; Deans, A.R.; Janzen, D.H.; Hallwachs, W.; Hebert, P.D. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc. Natl. Acad. Sci. USA 2008, 105, 12359–12364. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, R.; Stone, G.N.; Hearn, J.; Lohse, K.; Roslin, T. Revealing secret liaisons: DNA barcoding changes our understanding of food webs. Ecol. Ent. 2010, 35, 623–638. [Google Scholar] [CrossRef]
- Hrček, J.; Godfray, H.C.J. What do molecular methods bring to host–parasitoid food webs? Trends Parasitol. 2015, 31, 30–35. [Google Scholar] [CrossRef]
- Sheikh, S.I.; Ward, A.K.; Zhang, Y.M.; Davis, C.K.; Zhang, L.; Egan, S.P.; Forbes, A.A. Ormyrus labotus Walker (Hymenoptera: Ormyridae): Another generalist that should not be a generalist is not a generalist. Insect Syst. Diver. 2022, 6, 1–14. [Google Scholar]
- Dyer, L.A.; Wagner, D.L.; Greeney, H.F.; Smilanich, A.M.; Massad, T.J.; Robinson, M.L.; Fox, M.S.; Hazen, R.F.; Glassmire, A.E.; Pardikes, N.A.; et al. Novel insights into tri- trophic interaction diversity and chemical ecology using 16 years of volunteer-supported research. Am. Entomol. 2012, 58, 15–19. [Google Scholar] [CrossRef][Green Version]
- Dyer, L.A.; Singer, M.S.; Lill, J.T.; Stireman, J.O.; Gentry, G.L.; Marquis, R.J.; Ricklefs, R.E.; Greeney, H.F.; Wagner, D.L.; Morais, H.C.; et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature 2007, 448, 696–699. [Google Scholar] [CrossRef]
- Levin, D.A.; York, B.M., Jr. The toxicity of plant alkaloids: An ecogeographic perspective. Bioch. Syst. Ecol. 1978, 6, 61–76. [Google Scholar] [CrossRef]
- Gauld, I.D.; Gaston, K.J.; Janzen, D.H. Plant allelochemicals, tritrophic interactions and the anomalous diversity of tropical parasitoids: The “nasty” host hypothesis. Oikos 1992, 65, 353–357. [Google Scholar] [CrossRef]
