Will They Still Be Together? Distribution Modeling of Six Co-Occurring Species of Swertia (Gentianaceae) in Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Cleaning
2.2. Ecological Niche Modeling
2.3. Effect of Phylogenetic Distance on Niches
3. Results
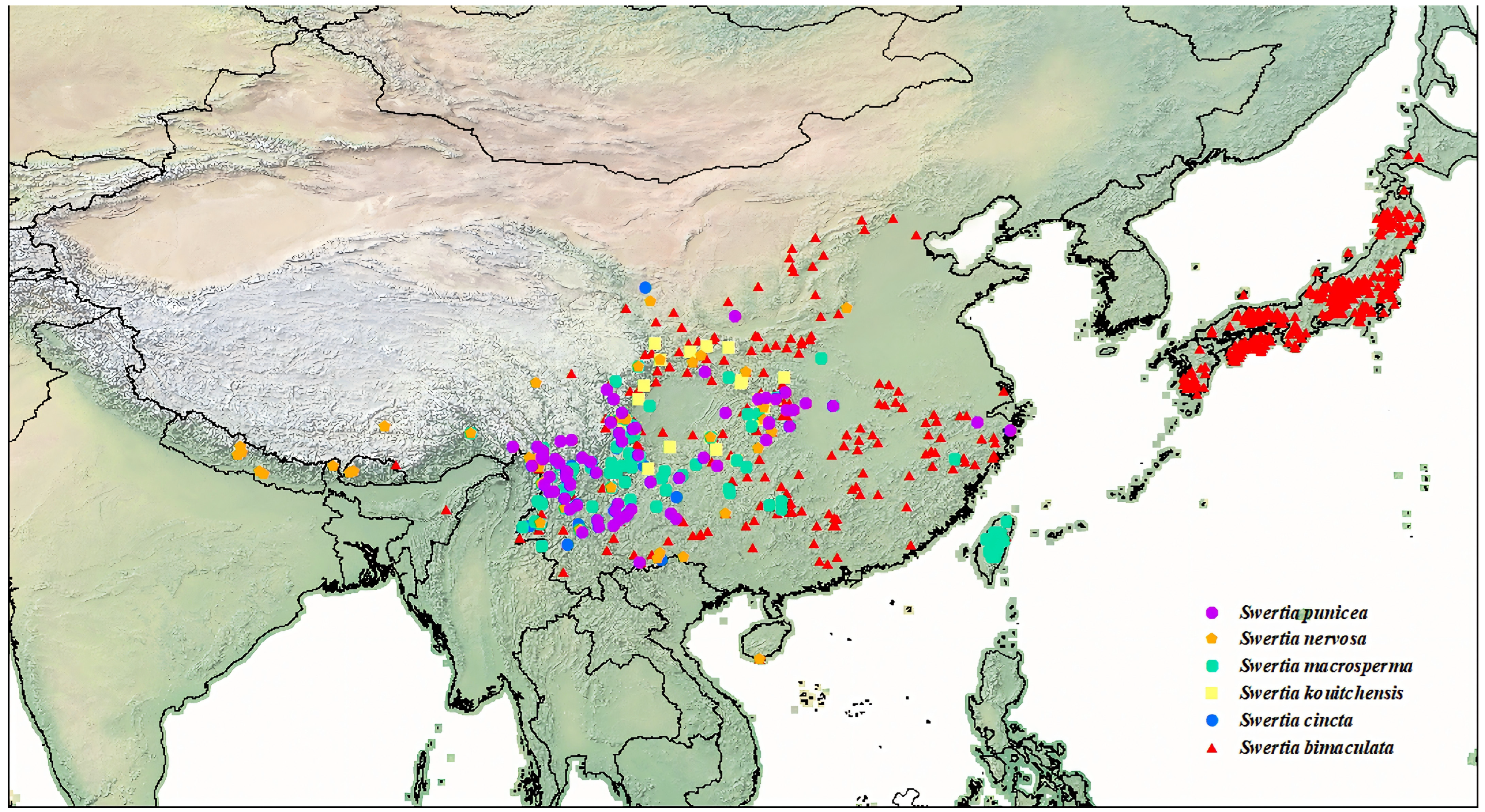
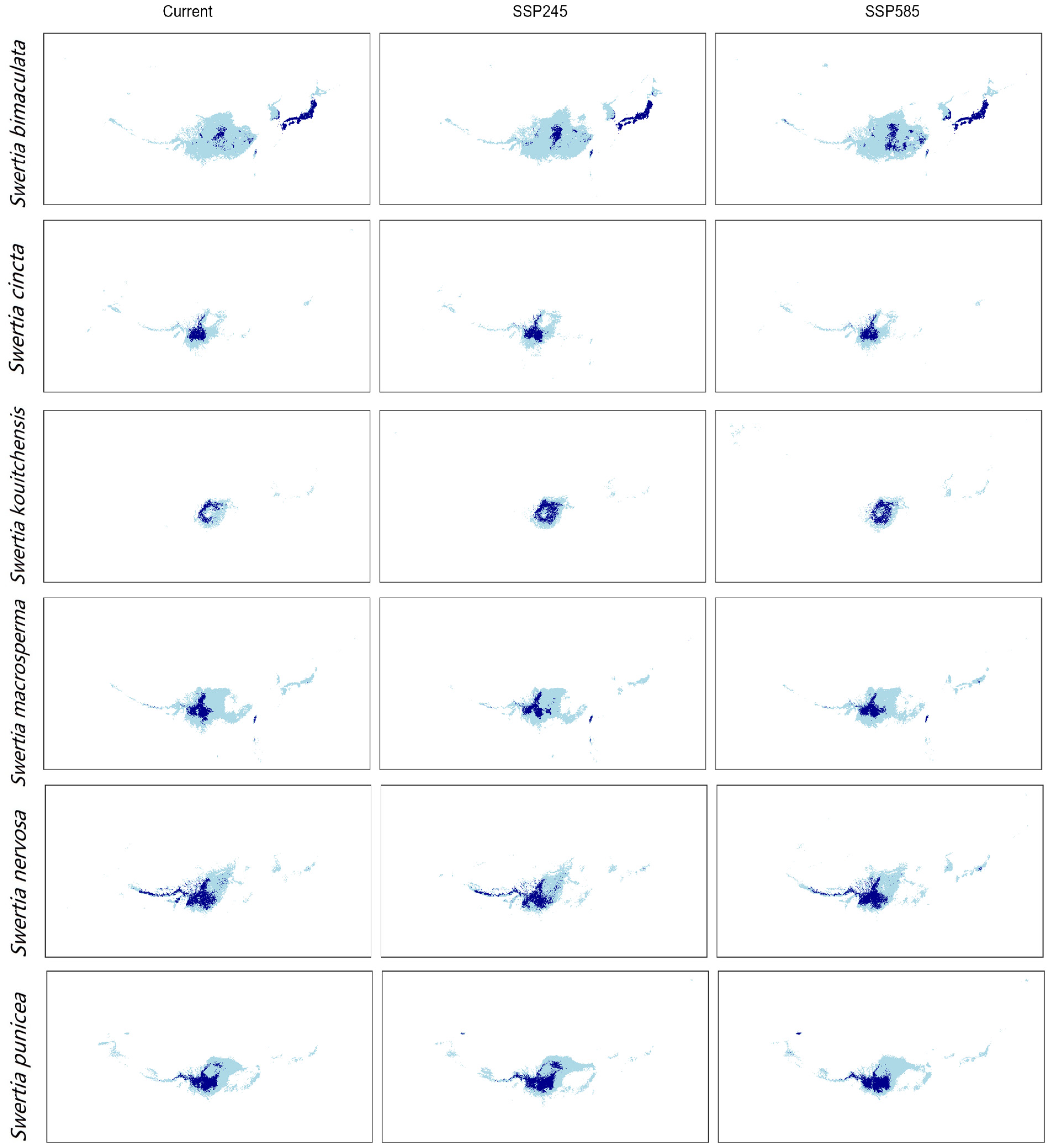
3.1. Current Distribution of Six Species in the Genus Swertia
3.2. MaxEnt Model Availability
3.3. Dominant Environmental Variables
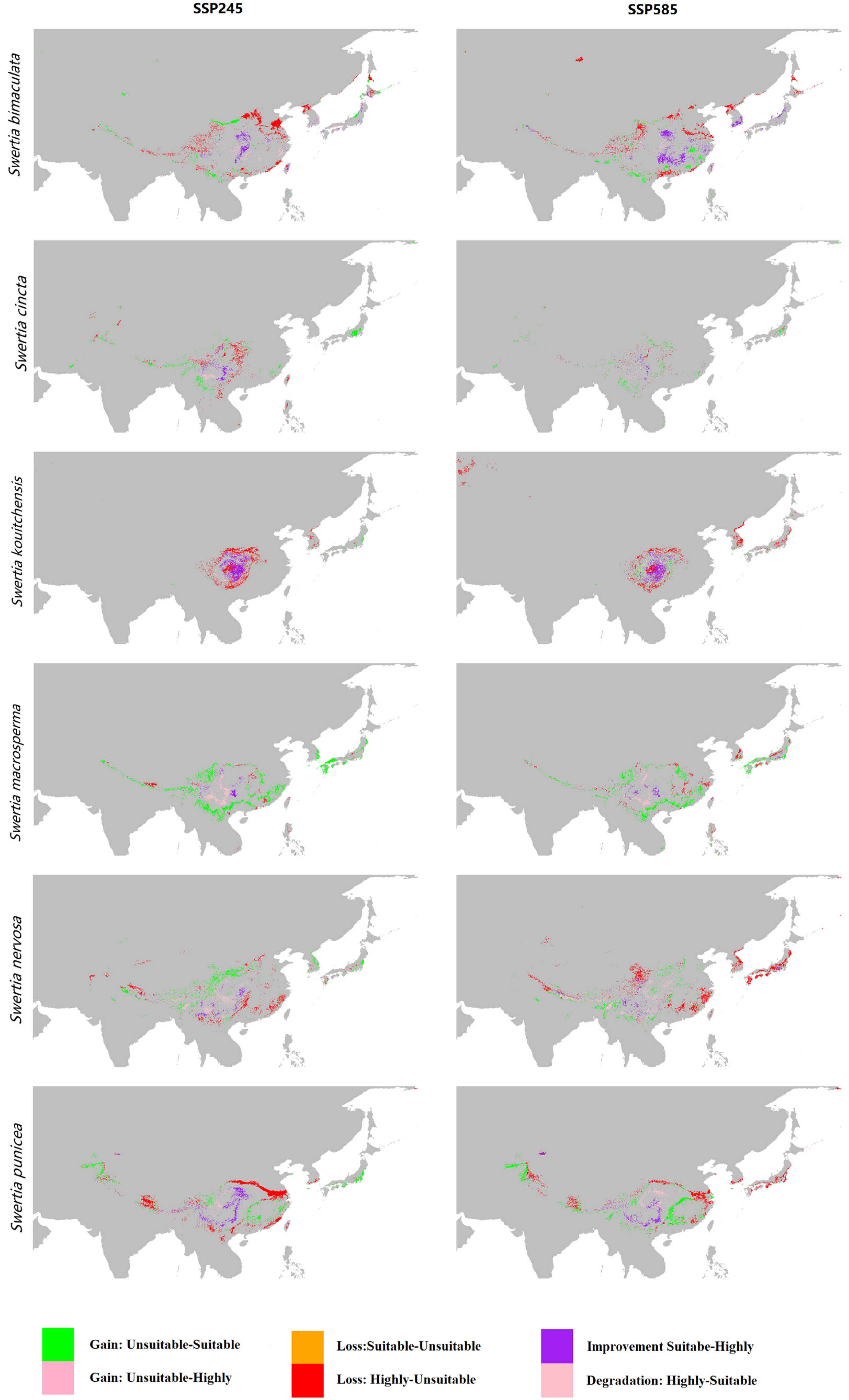
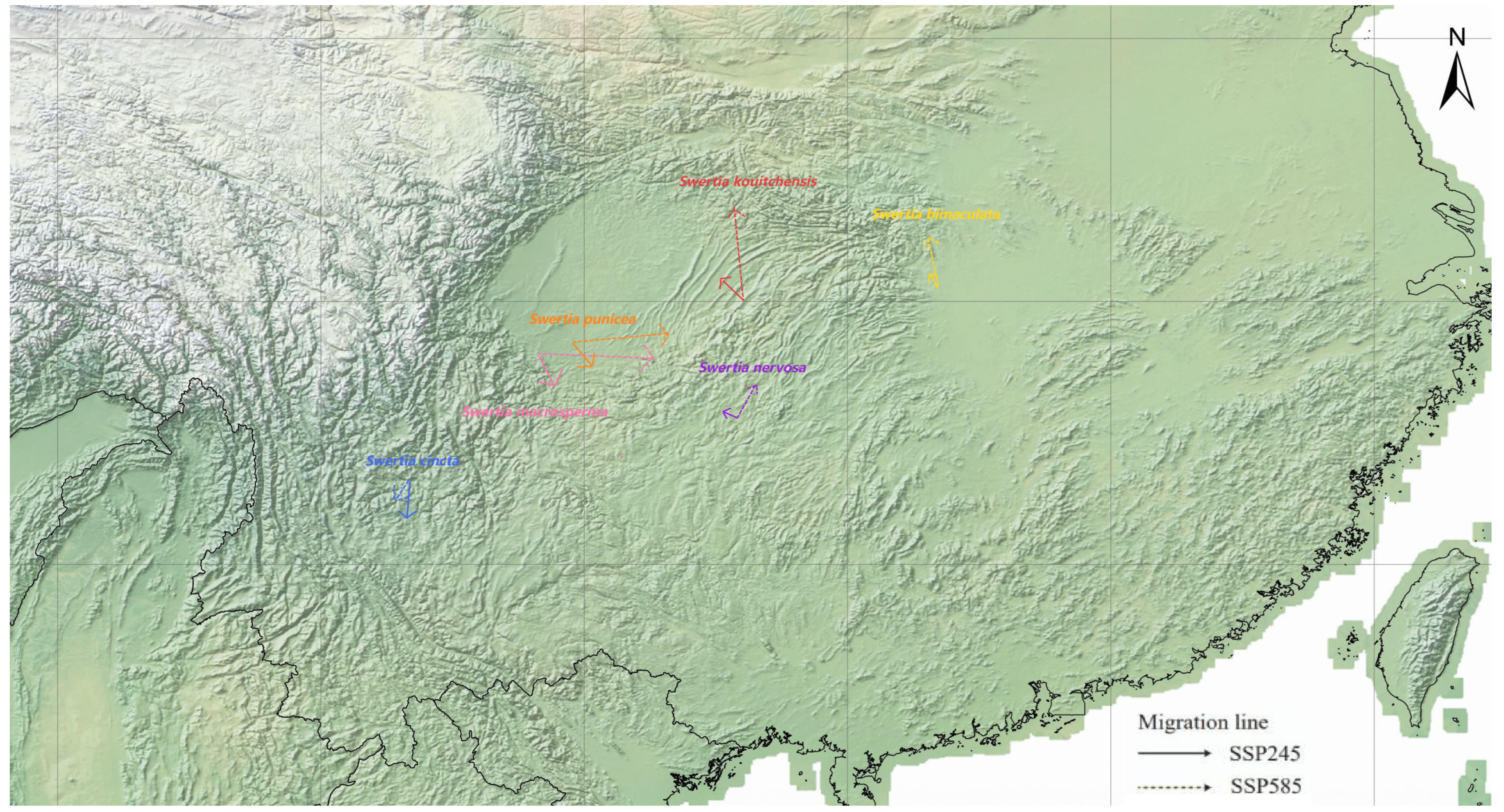
3.4. Range Changes Under Future Climate Change
3.5. Effect of Phylogenetic on Niches
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2008. [Google Scholar]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projected timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef]
- Holm, J.A.; Kueppers, L.M.; Chamber, J.Q. Novel tropical forests: Response to global change. New Phytol. 2017, 213, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Kèorner, C. Alpine Plant Life. Functional Plant Ecology of High Mountain Ecosystems; Springer: Berlin, Germany, 1999; pp. 247–257. [Google Scholar]
