Molecular Identification of Cryptic Cysticercosis: Taenia spp. in Wild and Domestic Intermediate Hosts in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Areas and Sampling
2.3. Parasitological Methods
2.4. DNA Extraction
2.5. PCR and Sequencing
2.6. Bioinformatics and Statistic Analysis
3. Results
3.1. Morphological Description and Identification
3.2. Molecular and Phylogenetic Analysis of Taenia spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| N | number |
| bp | base pairs |
| CI | confidence interval |
| cox1 | cytochrome c oxidase subunit 1 |
| DNA | deoxyribonucleic acid |
| ML | Maximum Likelihood |
| nad1 | NADH dehydrogenase subunit 1 |
| OR | Odds Ratio |
| SD | standard deviation |
| TBE | Tris-borate-EDTA buffer |
References
- Samuel, W.; Zewde, G.G. Prevalence, risk factors, and distribution of Cysticercus tenuicollis in visceral organs of slaughtered sheep and goats in central Ethiopia. Trop. Anim. Health Prod. 2010, 42, 1049–1051. [Google Scholar] [CrossRef]
- Scala, A.; Urrai, G.; Varcasia, A.; Nicolussi, P.; Mulas, M.; Goddi, L.; Pipia, A.P.; Sanna, G.; Genchi, M.; Bandino, E. Acute visceral cysticercosis by Taenia hydatigena in lambs and treatment with praziquantel. J. Helminthol. 2016, 90, 113–116. [Google Scholar] [CrossRef]
- Oryan, A.; Goorgipour, S.; Moazeni, M.; Shirian, S. Abattoir prevalence, organ distribution, public health and economic importance of major metacestodes in sheep, goats and cattle in Fars, southern Iran. Trop. Biomed. 2012, 29, 349–359. [Google Scholar]
- Mekuria, E.; Shimelis, S.; Bekele, J.; Sheferaw, D. Sheep and goats Cysticercus tenuicollis prevalence and associated risk factors. Afr. J. Agric. Res. 2013, 8, 3121–3125. [Google Scholar] [CrossRef]
- Bariselli, S.; Maioli, G.; Pupillo, G.; Calzolari, M.; Torri, D.; Cirasella, L.; Luppi, A.; Torreggiani, C.; Garbarino, C.; Barsi, F.; et al. Identification and phylogenetic analysis of Taenia spp. parasites found in wildlife in the Emilia-Romagna region, northern Italy (2017–2022). Int. J. Parasitol. Parasites Wildl. 2023, 22, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kibona, T.; Buza, J.; Shirima, G.; Lankester, F.; Ngongolo, K.; Hughes, E.; Cleaveland, S.; Allan, K.J. The Prevalence and Determinants of Taenia multiceps Infection (Cerebral Coenurosis) in Small Ruminants in Africa: A Systematic Review. Parasitologia 2022, 2, 137–146. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Urwin, N.A.R.; Williams, T.M.; Mitchell, K.L.; Lievaart, J.J.; Armua-Fernandez, M.T. Red foxes (Vulpes vulpes) and wild dogs (dingoes (Canis lupus dingo) and dingo/domestic dog hybrids), as sylvatic hosts for Australian Taenia hydatigena and Taenia ovis. Int. J. Parasitol. Pazasites Wildl. 2014, 3, 75–80. [Google Scholar] [CrossRef]
- Lavikainen, A.; Laaksonen, S.; Beckmen, K.; Oksanen, A.; Isomursu, M.; Meri, S. Molecular identification of Taenia spp. in wolves (Canis lupus), brown bears (Ursus arctos) and cervids from North Europe and Alaska. Parasitol. Int. 2011, 60, 289–295. [Google Scholar] [CrossRef]
- Deplazes, P.; Eichenberger, R.M.; Grimm, F. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int. J. Parasitol. Parasites Wildl. 2019, 9, 342–358. [Google Scholar] [CrossRef]
- Dorbek-Kolin, E.; Ahlberg, T.; Tummeleht, L.; Tappe, D.; Johansen, M.V.; Lassen, B. Prevalence of cysticercosis in Estonian pigs and cattle. Parasitol. Res. 2018, 117, 591–595. [Google Scholar] [CrossRef]
- Trevisan, C.; Sotiraki, S.; Laranjo-González, M.; Dermauw, V.; Wang, Z.; Kärssin, A.; Cvetkovikj, A.; Winkler, A.S.; Abraham, A.; Bobić, B.; et al. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: Eastern Europe. Parasites Vectors 2018, 11, 569. [Google Scholar] [CrossRef]
- Varcasia, A.; Tamponi, C.; Ahmed, F.; Cappai, M.G.; Porcu, F.; Mehmood, N.; Dessì, G.; Scala, A. Taenia multiceps coenurosis: A review. Parasites Vectors 2022, 15, 84. [Google Scholar] [CrossRef]
- Uakhit, R.; Smagulova, A.; Lider, L.; Leontyev, S.; Kiyan, V. Epizootiological monitoring of wolf helminths in Northern and Central Kazakhstan. Vet. World 2024, 17, 1648–1654. [Google Scholar] [CrossRef]
- Flisser, A.; Craig, P.S.; Ito, A. Cysticercosis and Taeniosis: Taenia solium, Taenia saginata and Taenia asiatica; Palmer, S.R., Soulsby, L., Torgerson, P.R., Brown, D.W.G., Eds.; Oxford Textbook of Zoonoses Biology, Clinical Practice, and Public Health Control; Oxford University Press: Oxford, UK, 2011; pp. 625–649. [Google Scholar]
