Integrating Egg Case Morphology and DNA Barcoding to Discriminate South American Catsharks, Schroederichthys bivius and S. chilensis (Carcharhiniformes: Atelomycteridae)
Abstract
1. Introduction
2. Materials and Methods
3. Results
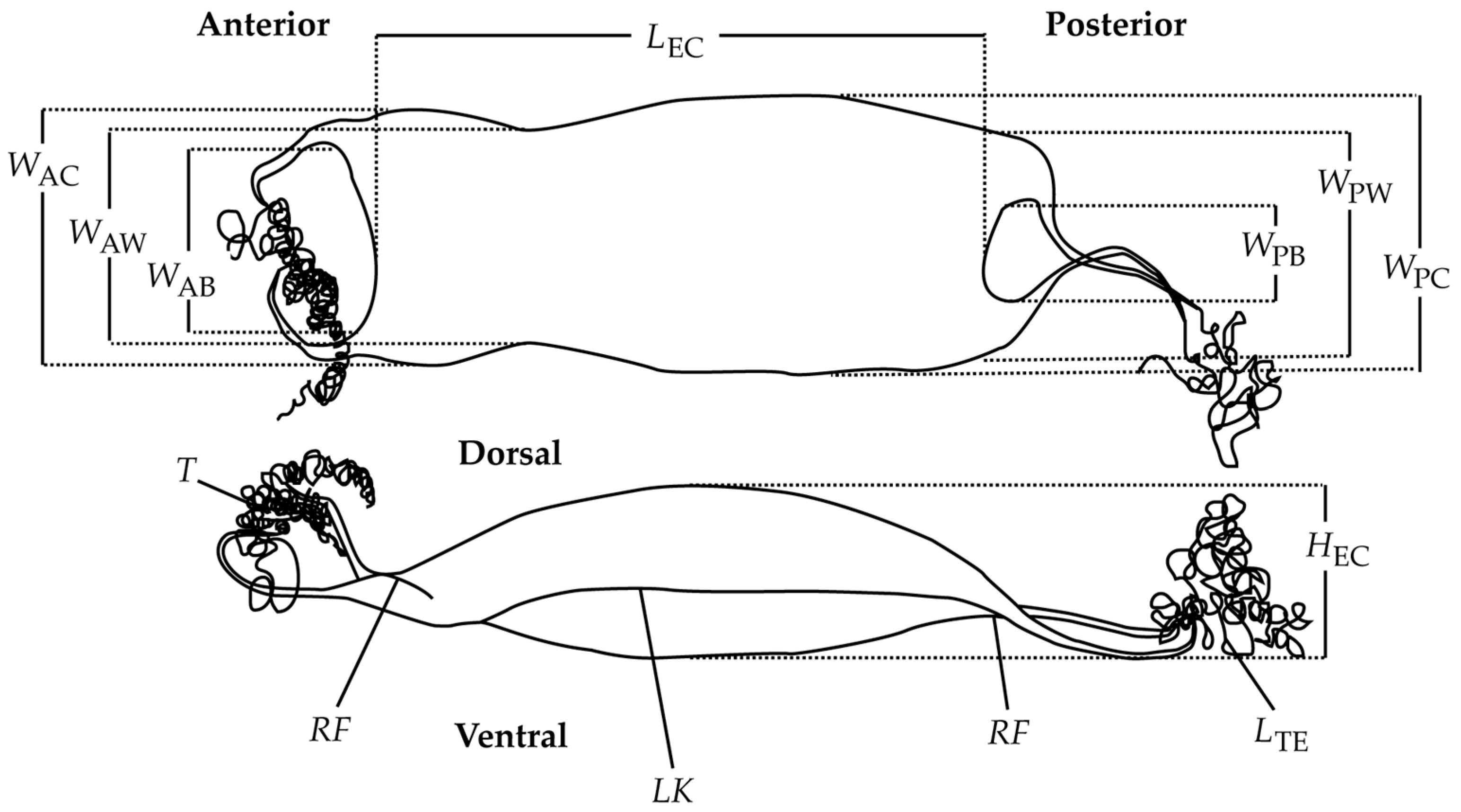
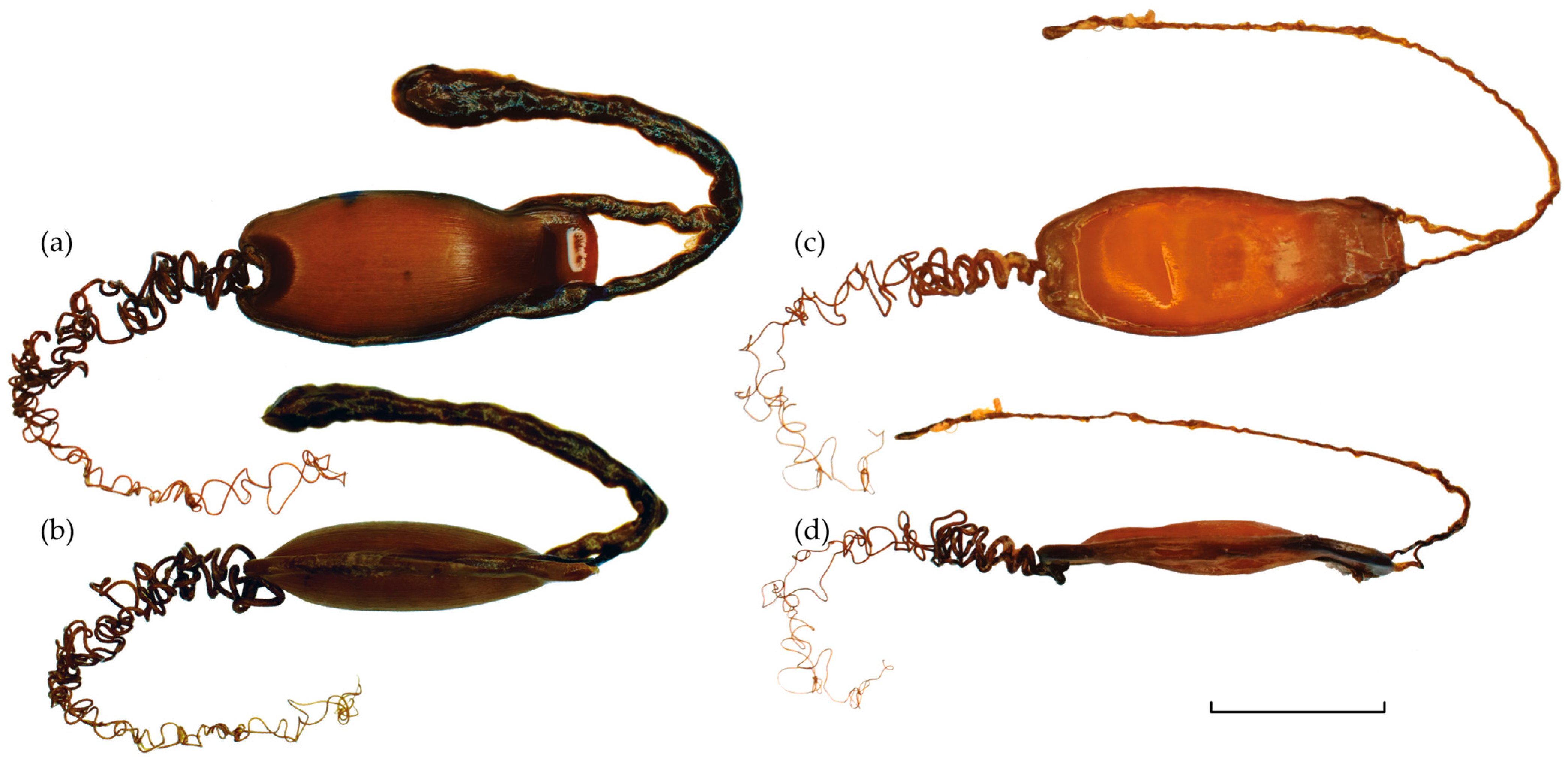
3.1. Description of the Egg Cases
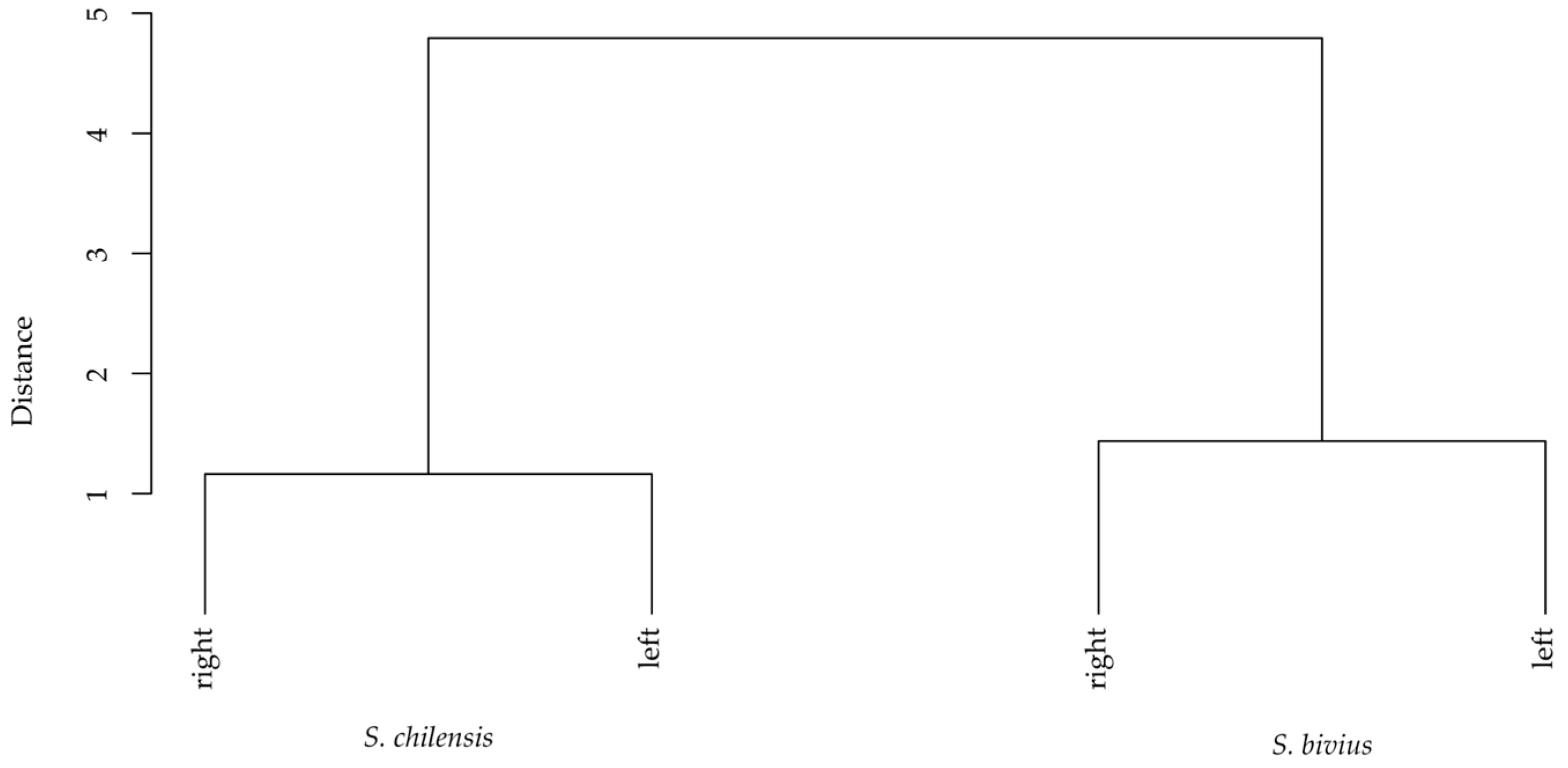
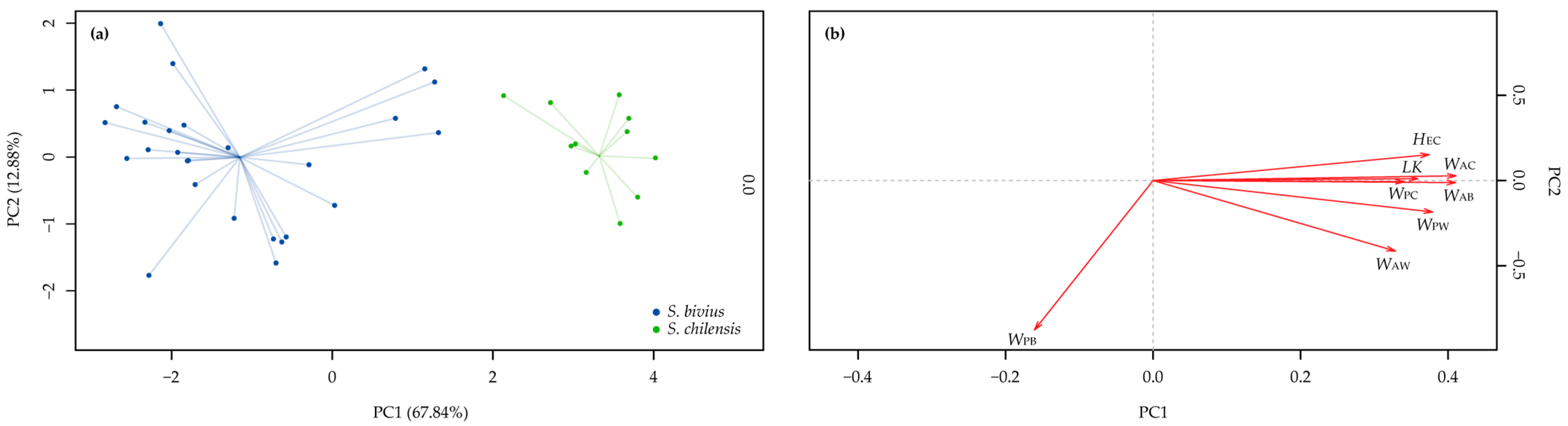
3.2. Morphometric Analyses
3.3. Molecular Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787.e8. [Google Scholar] [CrossRef]
- Pacoureau, N.; Carlson, J.K.; Kindsvater, H.K.; Rigby, C.L.; Winker, H.; Simpfendorfer, C.A.; Charvet, P.; Pollom, R.A.; Barreto, R.; Sherman, C.S.; et al. Conservation successes and challenges for wide-ranging sharks and rays. Proc. Natl. Acad. Sci. USA 2023, 120, e2216891120. [Google Scholar] [CrossRef] [PubMed]
- Sherman, C.S.; Simpfendorfer, C.A.; Pacoureau, N.; Matsushiba, J.H.; Yan, H.F.; Walls, R.H.L.; Rigby, C.L.; VanderWright, W.J.; Jabado, R.W.; Pollom, R.A.; et al. Half a century of rising extinction risk of coral reef sharks and rays. Nat. Commun. 2023, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Dulvy, N.K.; Pacoureau, N.; Matsushiba, J.H.; Yan, H.F.; VanderWright, W.J.; Rigby, C.L.; Finucci, B.; Sherman, C.S.; Jabado, R.W.; Carlson, J.K.; et al. Ecological erosion and expanding extinction risk of sharks and rays. Science 2024, 386, eadn1477. [Google Scholar] [CrossRef] [PubMed]
- White, W.T.; Fahmi; Weigmann, S. A new genus and species of catshark (Carcharhiniformes: Scyliorhinidae) from eastern Indonesia. Zootaxa 2019, 4691, 444–460. [Google Scholar] [CrossRef]