- Kuo, C.C.; Liu, Y.C.; Su, Y.; Liu, H.Y.; Lin, C.T. Responses of alpine summit vegetation under climate change in the transition zone between subtropical and tropical humid environment. Nature 2022, 12, 13352. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Li, Y.; Man, Z. Natural Vegetation Distribution and Climate Ecology Prediction Based on Geographic Information System. Environ. Eng. Manag. J. 2020, 19, 1623–1630. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Hong, Z.; Yuan, Y.; Tan, Z.; Wang, Y.; Chen, Z.; Zheng, J.; Zhang, Z.; Zhang, L.; et al. Geographic distribution and impacts of climate change on the suitable habitats of Glycyrrhiza species in China. Environ. Sci. Pollut. Res. 2023, 30, 55625–55634. [Google Scholar] [CrossRef]
- Des, M.; Martínez, B.; deCastro, M.; Viejo, R.M.; Sousa, M.C.; Gómez-Gesteira, M. The impact of climate change on the geographical distribution of habitat-forming macroalgae in the Rías Baixas. Mar. Environ. Res. 2020, 161, 105074. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Pan, J.; Wu, Z.; Chen, A. Predicting the potential distribution of Campsis grandiflora in China under climate change. Environ. Sci. Pollut. Res. 2022, 29, 63629–63639. [Google Scholar] [CrossRef]
- Huang, E.; Chen, Y.; Fang, M.; Zheng, Y.; Yu, S. Environmental drivers of plant distributions at global and regional scales. Glob. Ecol. Biogeogr. 2021, 30, 697–709. [Google Scholar] [CrossRef]