- Muku, R.J.; Yan, H.B.; Ohiolei, J.A.; Saaid, A.A.; Ahmed, S.; Jia, W.Z.; Fu, B.Q. Molecular Identification of Taenia hydatigena from Sheep in Khartoum, Sudan. Korean J. Parasitol. 2020, 58, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Filip, K.J.; Pyziel, A.M.; Jeżewski, W.; Myczka, A.W.; Demiaszkiewicz, A.W.; Laskowski, Z. First Molecular Identification of Taenia hydatigena in Wild Ungulates in Poland. EcoHealth 2019, 16, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Corda, A.; Dessì, G.; Varcasia, A.; Carta, S.; Tamponi, C.; Sedda, G.; Scala, M.; Marchi, B.; Salis, F.; Scala, A.; et al. Acute visceral cysticercosis caused by Taenia hydatigena in lambs: Ultrasonographic findings. Parasites Vectors 2020, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.A.; Ohiolei, J.A.; Saqib, M.; Tayyab, M.H.; Zafar Khan, M.U.; Li, L.; Aqib, A.I.; Hassan, A.; Alvi, A.A.; Qamar, W.; et al. First Report on Molecular Characterization of Taenia multiceps Isolates from Sheep and Goats in Faisalabad, Pakistan. Front. Vet. Sci. 2020, 7, 594599. [Google Scholar] [CrossRef]
- Pouchet, C.; Fernandez-Prada, C.; Dussault, C.; Leclerc, M.; Tremblay, J.P.; Côté, S.D. Variation in prevalence and intensity of macroparasites in moose and their interactions with winter tick load in eastern Canada. Wildl. Biol. 2024, e01205. [Google Scholar] [CrossRef]
- Žele Vengušt, D.; Kuhar, U.; Jerina, K.; Vengušt, G. Twenty Years of Passive Disease Surveillance of Roe Deer (Capreolus capreolus) in Slovenia. Animals 2021, 11, 407. [Google Scholar] [CrossRef]
- Morandi, B.; Bazzucchi, A.; Gambini, S.; Crotti, S.; Cruciani, D.; Morandi, F.; Napoleoni, M.; Piseddu, T.; Di Donato, A.; Gavaudan, S. A novel intermediate host for Taenia serialis (Gervais, 1847): The European roe deer (Capreolus capreolus L. 1758) from the Monti Sibillini National Park (MSNP), Italy. Int. J. Parasitol. Parasites Wildl. 2021, 17, 110–113. [Google Scholar] [CrossRef]
- Kautto, A.H.; Grandi, G.; Höglund, J. Taenia lynciscapreoli in semi-domesticated reindeer (Rangifer tarandus tarandus, L.) in Sweden. Int. J. Parasitol. Parasites Wildl. 2022, 18, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Pipia, A.P.; Dore, F.; Sanna, G.; Tamponi, C.; Marrosu, R.; Bandino, E.; Carmona, C.; Boufana, B.; Varcasia, A. Epidemiological updates and economic losses due to Taenia hydatigena in sheep from Sardinia, Italy. Parasitol. Res. 2015, 114, 3137–3143. [Google Scholar] [CrossRef]
- Deressa, A.; Tilahun, T.; Tadesse, A.; Beyene, M.; Gebrewold, G.; Pal, M. Assessment of Coenurus cerebralis and its economic impact in sheep brain harvested at Ethiopian Health and Nutrition Research Institute, Ethiopia. Int. J. Livest. Res. 2012, 2, 217–226. [Google Scholar]
- Shiferaw, A.; Abdela, N. Public health and economic significance of cerebral coenurosis in sheep and goat: A review. Acta Parasitol. Glob. 2016, 7, 54–65. [Google Scholar] [CrossRef]
- Braae, U.C.; Kabululu, M.; Nørmark, M.E.; Nejsum, P.; Ngowi, H.A.; Johansen, M.V. Taenia hydatigena cysticercosis in slaughtered pigs, goats, and sheep in Tanzania. Trop. Anim. Health Prod. 2015, 47, 1523–1530. [Google Scholar] [CrossRef]
- Ohiolei, J.A.; Luka, J.; Zhu, G.Q.; Yan, H.B.; Li, L.; Magaji, A.A.; Alvi, M.A.; Wu, Y.T.; Li, J.Q.; Fu, B.Q.; et al. First molecular description, phylogeny and genetic variation of Taenia hydatigena from Nigerian sheep and goats based on three mitochondrial genes. Parasites Vectors 2019, 12, 520. [Google Scholar] [CrossRef]
- Ohiolei, J.A.; Yan, H.B.; Li, L.; Li, W.H.; Wu, Y.D.; Alvi, M.A.; Zhang, N.Z.; Fu, B.Q.; Wang, X.L.; Jia, W.Z. A new molecular nomenclature for Taenia hydatigena: Mitochondrial DNA sequences reveal sufficient diversity suggesting the assignment of major haplotype divisions. Parasitology 2021, 148, 311–326. [Google Scholar] [CrossRef]
- Jarošová, J.; Antolová, D.; Iglodyová, A.; Königová, A.; Dolinská, M.U.; Víchová, B. Molecular identification of Taenia hydatigena from domestic and free-living animals in Slovakia, Central Europe. Parasitol. Res. 2022, 121, 1345–1354. [Google Scholar] [CrossRef]
