Ichthyoplankton Composition and Environmental Drivers in the Sanquianga Tapaje Estuarine System, Eastern Tropical Pacific
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Procedures
2.2. Data Analysis
3. Results
3.1. Ichthyoplankton Composition and Zooplankton Biomass
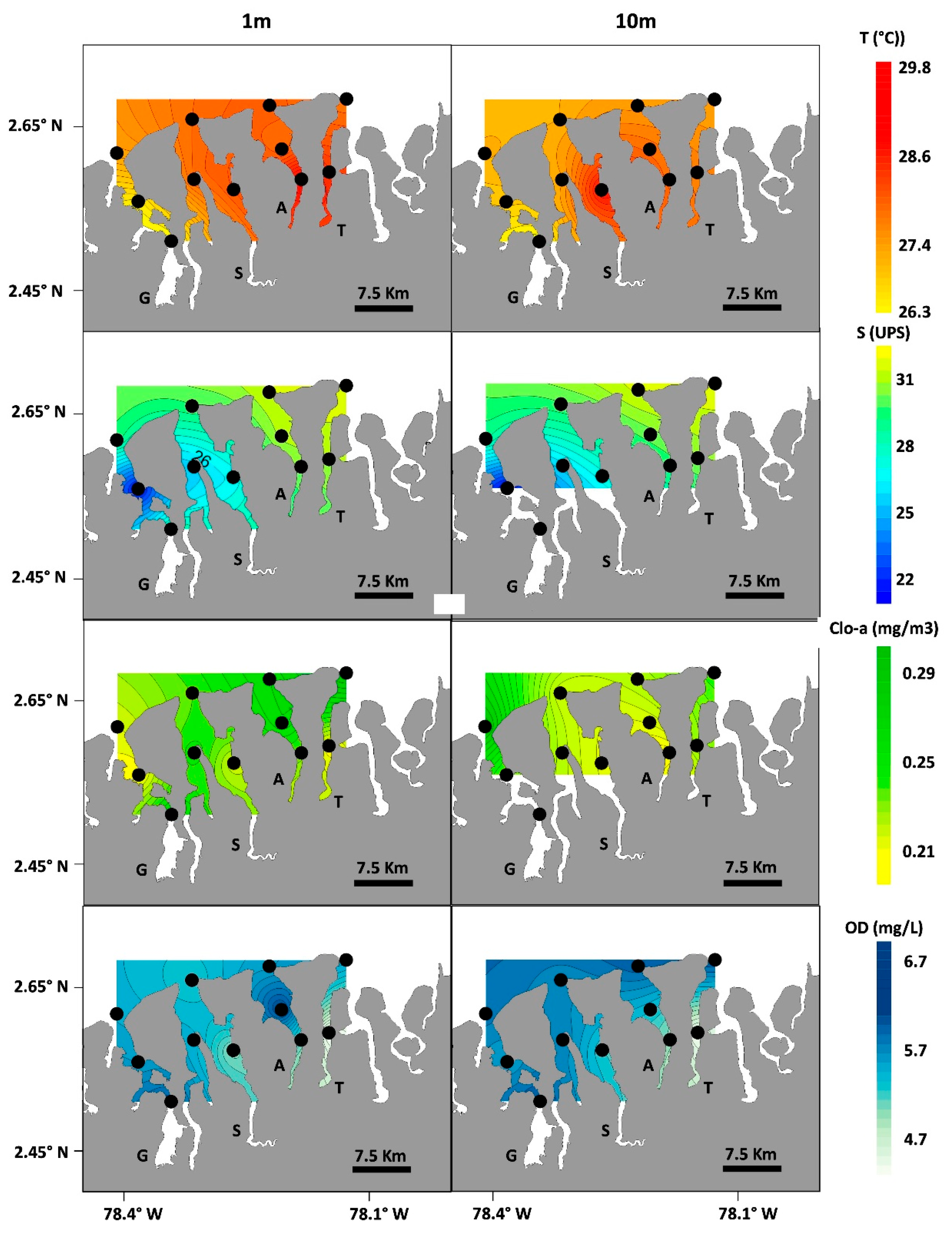
3.2. Environmental Conditions
3.3. Statistical Associations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Able, K.W. A re-examination of fish estuarine dependence: Evidence for connectivity between estuarine and ocean habitats. Estuar. Coast. Shelf Sci. 2005, 64, 5–17. [Google Scholar] [CrossRef]
- de Lima, A.R.A.; Barletta, M.; Dantas, D.V.; Ramos, J.A.A.; Costa, M.F. Early development of marine catfishes (Ariidae): From mouth brooding to the release of juveniles in nursery habitats. J. Fish Biol. 2013, 82, 1990–2014. [Google Scholar] [CrossRef]
- Gomes, E.A.P.; Campos, P.N.; Bonecker, A.C.T. Occurrence of Gobiidae larvae in a tropical Brazilian estuary, with particular emphasis on the use of size classes to categorize species guilds. J. Fish Biol. 2014, 84, 996–1013. [Google Scholar] [CrossRef]
- Martino, E.J.; Houde, E.D. Recruitment of striped bass in Chesapeake Bay: Spatial and temporal environmental variability and availability of zooplankton prey. Mar. Ecol. Prog. Ser. 2010, 409, 213–228. [Google Scholar] [CrossRef]
- Guerreiro, M.A.; Martinho, F.; Baptista, J.; Costa, F.; Pardal, M.Â.; Primo, A.L. Function of estuaries and coastal areas as nursery grounds for marine fish early life stages. Mar. Environ. Res. 2021, 170, 105408. [Google Scholar] [CrossRef] [PubMed]
- Blaber, S.J.M. Fishes and fisheries in tropical estuaries: The last 10 years. Estuar. Coast. Shelf Sci. 2013, 135, 57–65. [Google Scholar] [CrossRef]
- Marques, S.C.; Azeiteiro, U.M.; Marques, J.C.; Neto, J.M.; Pardal, M.Â. Zooplankton and ichthyoplankton communities in a temperate estuary: Spatial and temporal patterns. J. Plankton Res. 2006, 28, 297–312. [Google Scholar] [CrossRef]
- Hoffmeyer, M.S.; Clara, M.M.; Florencia, B.; Mabel, N.A.; Ramón, T.E. Ichthyoplankton spatial pattern in the inner shelf off Bahía Blanca Estuary, SW Atlantic Ocean. Estuar. Coast. Shelf Sci. 2009, 84, 383–392. [Google Scholar] [CrossRef]
- Williams, J.; Hindell, J.S.; Swearer, S.E.; Jenkins, G.P. Influence of freshwater flows on the distribution of eggs and larvae of black bream Acanthopagrus butcheri within a drought-affected estuary. J. Fish Biol. 2012, 80, 2281–2301. [Google Scholar] [CrossRef]
- Hou, G.; Wang, J.; Liu, L.; Chen, Y.; Pan, C.; Lin, J.; Zhang, H. Assemblage structure of the ichthyoplankton and its relationship with environmental factors in spring and autumn off the Pearl River Estuary. Front. Mar. Sci. 2021, 8, 732970. [Google Scholar] [CrossRef]