- Shi, Y.; Ren, Z.X.; Wang, W.; Xu, X.; Liu, J.; Zhao, Y.; Wang, H. Predicting the spatial distribution of three Astragalus species and their pollinating bumblebees in the Sino-Himalayas. Biodivers. Sci. 2021, 29, 79–769. [Google Scholar] [CrossRef]
- Deng, N.; Caixia, L.; Ma, F.; Song, Q.; Tian, Y. Understory vegetation diversity patterns of Platycladus orientalis and Pinus elliottii communities in Central and Southern China. Open Life Sci. 2023, 18, 791. [Google Scholar] [CrossRef]
- Vasconcelos, T.S.; Rodriguez, M.A.; Hawkins, B.A. Species distribution modelling as a macroecological tool: A case study using New World amphibians. Ecography 2012, 35, 539–548. [Google Scholar] [CrossRef]
- Mirhashemi, H.; Ahmadi, K.; Heydari, M.; Karami, O.; Valkó, P.; Nabaz, R.K. Climatic variables are more effective on the spatial distribution of oak forests than land use change across their historical range. Environ. Monit. Assess 2024, 196, 289. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Niu, Z.; Zhang, M.; Li, C.; Li, J. The effects of projected climate change and extreme climate on maize and rice in the Yangtze River Basin, China. Agric. For. Meteorol. 