- Moudgil, A.D.; Nehra, A.K.; Vohra, S.; Thakur, S.D.; Sharma, D. Prevalence and phylogeography of Taenia hydatigena metacestodes from goats of India. Parasitology 2022, 149, 1193–1198. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; González, M.F.; Villalobos, R.; Hidalgo, A. Parasitic findings on threatened pudu deer from Central Chile accounts first genetic characterization of lice parasitizing P. puda in Chile and the first molecular report of Taenia hydatigena metacestodes in this species. Vet. Q. 2024, 44, 1–8. [Google Scholar] [CrossRef]
- Abbas, I.; El-Alfy, E.S.; Janecek-Erfurth, E.; Strube, C. Molecular characterization of Cysticercus tenuicollis isolates from sheep in the Nile Delta, Egypt and a review on Taenia hydatigena infections worldwide. Parasitology 2021, 148, 913–933. [Google Scholar] [CrossRef]
- Ng-Nguyen, D.; Van Nguyen, T.; Van Nguyen, T.; Nguyen, H.Q.; Nguyen, V.T. Prevalence and risk factors of Taenia hydatigena in dogs, pigs, and cattle in the Central Highlands of Vietnam. Parasitol. Res. 2021, 120, 3245–3253. [Google Scholar] [CrossRef]
- Polaz, S.V. Helminths of wild ungulates living in different regions of Belarus. Russ. J. Parasitol. 2022, 16, 33–49. [Google Scholar] [CrossRef]
- Kirillova, N.Y.; Ruchin, A.B.; Kirillov, A.A.; Chikhlyaev, I.V.; Alpeev, M.A. Overview of Helminths in Land Vertebrates from the Mordovia Nature Reserve, European Russia. Nat. Environ. Pollut. Technol. 2023, 22, 1667–1690. [Google Scholar] [CrossRef]
- Alvi, M.A.; Ohiolei, J.A.; Saqib, M.; Li, L.; Muhammad, N.; Tayyab, M.H.; Qamar, W.; Alvi, A.A.; Wu, Y.D.; Li, X.R.; et al. Preliminary information on the prevalence and molecular description of Taenia hydatigena isolates in Pakistan based on mitochondrial cox1 gene. Infect. Genet. Evol. 2020, 85, 104481. [Google Scholar] [CrossRef] [PubMed]
- Wakid, M.H.; Alsulami, M.N. Genotyping of Taenia hydatigena isolated from sheep and goats in KSA based on Cox1 gene. Saudi J. Biol. Sci. 2022, 29, 1270–1275. [Google Scholar] [CrossRef]
- Maslennikova, O.V. Elk parasites and their danger to humans. In Biodiagnostics of the State of Natural and Natural-Technogenic Systems, Proceedings of the XII All-Russian Scientific-Practical Conference with International Participation, Kirov, Russia, 2–3 December 2014; Vesi Publishing House: Kirov, Russia, 2014; pp. 255–257. (In Russian) [Google Scholar]
- Gavrilova, N.A.; Belova, L.M.; Zabrovskaya, A.V. Larval cestodiasis of elks in the Leningrad region. Curr. Issues Vet. Biol. 2024, 1, 15–18. (In Russian) [Google Scholar] [CrossRef]
- Pelgunov, A.N. Helminthofaunal complex of wild ungulates in biocenoses contaminated with radionuclides. Russ. Parasitol. J. 2010, 2, 11–15. (In Russian) [Google Scholar]
- Baitursinov, K.K. Factors of formation of community of helminth fauna of wild and domestic ungulates of Kazakhstan. Russ. Parasitol. J. 2008, 4, 5–12. (In Russian) [Google Scholar]
- Bulgakova, N.F. Larval cestodiasis of animals of Belarus. Vet. Sci. Abstr. J. 2005, 1, 315. (In Russian) [Google Scholar]
- Kiriltsov, E.V. Population studies of the main parasitic systems of wild animals in the south of the Transbaikal Region. Perm Agrar. Bull. 2024, 2, 110–118. (In Russian) [Google Scholar] [CrossRef]
- Baitursinov, K.K. Ecological bases of prevention of helminthiasis of wild ungulates in conditions of Kazakhstan. Russ. Parasitol. J. 2008, 1, 47–53. (In Russian) [Google Scholar]
- Shikhalieva, M.A.; Golubev, A.A.; Sarbasheva, M.M.; Bittirov, A.M. Epizootological assessment of helminthiasis of chamois, deer and roe deer in the Kabardino-Balkarian Republic. Actual Issues Vet. Biol. 2012, 4, 36–38. (In Russian) [Google Scholar]
- Abdybekova, A.M.; Zhaksylykova, A.A.; Kushaliyev, K.Z.; Kidiraliyev, E.Z.; Kozhayeva, A.R.; Kuzhebayeva, U.Z.; Grachev, A.; Shevtsov, A.; Budke, C.M. A survey of the parasites of Ural saiga antelopes and Turkmenian kulans of Kazakhstan. Int. J. Parasitol. Parasites Wildl. 2023, 21, 232–236. [Google Scholar] [CrossRef]
- Mørk, T.; Eira, H.I.; Rødven, R.; Nymo, I.H.; Blomstrand, B.M.; Guttormsen, S.; Davidson, R.K. Necropsy findings and causes of loss in semi-domesticated reindeer (Rangifer tarandus tarandus) in Northern Norway. Acta Vet. Scand. 2023, 66, 1. [Google Scholar] [CrossRef]
- Maslennikova, O.V.; Kotelnikov, S.A. Taranda cysticercosis of elk in the Udmurt Republic. In Ecology of the Native Land: Problems and Solutions, Proceedings of the XIII All-Russian Scientific and Practical Conference with International Participation, Kirov, Russia, 23–24 April 2018; Vyatka State University Publishing House: Kirov, Russia, 2018; pp. 225–229. (In Russian) [Google Scholar]