- Badú, M.L.A.; Lima, C.S.S.; Pessanha, A.L.M. Environmental influences on the ichthyoplankton in hypersaline estuaries located in a Semiarid Northeastern Brazilian coast. Neotrop. Ichthyol. 2022, 20, e210081. [Google Scholar] [CrossRef]
- Muñoz, N.; Bonilla, S.; Arocena, R.; Maciel, F.; Haakonsson, S.; Pedocchi, F.; Machado, I. Estuarine front dynamics drive ichthyoplankton assemblage in the tidal freshwater zone of the Río de la Plata, South America. Reg. Stud. Mar. Sci. 2024, 73, 103521. [Google Scholar] [CrossRef]
- Garrido, S.; Albo-Puigserver, M.; Moyano, M. Larval trophic ecology of small pelagic fishes: A review of recent advances and pathways to fill remaining knowledge gaps. Mar. Ecol. Prog. Ser. 2024, 741, 127–143. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Blaber, S.J.; Elliott, M.; Harrison, T.D. Trophic ecology of fishes in estuaries. Rev. Fish Biol. Fish. 2024, 34, 1371–1405. [Google Scholar] [CrossRef]
- Motos, L.; Cotano, U.; Coombs, S.H.; Álvarez, P.; Santos, M. Ichthyoplankton assemblages. Elsevier Oceanogr. Ser. 2004, 70, 425–454. [Google Scholar] [CrossRef]
- Reyier, E.A.; Shenker, J.M.; Christian, D. Role of an estuarine fisheries reserve in the production and export of ichthyoplankton. Mar. Ecol. Prog. Ser. 2008, 359, 249–260. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Jiang, C. Biological and ecological studies on marine ichthyoplankton. Front. Mar. Sci. 2022, 9, 948521. [Google Scholar] [CrossRef]
- Castellanos-Galindo, G.A.; Krumme, U.; Rubio, E.A.; Saint-Paul, U. Spatial variability of mangrove fish assemblage composition in the tropical eastern Pacific Ocean. Rev. Fish Biol. Fish. 2013, 23, 69–86. [Google Scholar] [CrossRef]
- Pelage, L.; Gonzalez, J.G.; Le Loc’h, F.; Ferreira, V.; Munaron, J.M.; Lucena-Fredou, F.; Fredou, T. Importance of estuary morphology for ecological connectivity with their adjacent coast: A case study in Brazilian tropical estuaries. Estuar. Coast. Shelf Sci. 2021, 251, 107184. [Google Scholar] [CrossRef]
- Valencia, B.; Rivera-Gómez, M.; Jerez-Guerrero, M.; Rondón-Ramos, M.; Giraldo, A. Temporal and spatial variability of ichthyoplankton assemblages in the Eastern Tropical Pacific off Colombia. Cont. Shelf Res. 2024, 275, 105228. [Google Scholar] [CrossRef]
- Aburto-Oropeza, O.; Erisman, B.; Galland, G.R.; Mascarenas-Osorio, I.; Sala, E.; Ezcurra, E. Large recovery of fish biomass in a no-take marine reserve. PLoS ONE 2011, 6, e23601. [Google Scholar] [CrossRef]
- Aburto-Oropeza, O.; Ezcurra, E.; Moxley, J.; Sánchez-Rodríguez, A.; Mascarenas-Osorio, I.; Sánchez-Ortiz, C.; Erisman, B.; Ricketts, T. A framework to assess the health of rocky reefs linking geomorphology, community assemblage, and fish biomass. Ecol. Indic. 2015, 52, 353–361. [Google Scholar] [CrossRef]