2020, 15, 282–283. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Subedi, S.C.; Drake, S.; Adhikari, B.; Coggeshall, M.V. Climate-change habitat shifts for the vulnerable endemic oak species (Quercus arkansana Sarg.). J. For. Res. 2024, 35, 23. [Google Scholar] [CrossRef]
- Hu, W.J.; Wang, Y.Y.; Zhang, D.; Yu, W.W.; Chen, G.C.; Xie, T.; Liu, Z.H.; Ma, Z.Y.; Du, J.G.; Chao, B.X.; et al. Mapping the potential of mangrove forestrestoration based on species distribution models: A case study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef]
- Vessella, F.; Schirone, B. Predicting potential distribution of Quercus suber in Italy based on ecological niche models: Conservation insights and reforestation involvements. Forest. Ecol. Manag. 2013, 304, 150–161. [Google Scholar] [CrossRef]
- Qiu, L.; Jacquemyn, H.; Burgess, K.; Zhang, L.; Zhou, Y.; Yang, B.; Tan, S. Contrasting range changes of terrestrial orchids under future climate change in China. Sci. Total Environ. 2023, 895, 165128. [Google Scholar] [CrossRef]
- Yin, X.; Jarvie, S.; Guo, W.Y.; Deng, T.; Mao, L.F.; Zhang, M.H.; Chu, C.J.; Qian, H.; Svenning, J.C.; He, F.L. Niche overlap and divergence times support niche conservatism in eastern Asia-Eastern North America disjunct plants. Glob. Ecol. Biogeogr. 2021, 30, 1990–2003. [Google Scholar] [CrossRef]