- Coley, P.D.; Barone, J.A. Herbivory and plant defenses in tropical forests. Ann. Rev. Ecol. Syst. 1996, 27, 305–335. [Google Scholar] [CrossRef]
- Moles, A.T.; Bonser, S.P.; Poore, A.G.; Wallis, I.R.; Foley, W.J. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct. Ecol. 2011, 25, 380–388. [Google Scholar] [CrossRef]
- Eggleton, P.; Gaston, K.J. Tachinid host ranges: A reappraisal (Diptera: Tachinidae). Entomol. Gaz. 1992, 43, 139–143. [Google Scholar]
- Memmott, J.; Godfray, H.C.J.; Gauld, I.D. The structure of a tropical host parasitoid community. J. Anim. Ecol. 2024, 63, 521–540. [Google Scholar] [CrossRef]
- Dyer, L.A. Tasty generalists and nasty specialists? Antipredator mechanisms in tropical lepidopteran larvae. Ecology 1995, 76, 1483–1496. [Google Scholar] [CrossRef]
- Farkas, T.E.; Singer, M.S. Can caterpillar density or host-plant quality explain host-plant-related parasitism of a generalist forest caterpillar assemblage? Oecologia 2013, 173, 971–983. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of herbivore-induced plant odours by host seeking parasitic wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; pp. 1–473. [Google Scholar]
- Degenhardt, J.; Gershenzon, J.; Baldwin, I.T.; Kessler, A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotech. 2003, 14, 169–176. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Lewis, W.J.; Pare, P.W.; Alborn, H.T.; Tumlinson, J.H. Herbivore-infested plants selectively attract parasitoids. Nature 1998, 393, 570–573. [Google Scholar] [CrossRef]
- Slinn, H.L.; Richards, L.A.; Dyer, L.A.; Hurtado, P.; Smilanich, A.M. Across multiple species, phytochemical diversity and herbivore diet breadth have cascading effects on herbivore immunity and parasitism in a tropical model system. Front. Plant Sci. 2018, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Feener, D.H.; Brown, B.V. Diptera as parasitoids. Annu. Rev. Ent. 1996, 42, 73–97. [Google Scholar] [CrossRef]