- Blaber, S.J.M.; Farmer, M.J.; Milton, D.A.; Pang, J.; Boon-Teck, O.; Wong, P. The ichthyoplankton of selected estuaries in Sarawak and Sabah: Composition, distribution and habitat affinities. Estuar. Coast. Shelf Sci. 1997, 45, 197–208. [Google Scholar] [CrossRef]
- Gordo, C.F.; Vargas, R.F.; Rodríguez, C.N.; Rodríguez, R.F.; Martínez, R.S. Ictioplancton de las costas de Jalisco y Colima, México (diciembre de 1995 a diciembre de 1996). Cienc. Mar. 1999, 25, 107–118. [Google Scholar] [CrossRef][Green Version]
- Navarro-Rodríguez, M.C.; González-Guevara, L.F.; Flores-Vargas, R.; Amparan-Salido, R.T. Variación espacio temporal del ictioplancton en la Laguna El Quelele, Nayarit, México. Rev. Bio Cienc. 2015, 3, 116–131. [Google Scholar] [CrossRef]
- Navarro-Rodríguez, N.R.; Lara-López, M.Á.; González-Guevara, L.F.; Flores-Vargas, R. Biomasa zooplanctónica y densidad espacio temporal del ictioplancton en Bahía de Banderas. Acta Pesq. 2018, 4, 1–11. [Google Scholar]
- Ramírez, A.R.; López, M.I.; Szelistowski, W.A. Composition and abundance of ichthyoplankton in a Gulf of Nicoya mangrove estuary. Rev. Biol. Trop. 1990, 38, 463–466. [Google Scholar]
- Molina-Urena, H. Ichthyoplankton assemblages in the Gulf of Nicoya and Golfo Dulce embayments, Pacific coast of Costa Rica. Rev. Biol. Trop. 1996, 44, 173–182. [Google Scholar]
- Medina-Contreras, D.; Cantera, J.; Escarria, E.; Mejía-Ladino, L.M. Distribución y densidad de ictioplancton en el Estuario de Bahía Málaga, pacífico colombiano (septiembre de 2009-febrero de 2010). Bol. Investig. Mar. Cost. 2014, 43, 107–119. [Google Scholar] [CrossRef]
- Valencia, B.; Giraldo, A.; Rivera-Gómez, M.; Izquierdo-Peña, V.; Cuellar-Chacón, A. Efectos de la surgencia estacional sobre la hidrografia y las comunidades del mesozooplancton en una ensenada tropical del Pacifico colombiano. Rev. Biol. Trop. 2019, 67, 945–963. [Google Scholar]
- Calderón-Peralta, G.; Ayora-Macias, G.; Solís-Coello, P. Variación espacio-temporal de larvas de peces en el Golfo de Guayaquil, Ecuador. Bol. Investig. Mar. Costeras 2020, 49, 135–156. [Google Scholar] [CrossRef]
- González-Jaramillo, J.L.; Mártinez-Mártinez, J.O.; Carvajal-Perico, J.H. Geomorfología y Aspectos Erosivos del Litoral Pacífico Colombiano; Ingeominas: Cali, Colombia, 1995; p. 52. [Google Scholar]
- Velasco, E.R.; López, G.I. Marco conceptual para investigaciones tsunamigénicas: Caso litoral Pacífico colombiano. Bol. Geol. 2016, 38, 79–106. [Google Scholar] [CrossRef]
- Vásquez, L.; Iriarte, J.; Sánchez, R. Determinación de la cota máxima de inundación en la frontera colombo-ecuatoriana aplicando modelación numérica. Bol. Cient. CIOH 2018, 37, 3–16. [Google Scholar] [CrossRef]