- Kumar, V.; Van Staden, J. A review of Swertia chirayita (Gentianaceae) as a traditional medicinal plant. Front. Pharmacol. 2016, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Uprety, Y.; Asselin, H.; Boon, E.; Yadav, S.; Shrestha, K. Indigenous use and bio-efficacy of medicinal plants in the Rasuwa District, Central Nepal. J. Ethnobiol. Ethnomedicine 2010, 6, 101168. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G.; Mondal, S.; Gangopadhyay, A.; Gorai, D.; Mukhopadhyay, B.; Saha, S.; Brahmachari, A. Swertia (Gentianaceae): Chemical and pharmacological aspects. Chem. Biodivers. 2004, 1, 1627–1651. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Huang, H.; Wang, Y. Phytochemistry and pharmacological activities of the genus Swertia (Gentianaceae): A review. Am. J. Chin. Med. 2017, 45, 667–736. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Sun, B.; Ikeda, T.; Nohara, T. Hepatoprotective activity of the constituents in Swertia pseudochinensis. Biol. Pharm. Bull. 2005, 28, 534–537. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, T.; Wang, L.; Shan, Q.; Jiang, L. The hepatoprotective effect and chemical constituents of total iridoids and xanthones extracted from Swertia mussotii Franch. J. Ethnopharmacol. 