- Mondor, E.B.; Roland, J. Host locating behaviour of Leschenaultia exul and Patelloa pachypyga: Two tachinid parasitoids of the forest tent caterpillar, Malacosoma disstria. Entomol. Exp. Applic. 1997, 85, 161–168. [Google Scholar] [CrossRef]
- Stireman, J.O., III. Host location and selection cues in a generalist tachinid parasitoid. Entomol. Exp. Applic. 2002, 103, 23–34. [Google Scholar] [CrossRef]
- Ichiki, R.T.; Ho, G.T.; Wajnberg, E.; Kainoh, Y.; Tabata, J.; Nakamura, S. Different uses of plant semiochemicals in host location strategies of the two tachinid parasitoids. Naturwissenschaften 2012, 99, 687–694. [Google Scholar] [CrossRef]
- Nakamura, S.; Ichiki, R.T.; Kainoh, Y. Chemical ecology of tachinid parasitoids. In Chemical Ecology of Insect Parasitoids; Wajnberg, E., Colazza, S., Eds.; Wiley & Sons: Oxford, UK, 2013; pp. 145–167. [Google Scholar]
- Salkeld, E.H. Microtype eggs of some Tachinidae (Diptera). Canad. Entomol. 1980, 112, 51–83. [Google Scholar] [CrossRef]
- Marini, M.; Campadelli, G. Ootaxonomy of Goniini (Diptera Tachinidae) with microtype eggs. Ital. J. Zool. 1994, 61, 271–283. [Google Scholar] [CrossRef]
- Sudta, C.; Salcido, D.M.; Forister, M.L.; Walla, T.R.; Villamarín-Cortez, S.; Dyer, L.A. Jack-of-all-trades paradigm meets long-term data: Generalist herbivores are more widespread and locally less abundant. Ecol. Letts 2022, 25, 948–957. [Google Scholar] [CrossRef]
- Kerkig, P.; Quicke, D.L.J.; Butcher, B.A. Molecular food web of lepidopteran hosts and their parasitoids in a tropical secondary forest in Thailand. Trop. Nat. Hist. 2025, 25, 37–56. [Google Scholar]