- Gomes, U.L.; Peters, G.O.; De Carvalho, M.R.; Gadig, O.B.F. Anatomical investigation of the slender catshark Schroederichthys tenuis Springer, 1966, with notes on intrageneric relationships (Chondrichthyes: Carcharhiniformes: Scyliorhinidae). Zootaxa 2006, 1119, 29–58. [Google Scholar] [CrossRef]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef]
- Ebert, D.A.; Dando, M.; Fowler, S. Sharks of the World. A Complete Guide; Princeton University Press: Princeton, NJ, USA, 2021; pp. 429–433. [Google Scholar]
- Bornatowski, H.; Santos, L.; Robert, M.D.C.; Weiser, P.A. Occurrence of the narrowmouth catshark Schroederichthys bivius (Chondrichthyes: Scyliorhinidae) in southern Brazil. Mar. Biodivers. Rec. 2014, 7, e51. [Google Scholar] [CrossRef]
- Lamilla, J.; Bustamante, C.; Roa, R.; Acuña, E.; Vargas-Caro, C. Estimación del Descarte de Condrictios en Pesquerías Artesanales; Universidad Austral de Chile: Valdivia, Chile, 2010. [Google Scholar]
- Bustamante, C.; Acuña, E.; Tapia-Jopia, C.; Vargas-Caro, C. Actualización del Plan de Acción Nacional Para la Conservación y Manejo de Tiburones de Chile; Universidad de Antofagasta: Antofagasta, Chile, 2023; pp. 211–232. [Google Scholar]
- Sánchez, F.; Marí, N.R.; Bernardele, J.C. Distribución, abundancia relativa y alimentación de pintarroja Schroederichthys bivius Müller & Henle, 1838 en el Océano Atlántico sudoccidental. Rev. Biol. Mar. Oceanogr. 2009, 44, 453–466. [Google Scholar] [CrossRef]
- Colonello, J.H.; Cortés, F.; Belleggia, M. Male-biased sexual size dimorphism in sharks: The narrowmouth catshark Schroederichthys bivius as case study. Hydrobiologia 2020, 847, 1873–1886. [Google Scholar] [CrossRef]
- Springer, S. A revision of the catsharks, family Scyliorhinidae. NOAA Tech. Rep. NMFS Circ. 1979, 422, 1–153. [Google Scholar]
- Soares, K.D.A.; Mathubara, K. Combined phylogeny and new classification of catsharks (Chondrichthyes: Elasmobranchii: Carcharhiniformes). Zool. J. Linn. Soc. 2022, 195, 761–814. [Google Scholar] [CrossRef]