- Myczka, A.W.; Jeżewski, W.; Filip-Hutsch, K.J.; Pyziel, A.M.; Kowal, J.; Demiaszkiewicz, A.W.; Laskowski, Z. The morphological and molecular identification of the tapeworm, Taenia lynciscapreoli, in intermediate and definitive hosts in Poland. Int. J. Parasitol. Parasites Wildl. 2020, 11, 213–220. [Google Scholar] [CrossRef]
- Scala, A.; Varcasia, A. Updates on morphobiology, epidemiology and molecular characterization of coenurosis in sheep. Parassitologia 2006, 48, 61–63. [Google Scholar]
- Luo, H.; Zhang, H.; Li, K.; Rehman, M.U.; Mehmood, K.; Lan, Y.; Huang, S.; Li, J. Epidemiological survey and phylogenetic characterization of Cysticercus tenuicollis isolated from Tibetan pigs in Tibet, China. Biomed. Res. Int. 2017, 2017, 7857253. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Gabriël, S.; Abatih, E.N.; Dorny, P. A systematic review on the global occurrence of Taenia hydatigena in pigs and cattle. Vet. Parasitol. 2016, 226, 97–103. [Google Scholar] [CrossRef]
- Akbari, M.; Moazeni, M.; Oryan, A.; Sharifiyazdi, H.; Amrabadi, O. Experimen tal cerebral and non-cerebral coenurosis in goats: A comparative study on the morphological and molecular characteristics of the parasite. Vet. Parasitol. 2015, 211, 201–207. [Google Scholar] [CrossRef]
- Varcasia, A.; Tamponi, C.; Tosciri, G.; Pipia, A.P.; Dore, F.; Schuster, R.K.; Kandil, O.M.; Manunta, M.L.; Scala, A. Is the red fox (Vulpes vulpes) a competent definitive host for Taenia multiceps? Parasit. Vectors 2015, 8, 491. [Google Scholar] [CrossRef]
- Varcasia, A.; Pipia, A.P.; Dessì, G.; Zidda, A.; Tamponi, C.; Pau, M.; Scala, A.; Boufana, B. Morphology and genetic variability within Taenia multiceps in ruminants from Italy. Vet. Parasitol. 2016, 223, 181–185. [Google Scholar] [CrossRef]
- Scott, P.R. Diagnosis and treatment of coenurosis in sheep. Vet. parasitol. 2012, 189, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Varcasia, A.; Tosciri, G.; Coccone, G.N.S.; Pipia, A.P.; Garippa, G.; Scala, A.; Damien, V.; Vural, G.; Gauci, C.G.; Lightowlers, M.W. Preliminary field trial of a vaccine against coenurosis caused by Taenia multiceps. Vet. Parasitol. 2009, 162, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Akbari, M.; Moazeni, M.; Amrabadi, O.R. Cerebral and non-cerebral coenurosis in small ruminants. Trop. Biomed. 2014, 31, 1–16. [Google Scholar] [PubMed]
- Rostami, S.; Salavati, R.; Beech, R.N.; Sharbatkhori, M.; Babaei, Z.; Saedi, S.; Harandi, M.F. Cytochrome c oxidase subunit 1 and 12S ribosomal RNA characterization of Coenurus cerebralis from sheep in Iran. Vet. Parasitol. 2013, 197, 141–151. [Google Scholar] [CrossRef]
- Scala, A.; Cancedda, G.M.; Varcasia, A.; Ligios, C.; Garippa, G.; Genchi, C. A survey of Taenia multiceps coenurosis in Sardinian sheep. Vet. Parasitol. 2007, 143, 294–298. [Google Scholar] [CrossRef]
- Varma, T.K.; Malviya, H.C. Prevalence of coenuriosis in sheep, goat and pigs in Bareilly, Utar Pradesh. J. Vet. Parasitol. 1989, 3, 69–71. [Google Scholar]
- Tavassoli, M.; Malekifard, F.; Soleimanzadeh, A.; Tajik, H. Prevalence of Coenurus cerebralis in sheep in northwest Iran. Vet. Res. Forum 2011, 2, 274–276. [Google Scholar]
- Nooruddin, M.; Dey, A.; Ali, M.A. Coenurosis in Bengal goats of Bangladesh. Small Rumin. Res. 1996, 19, 77–81. [Google Scholar] [CrossRef]
- Ozmen, O.; Sahinduran, S.; Haligur, M.; Sezer, K. Clinicopathologic observa tions on Coenurus cerebralis in naturally infected sheep. Schweiz. Arch. Tierheilkd. 2005, 147, 129–134. [Google Scholar] [CrossRef]
- Miran, M.B.; Nzalawahe, J.; Kassuku, A.A.; Swai, E.S. Prevalence of coenurosis in sheep and goats at three slaughter slabs in Ngorongoro District, Tanzania. Trop. Anim. Health Prod. 2015, 47, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Gunyakti Kilinc, S.; Celik, F.; Kesik, H.K.; Simsek, S. In silico analysis of the biodiversity and conservation status of mitochondrial cytochrome C oxidase subunit 1 (CO1) gene of Taenia multiceps. Acta Parasitol. 2020, 65, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Varcasia, A.; Lightowlers, M.W.; Cattoli, G.; Cancedda, G.M.; Canu, S.; Garippa, G.; Scala, A. Genetic variation within Taenia multiceps in Sardinia, western Mediterranean (Italy). Parasitol. Res. 2006, 99, 622626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Jia, W.Z.; Qu, Z.G.; Xie, Z.Z.; Luo, J.X.; Yin, H.; Sun, X.L.; Blaga, R.; Fu, B.Q. Molecular characterization of Taenia multiceps isolates from Gansu Province, China by sequencing of mitochondrial cytochrome C oxidase subunit 1. Korean J. Parasitol. 2013, 51, 197–201. [Google Scholar] [CrossRef]