2014, 154, 259–266. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Liu, S. A Worldwide Monograph of Swertia and Its Allies; Beijing Science Press: Beijing, China, 2015. [Google Scholar]
- Chaudhuri, R.K.; Amita, P.; Timir, B.J. Production of genetically uniform plants from nodal explants of Swertia chirata Buch.-Ham. ex Wall.—An endangered medicinal herb. Vitr. Cell. Dev. Biol. 2007, 46, 467–472. [Google Scholar] [CrossRef]
- Cao, Q.; Xu, L.; Wang, J.; Zhang, F.; Chen, S. Molecular phylogeny of subtribe Swertiinae. Bull. Bot. Res. 2021, 41, 408–418. [Google Scholar]
- Hagen, K.B.V.; Kadereit, J.W. The phylogeny of Gentianella (Gentianaceae) and its colonization of the southern hemisphere as revealed by nuclear and chloroplast DNA sequence variation. Org. Divers. Evol. 2001, 1, 61–79. [Google Scholar]
- Wen, S.J.; Deng, M.X.; Ding, W.; Wang, Z.Y.; Ren, Z.X. Comparative floral nectar attributes in four Swertia species (Gentianaceae). Biodivers. Sci. 2024, 32, 23297. [Google Scholar] [CrossRef]
- The Global Biodiversity Information Facility (2023). Available online: https://www.gbif.org/ (accessed on 15 December 2023).