- Guerrero-Castillo, A.; Sánchez, E.R. Evaluación de la amenaza por tsunami en poblaciones del Sur, Centro y Norte del litoral Pacífico Colombiano. Bol. Cient. CIOH 2019, 38, 10–25. [Google Scholar] [CrossRef]
- MADS-PNN. Plan de Manejo Parque Nacional Natural Sanquianga: Territorio Ancestral y Colectivo; Ministerio de Ambiente y Desarrollo Sostenible (MADS)–Parques Nacionales Naturales de Colombia: Cali, Colombia, 2017; p. 307. [Google Scholar]
- Rangel-Ch, O.J.; Arellano-P, H. Clima del Chocó biogeográfico/Costa pacífica de Colombia. In Colombia Diversidad Biótica IV: El Chocó Biogeográfico/Costa Pacífica; Instituto de Ciencias Naturales-Conservación Internacional: Bogotá, Colombia, 2004; pp. 39–82. [Google Scholar]
- Devis-Morales, A.; Montoya-Sánchez, R.A. Variabilidad Espacio-Temporal del Sistema Océano-Atmosférico en el Pacífico Ecuatorial Oriental Con Énfasis en la Región Marino-Costera de Gorgona y Sanquianga; WWF: Cali, Colombia, 2016; p. 52. [Google Scholar]
- Del Valle Arango, J.I. Anotaciones sobre el clima de los bosques de guandal del delta del río Patía. Rev. Fac. Nac. Agron. Medellín 1994, 47, 145–159. [Google Scholar]
- Palacios, M.L.; Cantera, J.R.; Peña, E.J. Carbon stocks in mangrove forests of the Colombian Pacific. Estuar. Coast. Shelf Sci. 2019, 227, 106299. [Google Scholar] [CrossRef]
- Beltrán-León, B.S.; Ríos Herrera, R. Estadios Tempranos de Peces del Pacífico Colombiano; INPA: Cali, Colombia, 2000; p. 727. [Google Scholar]
- Richards, W.J. Early Stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic; CRC Press: Boca Raton, FL, USA, 2005; p. 2640. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Education: Hoboken, NJ, USA, 2010; p. 944. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Zahl, S. Jackknifing an index of diversity. Ecology 1977, 58, 907–913. [Google Scholar] [CrossRef]
- Chuang, C.J. Estimation of similarity indices via two-sample jackknife procedure. J. Appl. Sci. Eng. 2012, 15, 301–310. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Malden, MA, USA, 2004; p. 256. [Google Scholar]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Barletta, M.; Barletta-Bergan, A. Endogenous activity rhythms of larval fish assemblages in a mangrove-fringed estuary in North Brazil. Open Fish Sci. J. 2009, 2, 15–24. [Google Scholar] [CrossRef]
- Strydom, N.A. Patterns in larval fish diversity, abundance, and distribution in temperate South African estuaries. Estuaries Coast. 2015, 38, 268–284. [Google Scholar] [CrossRef]
- Beck, M.W.; Heck, K.L.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Weinstein, M.P. The role of nearshore ecosystems as fish and shellfish nurseries. Issues Ecol. 2003, 11, 1–12. [Google Scholar]