- Ishiyama, R. Studies on the rays and skates belonging to the family Rajidae, found in Japan and adjacent regions. 1 Egg-capsule of ten species. Jap. J. Ichthyol. 1950, 1, 30–36. [Google Scholar]
- Nakaya, K. Taxonomy, comparative anatomy and phylogeny of Japanese catsharks, Scyliorhinidae. Mem. Fac. Fish. Hokkaido Univ. 1975, 23, 1–94. [Google Scholar]
- Musick, J.A.; Ellis, J.K. Reproductive evolution of chondrichthyans. In Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Rays and Chimaeras; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 45–79. [Google Scholar]
- Ebert, D.A.; Compagno, L.J.V.; Cowley, P.D. Reproductive biology of catsharks (Chondrichthyes: Scyliorhinidae) off the west coast of southern Africa. ICES J. Mar. Sci. 2006, 63, 1053–1065. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Reynolds, J.D. Evolutionary transitions among egg-laying, live-bearing and maternal inputs in sharks and rays. Proc. R. Soc. Lond. B 1997, 264, 1309–1315. [Google Scholar] [CrossRef]
- Flammang, B.E.; Ebert, D.A.; Cailliet, G.M. Egg cases of the genus Apristurus (Chondrichthyes: Scyliorhinidae): Phylogenetic and ecological implications. Zoology 2007, 110, 308–317. [Google Scholar] [CrossRef]
- Kyne, P.M.; Simpfendorfer, C.A. Deepwater Chondrichthyans. In Biology of Sharks and Their Relatives II: Biodiversity, Adaptive Physiology, and Conservation; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 37–114. [Google Scholar]
- Mabragaña, E.; Figueroa, D.; Scenna, L.; Díaz de Astarloa, J.; Colonello, J.; Delpiani, G. Chondrichthyan egg cases from the south-west Atlantic Ocean. J. Fish Biol. 2011, 79, 1261–1290. [Google Scholar] [CrossRef]
- Bustamante, C.; Kyne, P.M.; Bennett, M.B. Comparative morphology of the egg cases of Asymbolus analis, Asymbolus rubiginosus and Figaro boardmani (Carcharhiniformes: Scyliorhinidae) from southern Queensland, Australia. J. Fish Biol. 2013, 83, 133–143. [Google Scholar] [CrossRef]