- O’Neill, B.C.; Kriegler, E.; Riahi, K.; Ebi, K.L.; Hallegatte, S.; Carter, T.R.; Mathur, R.; van Vuuren, D.P. A new scenario framework for climate change research: The concept of shared socioeconomic pathways. Clim. Chang. 2014, 122, 387–400. [Google Scholar] [CrossRef]
- Liu, H.C.; Jacquemyn, H.; He, X.Y.; Chen, W.; Huang, Y.Q.; Yu, S.; Lu, Y.P.; Zhang, Y. The impact of human pressure and climate change on the habitat availability and protection of Cypripedium (Orchidaceae) in northeast China. Plants 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.H.; Lin, H.P.; Bai, S.H.; Chen, J.; Chen, A.L. Simulation the potential distribution of Dendrolimus houi and its hosts, Pinus yunnanensis and Cryptomeria fortunei under climate change in China. Front. Plant Sci. 2022, 13, 1054710. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1998, 240, 1285–1293. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.W.; Deng, T.; Boufford, D.E. Origins and evolution of plant diversity in the Hengduan Mountains, China. Plant Divers. 2017, 39, 161–166. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, X. Spatiotemporal change in geographical distribution of global climate types in the context of climate warming. Clim. Dyn. 2014, 43, 595–605. [Google Scholar] [CrossRef]
- Zhang, K.L.; Liu, H.N.; Pan, H.L.; Shi, W.H.; Zhao, Y.; Li, S.L.; Liu, J.C.; Tao, J. Shifts in potential geographical distribution of Pterocarya stenoptera under climate change scenarios in China. Ecol. Evol. 2020, 10, 4828–4837. [Google Scholar] [CrossRef]
- Chen, I.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024. [Google Scholar] [CrossRef]
- Kou, J.; Wang, T.J.; Yu, F.Y.; Sun, Y.W.; Feng, C.; Shao, X.M. The moss genus Didymodon as an indicator of climate change on the Tibetan Plateau. Ecol. Indic 2020, 113, 106204. [Google Scholar] [CrossRef]
- Qian, H.; Ricklefs, R.E. Geographical distribution and ecological conservatism of disjunct genera of vascular plants in eastern Asia and eastern North America. J. Ecol. 2004, 92, 253–265. [Google Scholar] [CrossRef]
- Holt, R.D. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. USA 2009, 106, 19659–19665. [Google Scholar] [CrossRef]
- Liang, H.; Ren, Z.X.; Tao, Z.B.; Zhao, Y.H.; Bernhardt, P.; Wang, H. Impact of pre- and post-pollination barriers on pollen transfer and reproductive isolation among three sympatric Pedicularis species. Plant Biol. 2018, 20, 662–673. [Google Scholar] [CrossRef]
- Xu, X.; Liang, H.; Ren, Z.X.; Maruyama, P.K.; Rech, A.R.; Trunschke, J.; Zhao, Y.H.; Li, H.D.; Wang, H. Generalised bumblebee-flower interactions demonstrate weak floral niche partitioning despite a high bee diversity. Ecography 2025. [Google Scholar] [CrossRef]
- Wang, S.; Fu, W.-L.; Du, W.; Zhang, Q.; Li, Y.; Lyu, Y.-S.; Wang, X.-F. Nectary tracks as pollinator manipulators: The pollination ecology of Swertia bimaculata (Gentianaceae). Ecol. Evol. 2018, 8, 3187–3207. [Google Scholar] [CrossRef]
- Lioy, S.; Bergamino, C.; Porporato, M. The invasive hornet Vespa velutina: Distribution, impacts and management options. CABI Rev. 2022. [Google Scholar] [CrossRef]
| Species | Breeding Systems | Pollinators | Number of Records | GenBank Accession Numbers |