- Warne, C.P.; Hallwachs, W.; Janzen, D.H.; Smith, M.A. Functional and genetic diversity changes through time in a cloud forest ant assemblage. Biotropica 2020, 52, 1084–1091. [Google Scholar] [CrossRef]
- Rivers, D.B.; Thompson, C.; Brogan, R. Physiological trade-offs of forming maggot masses by necrophagous flies on vertebrate carrion. Bull. Entomol. Res. 2011, 101, 599–611. [Google Scholar] [CrossRef]
- Rotheray, G.E.; Lyszkowski, R. Diverse mechanisms of feeding and movement in cyclorrhaphan larvae (Diptera). J. Nat. Hist. 2015, 49, 2139–2211. [Google Scholar] [CrossRef]
- Rotheray, G.E. Saprophagy, developing on decay. In Ecomorphology of Cyclorrhaphan Larvae (Diptera); Rotheray, G.E., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 141–173. [Google Scholar]
- Ferrar, P.A. Guide to the Breeding Habits and Immature Stages of Diptera Cyclorrhapha: Volume 1; Brill: Leiden, The Netherlands, 2004; 907p. [Google Scholar]
- Quicke, D.L.J.; Butcher, B.A. Review of venoms of non-polydnavirus carrying ichneumonoid wasps. Biology 2021, 10, 50. [Google Scholar] [CrossRef]
- Whitfield, J. Parasitoids, polydnaviruses and endosymbiosis. Parasitol. Today 1990, 6, 381–384. [Google Scholar] [CrossRef]
- Whitfield, J.B.; O’Connor, J.M. Molecular systematics of wasp and polydnavirus genomes and their coevolution. In Parasitoid Viruses: Symbionts and Pathogens; Beckage, N.E., Drezen, J.-M., Eds.; Elsevier: London, UK, 2012; pp. 89–97. [Google Scholar]
- Strand, M.R.; Burke, G.R. Polydnaviruses as symbionts and gene delivery systems. PLoS Path. 2012, 8, e1002757. [Google Scholar] [CrossRef][Green Version]
- Muller, H.; Chebbi, M.A.; Bouzar, C.; Périquet, G.; Fortuna, T.; Calatayud, P.-A.; Le Ru, B.; Obonyo, J.; Kaiser, L.; Drezen, J.-M. Genome-wide patterns of bracovirus chromosomal integration into multiple host tissues during parasitism. J. Virol. 2021, 95, 10–128. [Google Scholar] [CrossRef]