- Sonmez, B.; Koroglu, E.; Simsek, S. Molecular characterization and detection of variants of Taenia multiceps in sheep in Turkey. Parasitology 2017, 144, 220–225. [Google Scholar] [CrossRef]
- Al-Riyami, S.; Ioannidou, E.; Koehler, A.V.; Hussain, M.H.; Al-Rawahi, A.H.; Giadinis, N.D.; Lafi, S.Q.; Papadopoulos, E.; Jabbar, A. Genetic characterisation of Taenia multiceps cysts from ruminants in Greece. Infect. Genet. Evol. 2016, 38, 110–116. [Google Scholar] [CrossRef]
- Boufana, B.; Scala, A.; Lahmar, S.; Pointing, S.; Craig, P.S.; Dessì, G.; Zidda, A.; Pipia, A.P.; Varcasia, A. A preliminary investigation into the genetic variation and population structure of Taenia hydatigena from Sardinia, Italy. Vet. Parasitol. 2015, 214, 67–74. [Google Scholar] [CrossRef]
- Adwan, K.; Jayousi, A.; Abuseir, S.; Abbasi, I.; Adwan, G.; Jarrar, N. Genetic diversity of Taenia hydatigena in the northern part of the West Bank, Palestine as determined by mitochondrial DNA sequences. Acta Parasitol. 2018, 63, 299–303. [Google Scholar] [CrossRef]
- Kilinc, S.G.; Kesik, H.K.; Simsek, S. Molecular characterization and haplotypes of sheep and goat isolates of Cysticercus tenuicollis in Turkey. Parasitology 2019, 146, 1047–1054. [Google Scholar] [CrossRef]
- Kazakhstan: Sustainable Livestock Development Program for Results. World Bank Group. Available online: https://www.worldbank.org/en/country/kazakhstan/brief/sustainable-livestock-development-program-for-results?utm_source=chatgpt.com (accessed on 10 September 2025).
- Skokov, R.; Dyussegalieva, B. Current state of livestock economy in Kazakhstan. In Proceedings of the International Scientific Conference “Fundamental and Applied Scientific Research in the Development of Agriculture in the Far East” (AFE–2023), Tashkent, Uzbekistan, 12 December 2023; Volume 462, p. e01023. [Google Scholar] [CrossRef]
- Key Indicators of Livestock Development in the Republic of Kazakhstan (January–December 2024). Available online: https://stat.gov.kz/ru/industries/business-statistics/stat-forrest-village-hunt-fish/publications/287928/ (accessed on 10 September 2025).
- Skrjabin, K.I. Method of Complete Helminthological Dissection of Vertebrates, Including Humans; Publishing House of 1st Moscow State University: Moscow, Russia, 1928; pp. 40–48. (In Russian) [Google Scholar]
- Deplazes, P.; Eckert, J.; Mathis, A.; Von Samson-Himmelstjerna, G.; Zahner, H. Parasitology in Veterinary Medicine, 3rd ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 224–239. [Google Scholar]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology. In Parasites of Sheep and Goats, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 436–523. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Al Malki, J.S.; Hussien, N.A. Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia. Open Life Sci. 2021, 16, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Samorek-Pieróg, M.; Karamon, J.; Brzana, A.; Bilska-Zajac, E.; Zdybel, J.; Cencek, T. Molecular Confirmation of Massive Taenia pisiformis Cysticercosis in One Rabbit in Poland. Pathogens 2021, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.; Elbeskawy, M. Molecular and phylogenetic status of Coenurus cerebralis infecting sheep from Dakahlia province, Egypt. J. Adv. Parasitol. 2016, 3, 117–124. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- McHugh, M.L. The chi-square test of independence. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef]
- Pybus, M.J. Survey of hepatic and pulmonary helminths of wild cervids in Alberta, Canada. J. Wildl. Dis. 1990, 26, 453–459. [Google Scholar] [CrossRef]
- Office International des Epizooties (OIE). Chapter 3.9.5 Cysticercosis. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees); Office International des Epizooties (OIE): Paris, France, 2018; pp. 1693–1704. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.09.05_CYSTICERCOSIS.pdf (accessed on 2 June 2025).