|---|---|---|---|---|
| Swertia bimaculata | Outcrossing | Flies | 643 | MW344296 |
| Swertia cincta | Outcrossing | Hornet wasps | 47 | MZ261898 |
| Swertia kouitchensis | Outcrossing | Hornet wasps | 26 | MZ261902 |
| Swertia macrosperma | Selfing | Flies and bees | 114 | MZ261903 |
| Swertia nervosa | Selfing | Flies and bees | 78 | NC057596 |
| Swertia punicea | Outcrossing | Hornet wasps | 69 | MZ261896 |
| Bioclimatic Variables | The Proportion of Environmental Factors in Different Models (%) | Contribution Ratio (%) | |||||
|---|---|---|---|---|---|---|---|
| Swertia bimaculata | Swertia cincta | Swertia kouitchensis | Swertia macrosperma | Swertia nervosa | Swertia punicea | ||
| aspect | 0.1 | 0 | 0 | 0 | 0.7 | 0 | 0.1 |
| bio2 (Mean diurnal range) | 0.6 | 4.8 | 6.4 | 3.2 | 1.2 | 0 | 2.7 |
| bio3 (Isothermality) | 1.2 | 0 | 0 | 1.9 | 0 | 0 | 0.5 |
| bio4 (Temperature seasonality) | 1.2 | 0 | 0 | 0 | 6.4 | 0 | 1.3 |
| bio6 (Min temperature of coldest month) | 0 | 26 | 0 | 0 | 0 | 0 | 4.3 |
| bio7 (Temperature annual range) | 0 | 22.3 | 5.2 | 11.1 | 0 | 41.8 | 13.4 |
| bio8 (Mean temperature of wettest quarter) | 0.6 | 0 | 0 | 0 | 0 | 0 | 0.1 |
| bio9 (Mean temperature of driest quarter) | 23.2 | 0 | 24.8 | 10.3 | 28.0 | 14.8 | 16.9 |
| bio15 (Precipitation seasonality) | 5.3 | 0.1 | 1.6 | 1.4 | 0 | 0.2 | 1.4 |
| bio18 (Precipitation of warmest quarter) | 54.8 | 0 | 23 | 40.4 | 35.8 | 0 | 25.7 |
| bio19 (Precipitation of coldest quarter) | 0 | 0.9 | 6.2 | 0 | 4.1 | 5.6 | 2.8 |
| elevation | 0 | 30.5 | 0 | 19.1 | 14.6 | 9.1 | 12.2 |
| slope | 2.5 | 0.5 | 7.1 | 0.7 | 8.1 | 1.5 | 3.4 |
| solar radiation | 9.6 | 14.9 | 25.7 | 11.9 | 1.1 | 26.9 | 15.0 |
| Rate of Change in Suitable Areas | Rate of Change in Highly Suitable Areas | |||
|---|---|---|---|---|
| SSP245 | SSP585 | SSP245 | SSP585 | |
| S. bimaculata | 6.59% | 4.35% | 6.39% | 38.29% |
| S. cincta | 0.69% | −3.63% | 8.81% | 2.78% |
| S. kouitchensis | 31.45% | 27.61% | 92.20% | 71.19% |
| S. macrosperma | −16.04% | −8.43% | −17.35% | −7.94% |
| S. nervosa | −0.72% | 10.20% | −11.81% | −8.35% |
| S. punicea | 9.24% | 1.30% | 24.26% | 5.34% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, M.-X.; Wen, S.-J.; Wu, D.; Wang, Z.; Ren, Z.-X. Will They Still Be Together? Distribution Modeling of Six Co-Occurring Species of Swertia (Gentianaceae) in Asia. Diversity 2025, 17, 657. https://doi.org/10.3390/d17090657
Deng M-X, Wen S-J, Wu D, Wang Z, Ren Z-X. Will They Still Be Together? Distribution Modeling of Six Co-Occurring Species of Swertia (Gentianaceae) in Asia. Diversity. 2025; 17(9):657. https://doi.org/10.3390/d17090657
Chicago/Turabian StyleDeng, Min-Xue, Shi-Jia Wen, Ding Wu, Zhiyong Wang, and Zong-Xin Ren. 2025. "Will They Still Be Together? Distribution Modeling of Six Co-Occurring Species of Swertia (Gentianaceae) in Asia" Diversity 17, no. 9: 657. https://doi.org/10.3390/d17090657
APA StyleDeng, M.-X., Wen, S.-J., Wu, D., Wang, Z., & Ren, Z.-X. (2025). Will They Still Be Together? Distribution Modeling of Six Co-Occurring Species of Swertia (Gentianaceae) in Asia. Diversity, 17(9), 657. https://doi.org/10.3390/d17090657