- Arthur, A.P.; Stainer, J.E.R.; Turnbull, A.L. An interaction between Orgilus obscurator (Nees) (Hymenoptera: Braconidae) and Temelucha interruptor (Grav.) (Hymenoptera: Ichneumonidae), parasites of the pine shoot moth, Rhyacionia buoliana (Schiff.) (Lepidoptera: Olethreutidae). Canad. Entomol. 1964, 96, 1030–1034. [Google Scholar] [CrossRef]
- Guzo, D.; Stoltz, D.B. Obligatory multiparasitism in the tussock moth, Orgyia leucostigma. Parasitol. 1985, 90, 1–10. [Google Scholar] [CrossRef]
- Quicke, D.L.J. The Braconid and Ichneumonid Parasitic Wasps: Biology, Systematics, Evolution and Ecology; Wiley Blackwell: Oxford, UK, 2015; pp. 1–688. [Google Scholar]
- Cusson, M.; Laforge, M.; Régnière, J.; Béliveau, C.; Trudel, D.; Thireau, J.-C.; Bellemare, G.; Keirstead, N.; Stolz, D. Multiparasitism of Choristoneura fumiferana by the ichneumonid Tranosema rostrale and the tachinid Actia interrupta: occurrence in the field and outcome of competition under laboratory conditions. Entomol. Exp. Appl. 2002, 102, 125–133. [Google Scholar] [CrossRef]
- Salt, G. Experimental studies in insect parasitism. XV. The means of resistance of a parasitoid larva. Proc. R. Soc. Lond. B 1970, 176, 105–114. [Google Scholar]
- Norton, W.N.; Vinson, S.B. Encapsulation of a parasitoid egg within its natural host: An ultrastructual investigation. J. Invert. Pathol. 1977, 30, 55–67. [Google Scholar] [CrossRef]
- Quicke, D.L.J. Parasitic Wasps; Chapman & Hall: London, UK, 1997; pp. 1–470. [Google Scholar]
- Salt, G. The defence reactions of insect metazoan parasites. Parasitol. 1963, 53, 527–642. [Google Scholar] [CrossRef]