- Perl, S.; Edery, N.; Bouznach, A.; Abdalla, H.; Markovics, A. Acute Severe Visceral Cysticercosis in Lambs and Kids in Israel. Isr. J. Vet. Med. 2015, 70, 49–53. [Google Scholar]
- Akramova, F.; Shakarbaev, U.; Mirzayeva, A.; Saidova, S.; Akbarova, M.; Uralova, F.; Hamrokulova, Z.; Ubbiniyazova, J.; Toremuratov, M.; Saparov, K.; et al. Helminths of domestic and wild artiodactyls (Mammalia, Artiodactyla) in Uzbekistan. Biosyst. Divers. 2025, 33, e2514. [Google Scholar] [CrossRef]
- Gaipova, M.E.; Akramova, F.D.; Saparov, K.A.; Azimov, D.A.; Shakarbaev, U.A. Fauna and ecology of helminths in cattle (Bos taurus Dom.) of Central Uzbekistan. Russ. J. Parasitol. 2016, 38, 447–453. (In Russian) [Google Scholar] [CrossRef]
- Razikov, S.S.; Sherkhonov, T. Reasons of prevalence of Cysticercus bovis in the zones of yak husbandry in the Republic of Tadjikistan. Theory Pract. Parasit. Dis. Anim. 2011, 12, 414–416. (In Russian) [Google Scholar]
- Murtazoev, D.M.; Valiev, H.G.; Pulotov, M.B.; Faizullaev, U.F. Epidemiological situation and problems of combating taeniasis in Tajikistan. Theory Pract. Combat. Parasit. Dis. 2015, 16, 276–278. (In Russian) [Google Scholar]
- Shakirov, A.B. Helminths and Helminth Diseases of Cattle in the Kyrgyz Republic and Methods for their Control. Ph.D. Dissertation, Dyishyeyeva Scientific Research Institute of Livestock, Veterinary and Pastures, Bishkek, Kyrgyzstan, 2004. (In Russian). [Google Scholar]
- Uakhit, R.; Bauer, C.; Smagulova, A.; Kiyan, V. First Reported Case of Accidental Gastric Myiasis Caused by Gasterophilus Larvae in a Gray Wolf. Acta Parasitol. 2025, 70, 1–5. [Google Scholar] [CrossRef]