- Mancusi, C.; Massi, D.; Baino, R.; Cariani, A.; Crobe, V.; Ebert, D.A.; Ferrari, A.; Gordon, C.A.; Hoff, G.R.; Iglesias, S.P.; et al. An identification key for Chondrichthyes egg cases of the Mediterranean and Black Sea. Eur. Zool. J. 2021, 88, 436–448. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.H.; Steinke, D.; Ward, R.W. Identification of shark and ray fins using DNA barcoding. Fish. Res. 2009, 95, 280–288. [Google Scholar] [CrossRef]
- Naylor, G.J.P.; Caira, J.N.; Jensen, K.; Rosana, K.A.M.; Straube, N.; Lakner, C. Elasmobranch Phylogeny: A Mitochondrial Estimate Based on 595 Species. In Biology of Sharks and Their Relatives, 2nd ed.; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 31–56. [Google Scholar]
- Veríssimo, A.; Zaera-Perez, D.; Leslie, R.; Iglésias, S.P.; Séret, B.; Grigoriou, P.; Sterioti, A.; Gubili, C.; Barría, C.; Duffy, C.; et al. Molecular diversity and distribution of eastern Atlantic and Mediterranean dogfishes Squalus highlight taxonomic issues in the genus. 2017. Zool. Scr. 2017, 46, 414–428. [Google Scholar] [CrossRef]
- Lamilla, J.; Bustamante, C. Guía Para el Reconocimiento de: Tiburones, Rayas y Quimeras de Chile; Oceana: Santiago, Chile, 2005; pp. 31–32. [Google Scholar]
- Gomes, U.L.; de Carvalho, M.R. Egg capsules of Schroederichthys tenuis and Scyliorhinus haeckelii (Chondrichthyes, Scyliorhinidae). Copeia 1995, 1995, 232–236. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef]