- Michalková, V.; Valigurová, A.; Dindo, M.L.; Vaňhara, J. Larval morphology and anatomy of the parasitoid Exorista larvarum (Diptera: Tachinidae), with an emphasis on cephalopharyngeal skeleton and digestive tract. J. Parasitol. 2009, 95, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Nakamura, S.; Furukawa, S. Cloak scavenges the reactive oxygen species around the larvae of Drino inconspicuoides (Diptera: Tachinidae). Insects 2023, 14, 602. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, J.; Gu, X.; Chi, F.; Zhao, Y.; Li, F.; Jiang, X.; Li, B.; Wei, J. The parasitoid Exorista sorbillans exploits host silkworm encapsulation to build respiratory funnel for survival. Insect Biochem. Molec. Biol. 2025, 177, 104255. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H.; Hallwachs, W. How a tropical country can DNA barcode itself. iBOL Barcode Bull. 2019, 9, 1–6. [Google Scholar] [CrossRef]




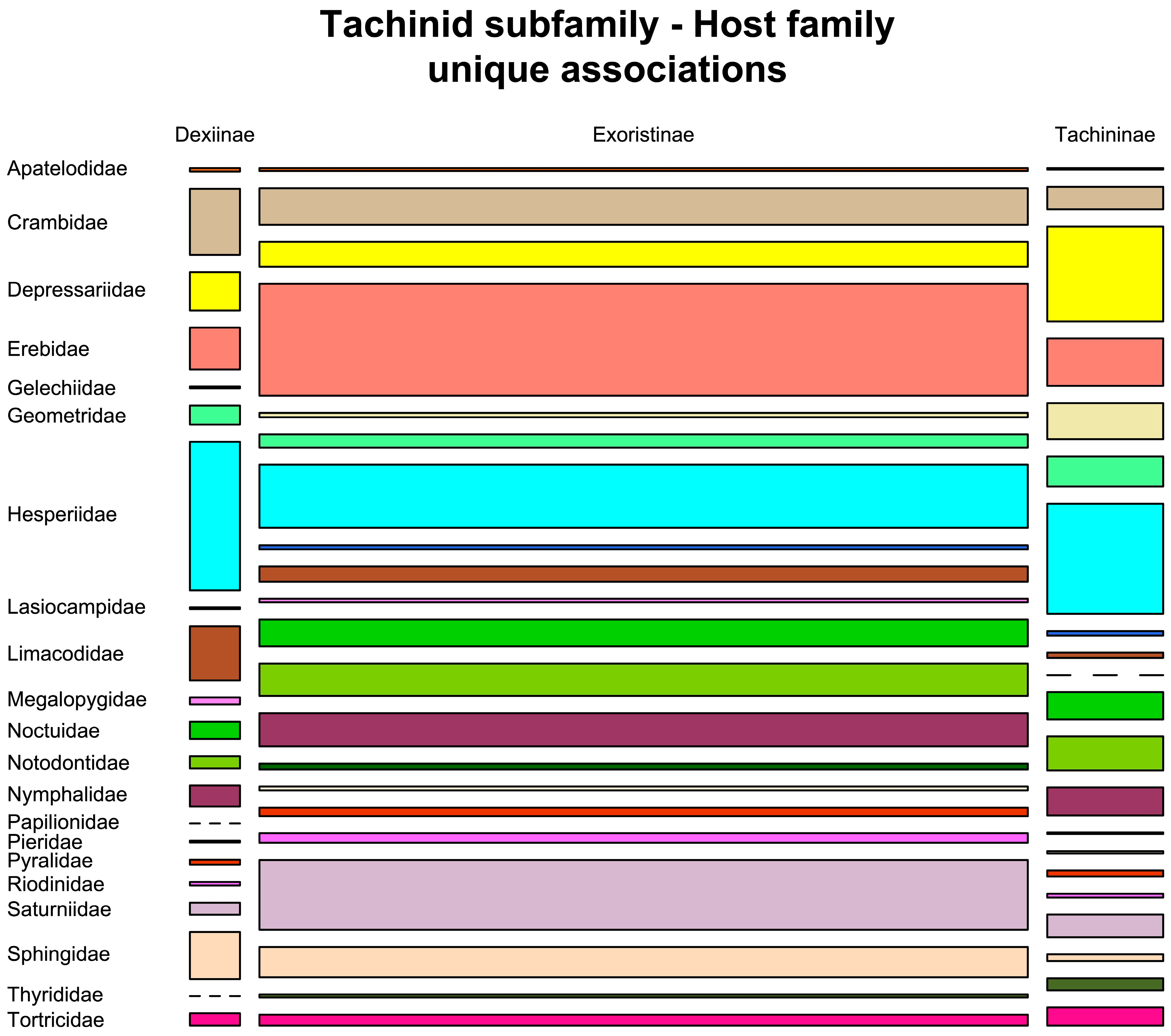
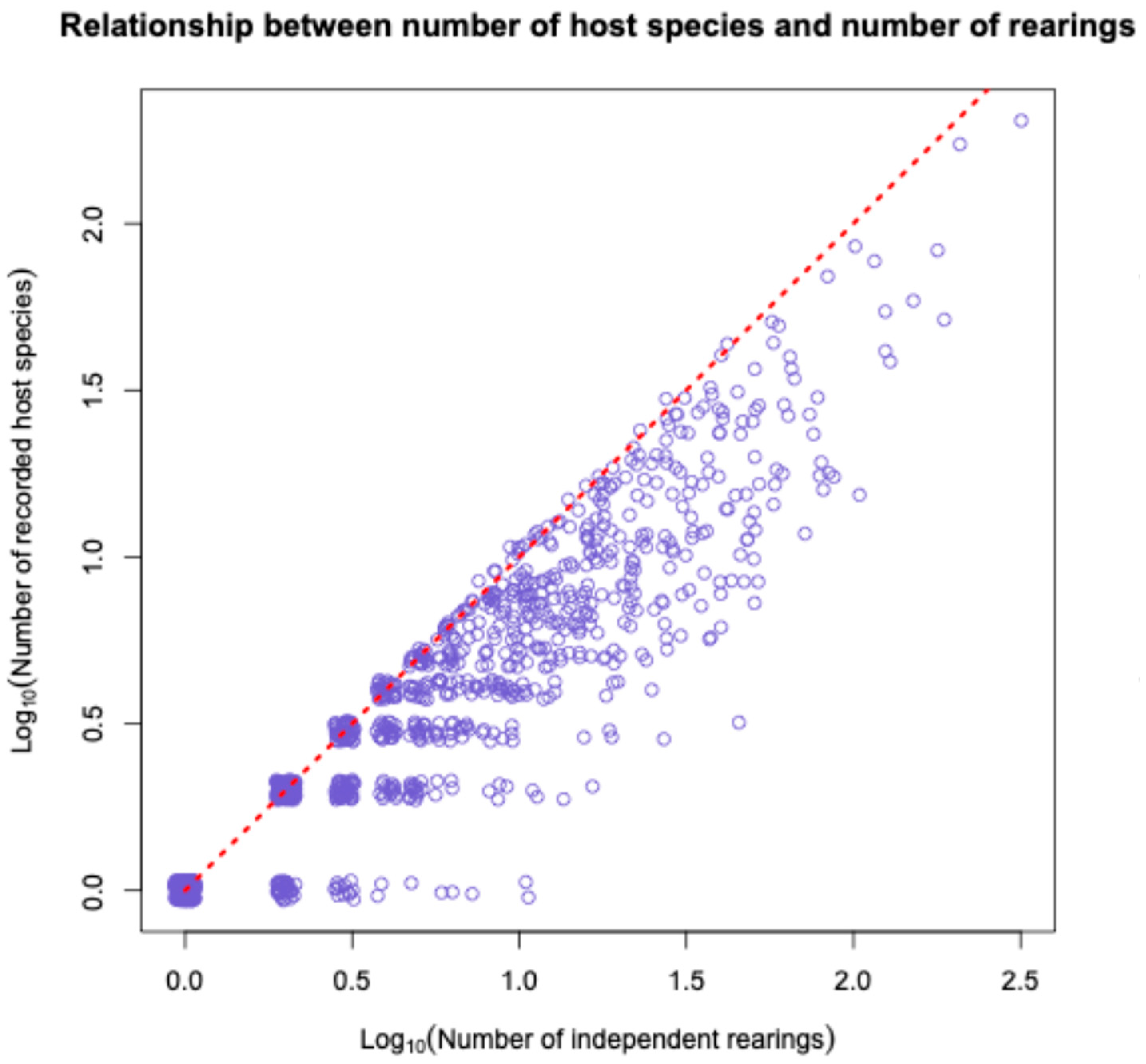
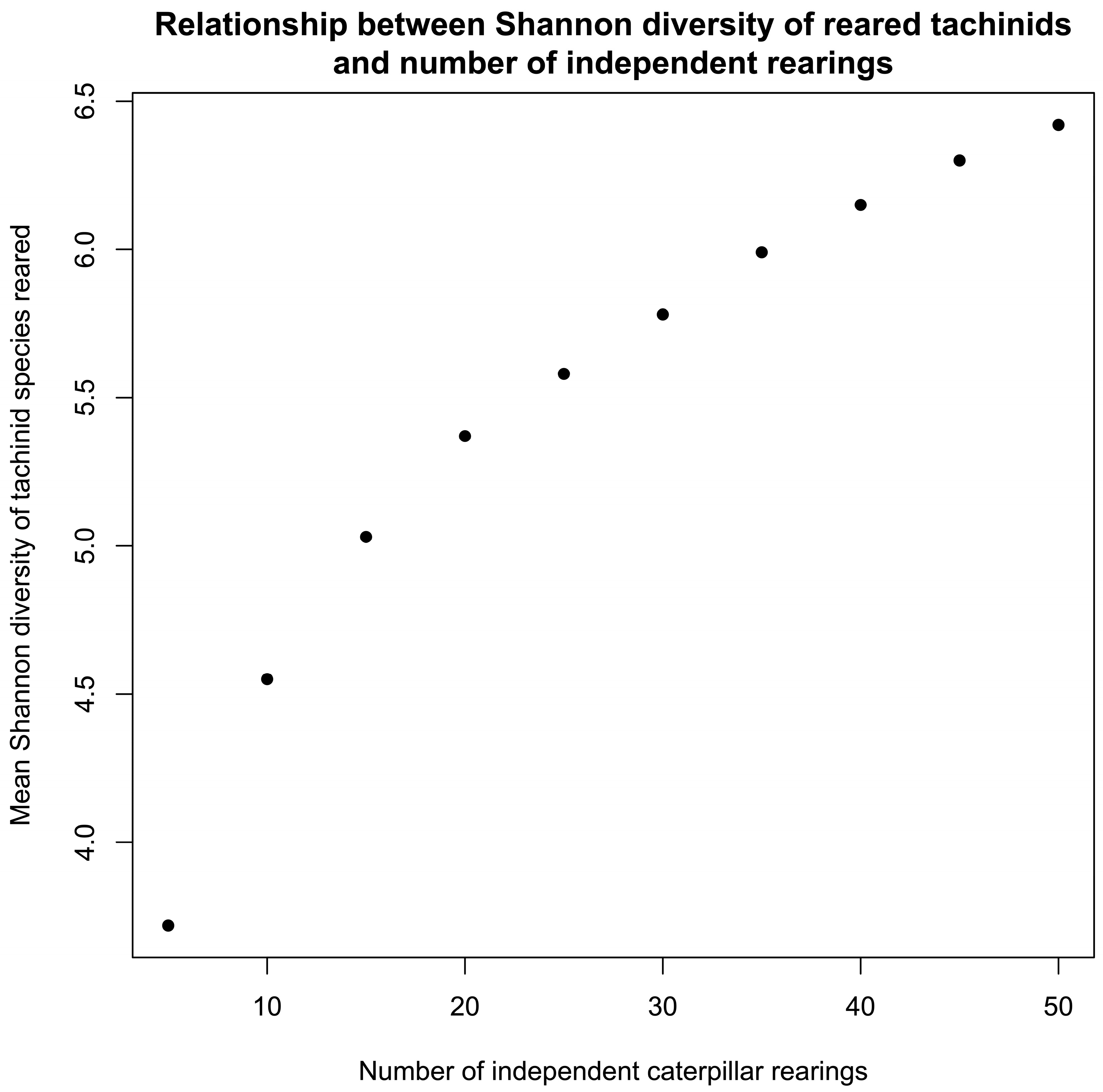
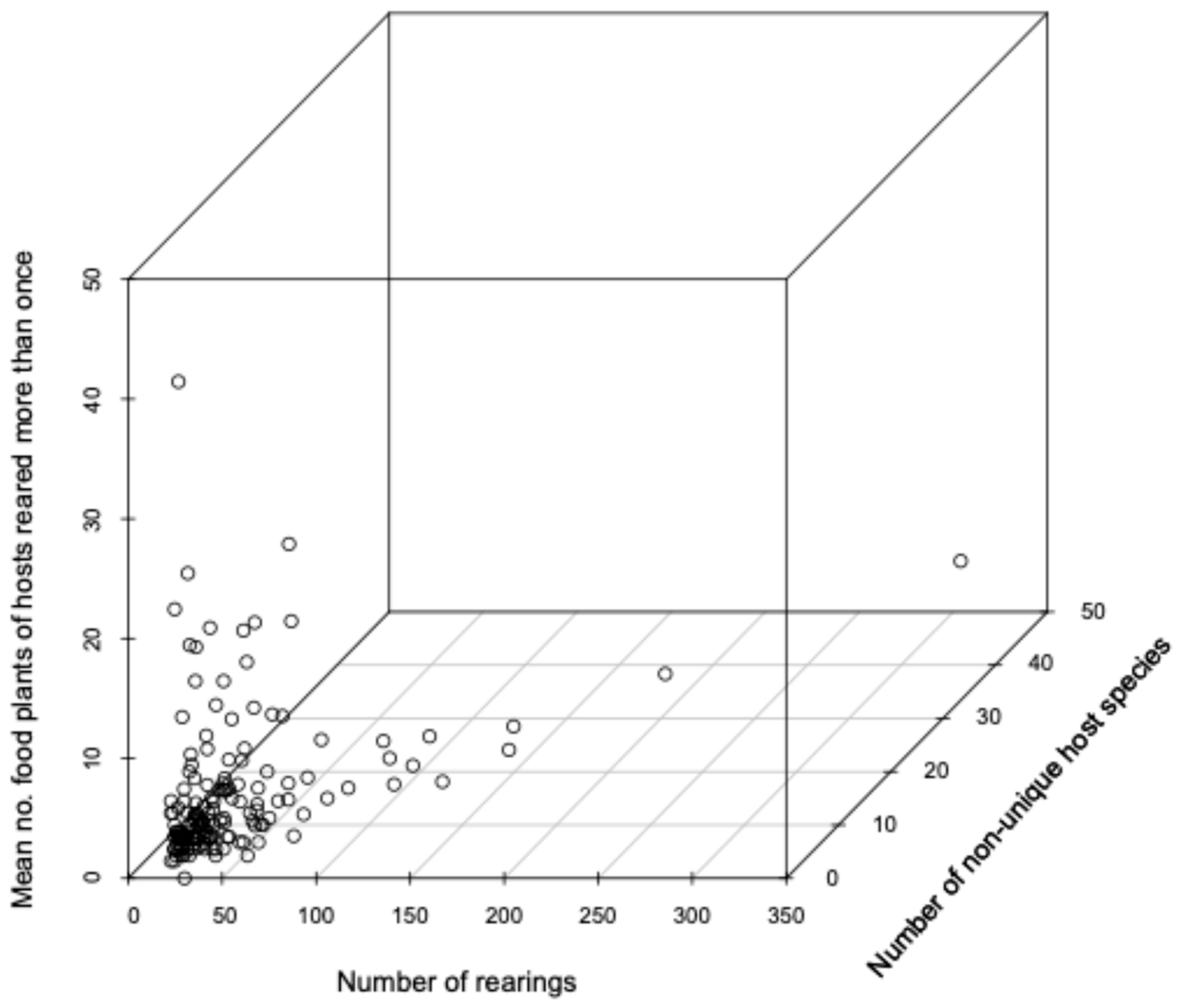


| Subfamily | Number After Data Cleaning | Number of Independent Rearings 1 | Number with BIN | Number of BINs | Host Families | Host Species | Plant Families | Plant Species |
|---|---|---|---|---|---|---|---|---|
| Dexiinae | 1195 | 836 | 535 | 85 | 30 | 314 | 71 | 298 |
| Exoristinae | 16,619 | 10,043 | 6974 | 687 | 43 | 1858 | 133 | 1078 |
| Tachininae | 2264 | 1757 | 1332 | 245 | 34 | 671 | 96 | 498 |
| Overall 2 | 20,078 | 12,636 | 8841 | 1017 | 43 | 2110 | 138 | 1194 |
| Observed | Chao1 Estimate | Multiplier (Completeness) | |
|---|---|---|---|
| Tachinid species | 1206 | 1406 | 1.17 (86%) |
| Caterpillar species | 2110 | 3028 | 1.44 (70%) |
| Food plant species | 1194 | 1313 | 1.18 (91%) |
| Subfamily | Fly–Caterpillar Bitrophic Interactions | Fly–Food Plant Bitrophic Interactions | Tritrophic Interactions |
|---|---|---|---|
| Dexiinae | 388 | 434 | 477 |
| Exoristinae | 5580 | 6064 | 6695 |
| Tachininae | 986 | 1058 | 1151 |