- Nagelkerken, I.S.J.M.; Blaber, S.J.M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef]
- Alebregtse, N.C.; de Swart, H.E.; Schuttelaars, H.M. Impact of intertidal habitats on hydrodynamics in tidally energetic, well-mixed estuaries. J. Phys. Oceanogr. 2025, 55, e2023JPO0172. [Google Scholar] [CrossRef]
- Barletta, M.; Barletta-Bergan, A.; Saint-Paul, U.S.G.H.; Hubold, G. The role of salinity in structuring the fish assemblages in a tropical estuary. J. Fish biol. 2005, 66, 45–72. [Google Scholar] [CrossRef]
- Castro, M.S.D.; Bonecker, A.C.T.; Valentin, J.L. Seasonal variation in fish larvae at the entrance of Guanabara Bay, Brazil. Braz. Arch. Biol. Technol. 2005, 48, 121–128. [Google Scholar] [CrossRef]
- de Lima, A.R.A.; Barletta, M.; Costa, M.F. Seasonal distribution and interactions between plankton and microplastics in a tropical estuary. Estuar. Coast. Shelf Sci. 2015, 165, 213–225. [Google Scholar] [CrossRef]
- Eby, L.A.; Crowder, L.B. Effects of hypoxic disturbances on an estuarine nekton assemblage across multiple scales. Estuaries 2004, 27, 342–351. [Google Scholar] [CrossRef]
- Lin, J.; Xu, H.; Cudaback, C.; Wang, D. Inter-annual variability of hypoxic conditions in a shallow estuary. J. Mar. Syst. 2008, 73, 169–184. [Google Scholar] [CrossRef]
- Tyler, R.M.; Brady, D.C.; Targett, T.E. Temporal and spatial dynamics of diel-cycling hypoxia in estuarine tributaries. Estuaries Coast. 2009, 32, 123–145. [Google Scholar] [CrossRef]
- Schmidt, S.; Diallo, I.I.; Derriennic, H.; Fallou, H.; Lepage, M. Exploring the susceptibility of turbid estuaries to hypoxia as a prerequisite to designing a pertinent monitoring strategy of dissolved oxygen. Front. Mar. Sci. 2019, 6, 352. [Google Scholar] [CrossRef]
- Santos, R.V.S.; Severi, W. Dynamics of early life-history stages of fish along an estuarine gradient. Fish. Oceanogr. 2019, 28, 402–418. [Google Scholar] [CrossRef]
- de Lima, L.G.; Araújo, F.G.; Macário, B.S.; Pessanha, A.L.M. Larval fish assemblages in selected Brazilian estuaries: Species-environment relationships under different anthropogenic influences. Mar. Pollut. Bull. 2024, 198, 115858. [Google Scholar] [CrossRef]
- de Morais, A.T.; de Morais, L.T. The abundance and diversity of larval and juvenile fish in a tropical estuary. Estuaries 1994, 17, 216–225. [Google Scholar] [CrossRef]
- Barletta-Bergan, A.; Barletta, M.; Saint-Paul, U. Community structure and temporal variability of ichthyoplankton in North Brazilian mangrove creeks. J. Fish Biol. 2002, 61, 33–51. [Google Scholar] [CrossRef]
- Barletta-Bergan, A.; Barletta, M.; Saint-Paul, U. Structure and seasonal dynamics of larval fish in the Caeté River Estuary in North Brazil. Estuar. Coast. Shelf Sci. 2002, 54, 193–206. [Google Scholar] [CrossRef]