- Šlenker, M.; Koutecký, P.; Marhold, K. MorphoTools2: An R package for multivariate morphometric analysis. Bioinformatics 2022, 38, 2954–2955. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Exploratory Multivariate Analysis. In Modern Applied Statistics with S, 4th ed.; Venables, W.N., Ripley, B.D., Eds.; Springer: New York, NY, USA, 2002; pp. 301–330. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary Rrte of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Trujillo, J.; Pardo, L.M.; Vargas-Chacoff, L.; Valdivia, N. Sharks in the forest: Relationships between kelp physical-complexity attributes and egg deposition sites of the red-spotted catshark. Mar. Ecol. Prog. Ser. 2018, 610, 125–135. [Google Scholar] [CrossRef]
- Cardeñosa, D.; Fields, A.T.; Shea, S.K.H.; Feldheim, K.A.; Chapman, D.D. Relative contribution to the shark fin trade of Indo-Pacific and Eastern Pacific pelagic thresher sharks. Anim. Conserv. 2021, 24, 367–372. [Google Scholar] [CrossRef]
- Domingues, R.R.; Bunholi, I.V.; Pinhal, D.; Antunes, A.; Mendonça, F.F. From molecule to conservation: DNA-based methods to overcome frontiers in the shark and ray fin trade. Conserv. Genet. Resour. 2021, 13, 231–247. [Google Scholar] [CrossRef]
- Soares, K.D.A.; de Carvalho, M.R. The catshark genus Scyliorhinus (Chondrichthyes: Carcharhiniformes: Scyliorhinidae): Taxonomy, morphology and distribution. Zootaxa 2019, 4601, 1–147. [Google Scholar] [CrossRef]
- Soares, K.D.A.; de Carvalho, M.R. Phylogenetic relationship of catshark species of the genus Scyliorhinus (Chondrichthyes, Carcharhiniformes, Scyliorhinidae) based on comparative morphology. Zoosyst. Evol. 2020, 96, 345–395. [Google Scholar] [CrossRef]
- Soares, K.D.A.; Zanini, F. Redescription and anatomical investigation of Schroederichthys maculatus Springer, 1966 and S. saurisqualus Soto, 2001 with comments on their systematics (Chondrichthyes: Carcharhiniformes: Atelomycteridae). Zool. Anz. 2023, 302, 224–238. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Acuña, E.; Bustamante, C.; Chiaramonte, G.E.; Cuevas, J.M.; Herman, K.; Pompert, J.; Velez-Zuazo, X. Schroederichthys bivius. In The IUCN Red List of Threatened Species; IUCN: Cambridge, UK, 2020; p. e.T39347A2906921. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Acuña, E.; Bustamante, C.; Herman, K.; Velez-Zuazo, X. Schroederichthys chilensis. In The IUCN Red List of Threatened Species; IUCN: Cambridge, UK, 2020; p. e.T44585A124433964. [Google Scholar] [CrossRef]
- Carr, M.H. Effects of Macroalgal Assemblages on the Recruitment of Temperate Zone Reef Fishes. J. Exp. Mar. Biol. Ecol. 1989, 126, 59–76. [Google Scholar] [CrossRef]
- Pérez-Matus, A.; Micheli, F.; Konar, B.; Shears, N.; Low, N.H.N.; Okamoto, D.K.; Wernberg, T.; Krumhansl, K.A.; Ling, S.D.; Kingsford, M.; et al. Kelp forests as nursery and foundational habitat for reef fishes. Ecology 2025, 106, e70007. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.J.; Pfirrmann, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.W.; Orth, R.J. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019, 12, e12645. [Google Scholar] [CrossRef]

| Species | Locality | Accession Number |
|---|---|---|
| Carcharhiniformes: Atelomycteridae | ||
| Atelomycterus baliensis | Indonesia: Bali | EU398568, EU398569 |
| Atelomycterus erdmanni | Indonesia | KP769787 |
| Atelomycterus fasciatus | Australia: Western Australia | EU398570 |
| Atelomycterus marmoratus | Philippines: Cebu | OQ386292, OQ386496 |
| Atelomycterus marnkalha | Australia: Queensland | EU398574, EU398577 |
| Aulohalaelurus labiosus | Australia: Western Australia | EU398581, HQ955988, JN312813 |
| Schroederichthys bivius | Argentina | EU074581–EU074586 |
| Schroederichthys bivius | Chile: Valdivia | PX250298–PX250303 |
| Schroederichthys bivius | Chile: Puerto Montt | PX250304–PX250309 |
| Schroederichthys chilensis | Chile: Coquimbo | MK982882, MK982902 |