| Overall totals | 6954 | 7556 | 8323 |
| Ecosystem | Caterpillar Species | Tachinid Species | Number of Links | C | RC | LD | Generality |
|---|---|---|---|---|---|---|---|
| All | 2110 | 1206 | 6963 | 1.27 × 10−3 | 2.74 × 10−3 | 2.10 | 5.77 |
| Dry forest | 718 | 608 | 1750 | 1.99 × 10−3 | 4.01 × 10−3 | 1.32 | 2.88 |
| Rain forest | 1654 | 1021 | 5156 | 1.44 × 10−3 | 3.06 × 10−3 | 1.93 | 5.05 |
| Cloud forest | 193 | 225 | 393 | 4.51 × 10−3 | 9.05 × 10−3 | 0.94 | 1.75 |
| Dexiinae | Exoristinae | Tachininae | |
|---|---|---|---|
| most host-specific | 28 (25.7) | 313 (38.8) | 82 (27.2) |
| intermediate | 42 (38.5) | 288 (35.7) | 95 (31.6) |
| most generalist | 39 (35.7) | 205 (25.4) | 124 (41.2) |
| Dataset | Unique Rearings | Explanatory Variable | Slope | Adjusted R2 | t-Value (154 d.f.) | p-Value |
|---|---|---|---|---|---|---|
| Only tachinid host caterpillars | Included | No. host species | −0.036 | 0.042 | −2.67 | 0.008 |
| “ | “ | No. rearings | 0.016 | “ | 2.34 | 0.021 |
| “ | Excluded | No. host species | −0.485 | 0.019 | −1.17 | N.S. |
| “ | “ | No. rearings | 0.050 | “ | 2.30 | 0.023 |
| Data on caterpillar host range from all data | Included | No. host species | 0.145 | −0.01 | 0.69 | N.S. |
| “ | “ | No. rearings | 0.068 | “ | 0.65 | N.S. |
| “ | Excluded | No. host species | 0.145 | −0.01 | 0.69 | N.S. |
| “ | “ | No. rearings | 0.067 | “ | 0.65 | N.S. |
| Parasitoid Family | Parasitoid Species | Host Species | Singleton Host Associations | Doubleton Host Associations | >2 Host Species |
|---|---|---|---|---|---|
| Tachinidae | 1206 | 2110 | 5808 (83%) | 494 (7%) | 661 (10%) |
| Braconidae | 1492 | 2006 | 2194 (60%) | 530 (15%) | 879 (25%) |