- Bauer, C.; Uakhit, R.; Smagulova, A.; Jazina, K.; Lyalchenko, A.; Kiyan, V. Where there are moose (Alces alces) in Eurasia, there are moose nose botflies: First morphological and molecular identification of Cephenemyia ulrichii (Brauer, 1862) in Kazakhstan. Int. J. Parasitol. Parasites Wildl. 2025, 27, 101086. [Google Scholar] [CrossRef]
- Zhaxylykov, A.; Uakhit, R.; Kiyan, V. Study of prevalence and morphological features of cestodes in foxes (Vulpes vulpes) in the territory of Kazakhstan. J. Biol. Res. 2024, 1, 31–38. [Google Scholar] [CrossRef]
- Morais, D.F.; Vilela, V.L.R.; Feitosa, T.F.; Santos, V.M.D.; Gouveia, V.R.; Athayde, A.C.R.; Azevêdo, S.S. Prevalence and risk factors for Cysticercus tenuicollis in goats and sheep in Paraíba, northeastern Brazil. Rev. Bras. Parasitol. Vet. 2017, 26, 235–238. [Google Scholar] [CrossRef]
- Bhat, R.A.; Tak, H.; Bhat, B.A.; Dar, J.A.; Ahmad, R. Gastrointestinal helminth parasites of wild ungulates in Hirpora Wildlife Sanctuary, Kashmir, India. J. Parasit. Dis. 2022, 46, 804–810. [Google Scholar] [CrossRef]
- Avise, J.C.P. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2001; p. 447. [Google Scholar]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
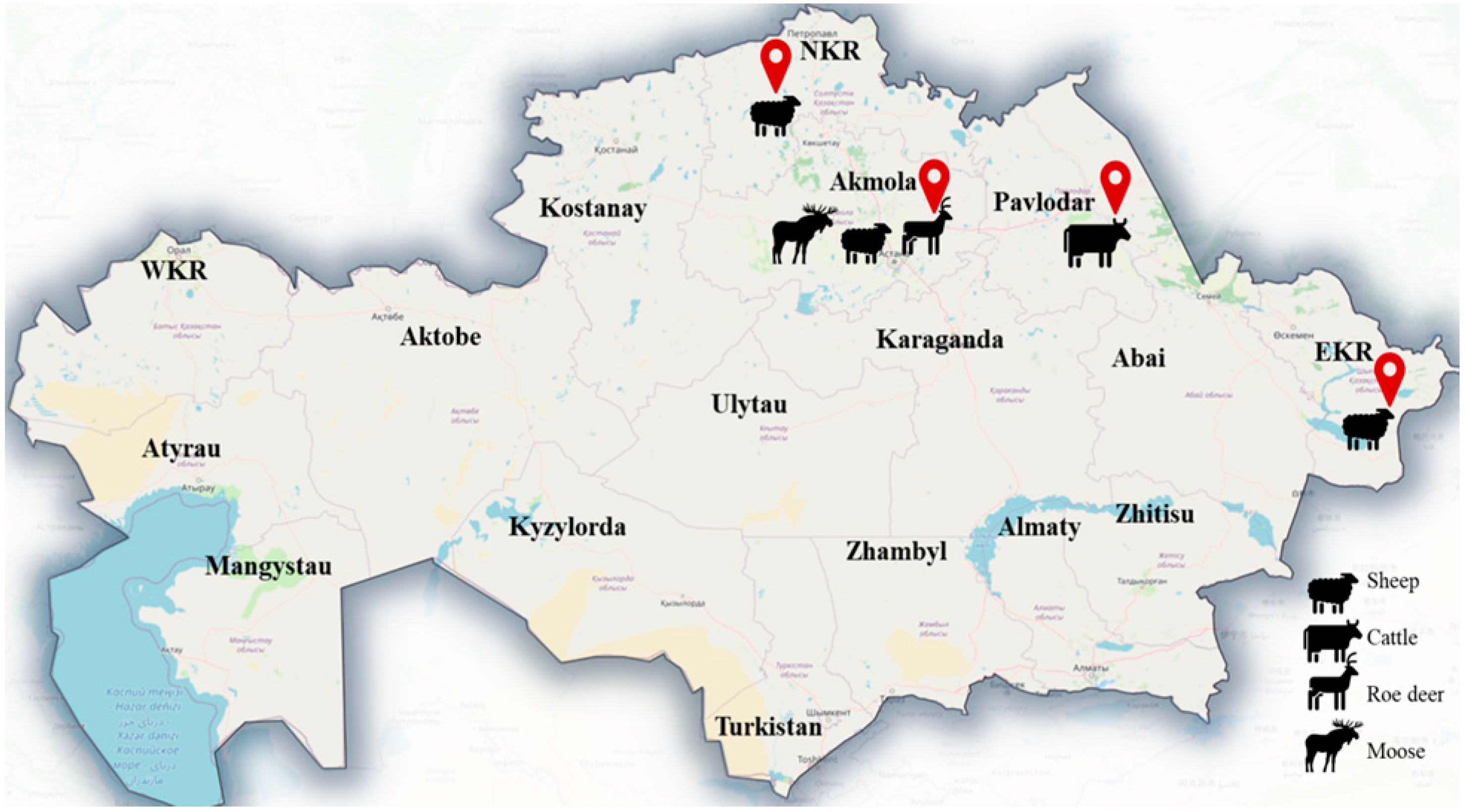

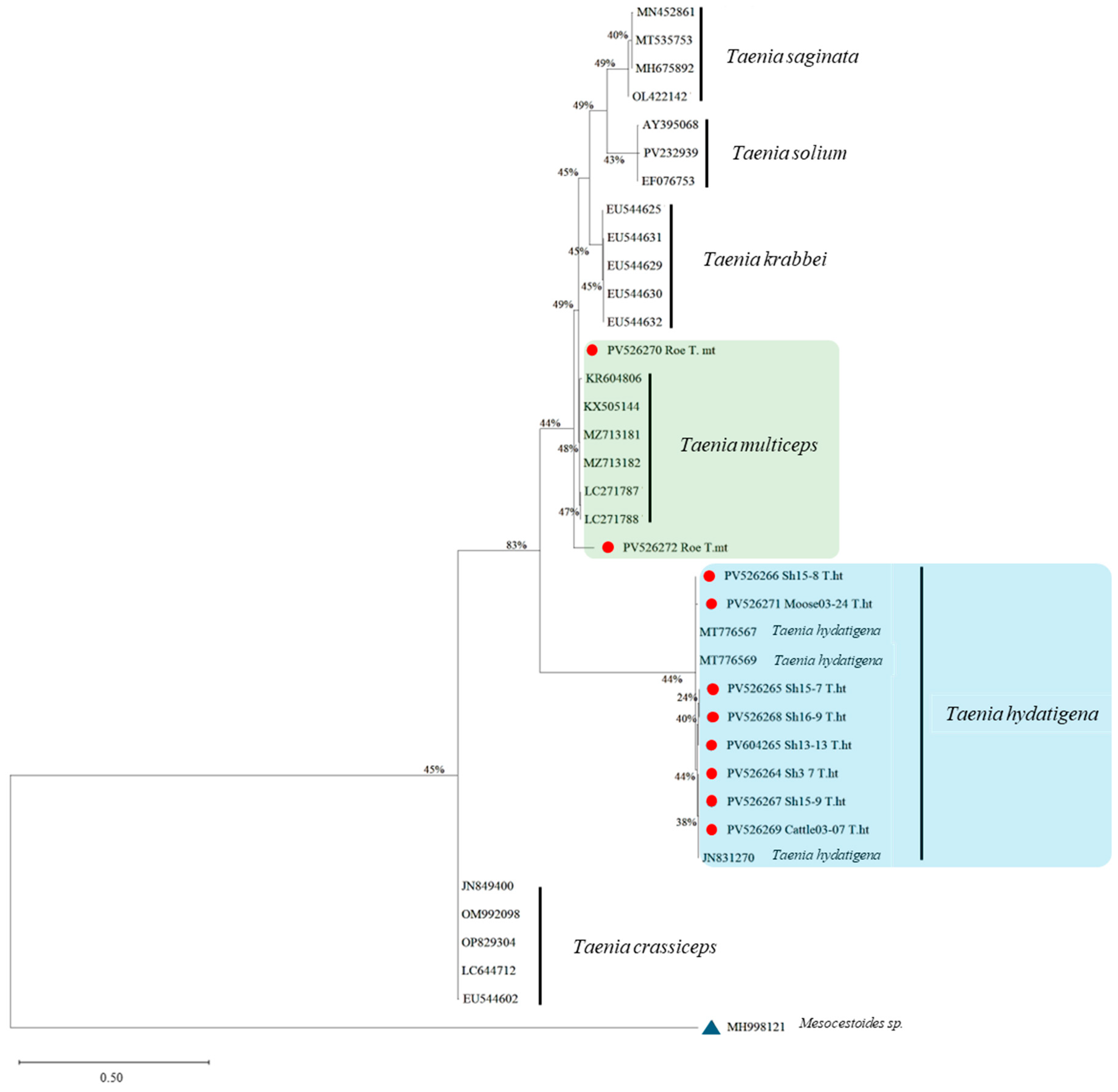
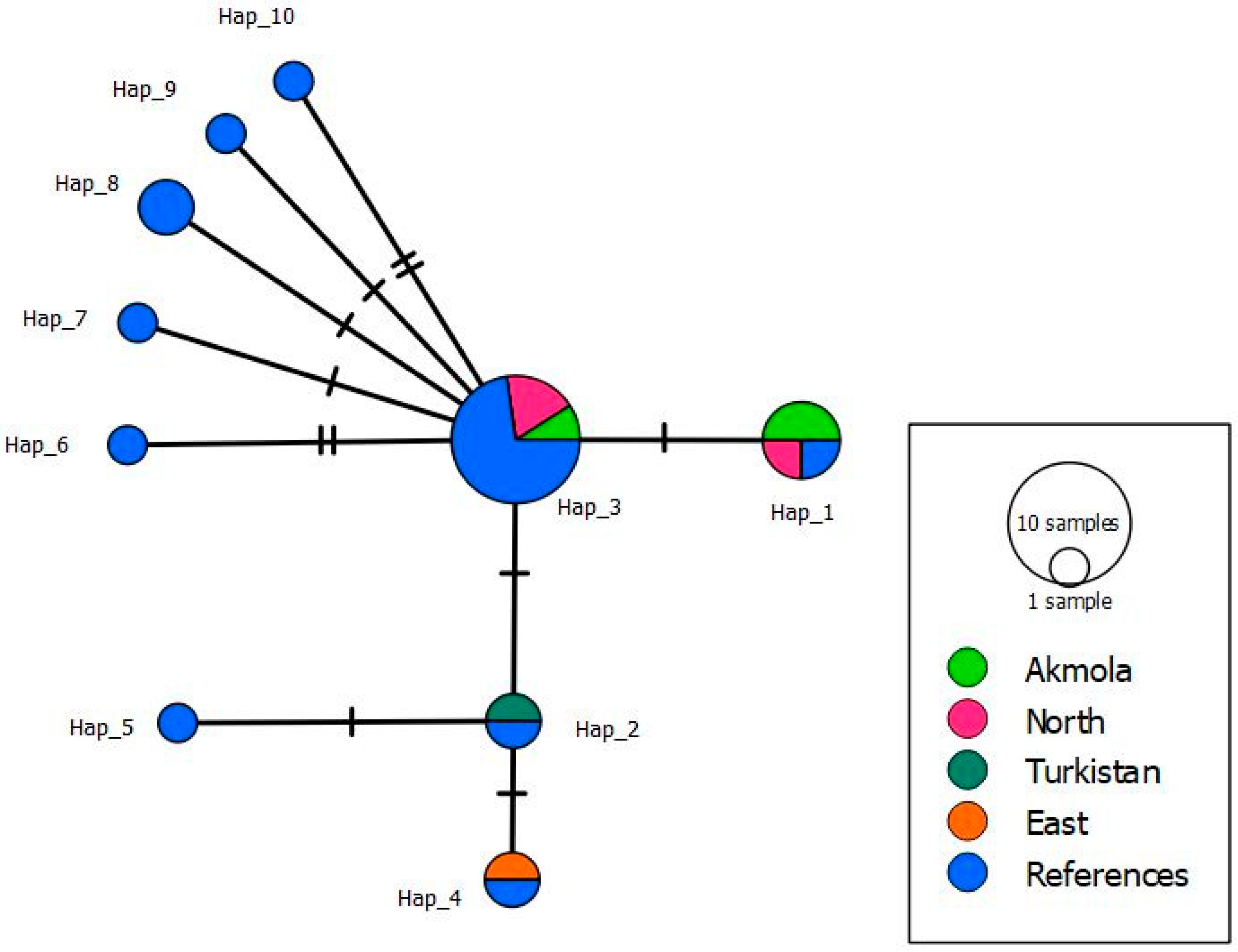
| Host | N infected/N Examined | % Prevalence (95% CI) | N Cyst Found | Range of Intensity | Mean (SD) Intensity | Cysticercosis Species Identified |
|---|---|---|---|---|---|---|
| cattle | 5/773 | 0.6 (0.2–1.5) | 18 | 2–5 | 3.6 (1.14) | T. hydatigena |
| sheep | 6/563 | 1.1 (0.3–2.3) | 24 | 2–6 | 4 (1.41) | T. hydatigena |