| Schroederichthys chilensis | Chile: Iquique | PX250310, PX250311 |
| Schroederichthys chilensis | Chile: Valparaíso | PX250312–PX250315 |
| Schroederichthys chilensis | Chile: San Antonio | PX250316–PX250321 |
| Carcharhiniformes: Scyliorhinidae | ||
| Cephaloscyllium laticeps | Australia: Victoria | HQ956280 |
| Cephaloscyllium pictum | Indonesia: Nusa Tenggara Barat | EU398676 |
| Cephaloscyllium silasi | India | KF899711 |
| Cephaloscyllium variegatum | Australia: Tasmania | HQ956282 |
| Poroderma africanum | South Africa: Western Cape | OR138385, OR138381 |
| Poroderma pantherinum | South Africa: Western Cape | OR138386, OR138391 |
| Scyliorhinus canicula | USA: Washington | KJ709897, JN641232 |
| Scyliorhinus capensis | South Africa: Western Cape | OR138392 |
| Scyliorhinus stellaris | Malta | KJ709900 |
| Carcharhiniformes: Pentanchidae | ||
| Apristurus brunneus | USA: Washington | JQ353982 |
| Apristurus melanoasper | Canada: Newfoundland | MW339366 |
| Apristurus nasutus | Chile: Coquimbo | KU737638 |
| Apristurus profundorum | Canada: Newfoundland | MW339382 |
| Holohalaelurus punctatus | South Africa | OR138367, OR138368 |
| Holohalaelurus regani | South Africa: Western Cape | OR138372, OR138373 |
| Asymbolus analis | Australia | HM902609 |
| Asymbolus parvus | Australia: Western Australia | EU398564 |
| Asymbolus rubiginosus | Australia | EU398567 |
| Carcharhiniformes: Carcharhinidae | ||
| Carcharhinus brachyurus 1 | South Korea: Jeju island | MT995631 |
| Measurement | Schroederichthys chilensis | Schroederichthys bivius | ||
|---|---|---|---|---|
| Mean (s.d.) | Range (mm) | Mean (s.d.) | Range (mm) | |
| LEC | 52.3 (3.8) | 45.2–57.1 | 52.8 (3.2) | 45.0–58.6 |
| HEC | 11.0 (1.8) | 7.8–13.7 | 4.9 (1.6) | 2.5–10.0 |
| LK | 3.3 (0.4) | 2.8–4.0 | 2.0 (0.9) | 0.1–3.7 |