| Ichneumonidae | 705 | 1262 | 1228 (64%) | 267 (14%) | 433 (22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quicke, D.L.J.; Fleming, A.J.; Wood, D.M.; Woodley, N.E.; Manjunath, R.; Naik, S.; Smith, M.A.; Sharkey, M.J.; Hallwachs, W.; Janzen, D.H.; et al. Tachinid Flies (Diptera), Caterpillar Hosts (Lepidoptera) and Their Food Plants, Reared in Área de Conservación Guanacaste (ACG), Northwestern Costa Rica: Documenting Community Structure with the Aid of DNA Barcodes. Diversity 2025, 17, 658. https://doi.org/10.3390/d17090658
Quicke DLJ, Fleming AJ, Wood DM, Woodley NE, Manjunath R, Naik S, Smith MA, Sharkey MJ, Hallwachs W, Janzen DH, et al. Tachinid Flies (Diptera), Caterpillar Hosts (Lepidoptera) and Their Food Plants, Reared in Área de Conservación Guanacaste (ACG), Northwestern Costa Rica: Documenting Community Structure with the Aid of DNA Barcodes. Diversity. 2025; 17(9):658. https://doi.org/10.3390/d17090658
Chicago/Turabian StyleQuicke, Donald L. J., Alan J. Fleming, D. Monty Wood, Norman E. Woodley, Ramya Manjunath, Suresh Naik, M. Alex Smith, Michael J. Sharkey, Winnie Hallwachs, Daniel H. Janzen, and et al. 2025. "Tachinid Flies (Diptera), Caterpillar Hosts (Lepidoptera) and Their Food Plants, Reared in Área de Conservación Guanacaste (ACG), Northwestern Costa Rica: Documenting Community Structure with the Aid of DNA Barcodes" Diversity 17, no. 9: 658. https://doi.org/10.3390/d17090658
APA StyleQuicke, D. L. J., Fleming, A. J., Wood, D. M., Woodley, N. E., Manjunath, R., Naik, S., Smith, M. A., Sharkey, M. J., Hallwachs, W., Janzen, D. H., Fernández-Triana, J., Whitfield, J. B., Hebert, P. D. N., & Butcher, B. A. (2025). Tachinid Flies (Diptera), Caterpillar Hosts (Lepidoptera) and Their Food Plants, Reared in Área de Conservación Guanacaste (ACG), Northwestern Costa Rica: Documenting Community Structure with the Aid of DNA Barcodes. Diversity, 17(9), 658. https://doi.org/10.3390/d17090658