| roe deer | 2/25 | 8.0 (0.9–2.6) | 15 | 6–9 | 7.5 (2.12) | T. multiceps |
| moose | 1/9 | 11.11 (0.2–48.2) | 1 | 1 | - | T. hydatigena |
| red deer | 0/2 | - | - | - | - | |
| Chi-square test | χ2 = 22.23, df = 4, p-value = 0.0001 |
| nad1 Gene (894) | |
|---|---|
| Number of mutations | 11 |
| Haplotypes number | 10 |
| Haplotype diversity | 0.820 |
| Nucleotide diversity | 0.00397 |
| Tajima’s test | −1.65005 |
| Fu’s test | −5.282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiyan, V.; Smagulova, A.; Uakhit, R.; Hermosilla, C.; Lider, L.; Jazina, K.; Manapov, N. Molecular Identification of Cryptic Cysticercosis: Taenia spp. in Wild and Domestic Intermediate Hosts in Kazakhstan. Diversity 2025, 17, 655. https://doi.org/10.3390/d17090655
Kiyan V, Smagulova A, Uakhit R, Hermosilla C, Lider L, Jazina K, Manapov N. Molecular Identification of Cryptic Cysticercosis: Taenia spp. in Wild and Domestic Intermediate Hosts in Kazakhstan. Diversity. 2025; 17(9):655. https://doi.org/10.3390/d17090655
Chicago/Turabian StyleKiyan, Vladimir, Ainura Smagulova, Rabiga Uakhit, Carlos Hermosilla, Lyudmila Lider, Karina Jazina, and Nurassyl Manapov. 2025. "Molecular Identification of Cryptic Cysticercosis: Taenia spp. in Wild and Domestic Intermediate Hosts in Kazakhstan" Diversity 17, no. 9: 655. https://doi.org/10.3390/d17090655
APA StyleKiyan, V., Smagulova, A., Uakhit, R., Hermosilla, C., Lider, L., Jazina, K., & Manapov, N. (2025). Molecular Identification of Cryptic Cysticercosis: Taenia spp. in Wild and Domestic Intermediate Hosts in Kazakhstan. Diversity, 17(9), 655. https://doi.org/10.3390/d17090655