| WAB | 14.0 (0.4) | 13.3–14.6 | 9.1 (1.3) | 7.2–13.4 |
| WAC | 17.4 (0.9) | 15.7–18.5 | 13.0 (1.5) | 10.2–16.6 |
| WAW | 16.9 (1.1) | 14.9–18.2 | 14.9 (1.1) | 13.0–18.1 |
| WPB | 5.8 (1.3) | 4.1–7.7 | 6.7 (1.8) | 3.2–9.2 |
| WPC | 24.2 (1.7) | 21.2–26.2 | 19.7 (2.3) | 12.2–23.8 |
| WPW | 18.7 (1.5) | 16.1–20.3 | 13.7 (2.0) | 10.7–18.1 |
| Taxon | n | as. S. chilensis | as. S. bivius | n Correct | Percentage Correct |
|---|---|---|---|---|---|
| S. chilensis | 12 | 12 | 0 | 12 | 100 |
| S. bivius | 24 | 0 | 24 | 24 | 100 |
| Total | 36 | 12 | 24 | 36 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante, C.; Vargas-Caro, C.; Indurain, M.J.; Silva, G. Integrating Egg Case Morphology and DNA Barcoding to Discriminate South American Catsharks, Schroederichthys bivius and S. chilensis (Carcharhiniformes: Atelomycteridae). Diversity 2025, 17, 651. https://doi.org/10.3390/d17090651
Bustamante C, Vargas-Caro C, Indurain MJ, Silva G. Integrating Egg Case Morphology and DNA Barcoding to Discriminate South American Catsharks, Schroederichthys bivius and S. chilensis (Carcharhiniformes: Atelomycteridae). Diversity. 2025; 17(9):651. https://doi.org/10.3390/d17090651
Chicago/Turabian StyleBustamante, Carlos, Carolina Vargas-Caro, María J. Indurain, and Gabriela Silva. 2025. "Integrating Egg Case Morphology and DNA Barcoding to Discriminate South American Catsharks, Schroederichthys bivius and S. chilensis (Carcharhiniformes: Atelomycteridae)" Diversity 17, no. 9: 651. https://doi.org/10.3390/d17090651
APA StyleBustamante, C., Vargas-Caro, C., Indurain, M. J., & Silva, G. (2025). Integrating Egg Case Morphology and DNA Barcoding to Discriminate South American Catsharks, Schroederichthys bivius and S. chilensis (Carcharhiniformes: Atelomycteridae). Diversity, 17(9), 651. https://doi.org/10.3390/d17090651