- Barletta, M.; Barletta-Bergan, A.; Saint-Paul, U.; Hubold, G. Seasonal changes in density, biomass, and diversity of estuarine fishes in tidal mangrove creeks of the lower Caeté Estuary (northern Brazilian coast, east Amazon). Mar. Ecol. Prog. Ser. 2003, 256, 217–228. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in estuarine ecology–a review of the Remane diagram with a suggested revised model for estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Primo, A.L.; Azeiteiro, U.M.; Marques, S.C.; Pardal, M.Â. Impact of climate variability on ichthyoplankton communities: An example of a small temperate estuary. Estuar. Coast. Shelf Sci. 2011, 91, 484–491. [Google Scholar] [CrossRef]
- Lunt, J.; Smee, D.L. Turbidity alters estuarine biodiversity and species composition. ICES J. Mar. Sci. 2020, 77, 379–387. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Liang, C.; Liu, S.; Xian, W. Estuarine Ichthyoplankton Studies—A Review. Front. Mar. Sci. 2022, 9, 794433. [Google Scholar] [CrossRef]
- Barletta, M.; Lima, A.R. Systematic review of fish ecology and anthropogenic impacts in South American estuaries: Setting priorities for ecosystem conservation. Front. Mar. Sci. 2019, 6, 237. [Google Scholar] [CrossRef]
- Silva, V.E.L.; Fabré, N.N. Rare species enhance niche differentiation among tropical estuarine fish assemblages. Estuaries Coasts 2019, 42, 890–899. [Google Scholar] [CrossRef]
- Ramos, J.A.; Costa, M.J.; Cabral, H.N. Diversity patterns of fish larvae in tropical estuarine gradients: Implications for nursery function and conservation. Mar. Ecol. Prog. Ser. 2022, 689, 45–60. [Google Scholar]
- Kariyawasam, K.M.G.M.M.; Kariyawasam, K.M.G.R.M.; Rathnayake, A.U.; Wickramasinghe, I. Nutrition of marine larvae. In Marine Larvae; CRC Press: Boca Raton, FL, USA, 2024; pp. 126–157. [Google Scholar]
- Joyeux, J.C.; Pereira, B.B.; de Almeida, H.G. The flood-tide ichthyoplanktonic community at the entrance into a Brazilian tropical estuary. J. Plankton Res. 2004, 26, 1277–1287. [Google Scholar] [CrossRef]
- Namiki, A.P.; Bonecker, F.T.; Bernardo, F. Larval fish composition of a tropical estuary in northern Brazil (2°18’–2°47’S/044°20’–044°25’W) during the dry season. Pan-Am. J. Aquat. Sci. 2007, 2, 235–241. [Google Scholar]
- Sarpedonti, V.; Anunciação, É.M.S.D.; Bordalo, A.O. Spatio-temporal distribution of fish larvae in relation to ontogeny and water quality in the oligohaline zone of a North Brazilian estuary. Biota Neotrop. 2013, 13, 55–63. [Google Scholar] [CrossRef]
- Ooi, A.L.; Chong, V.C. Larval fish assemblages in a tropical mangrove estuary and adjacent coastal waters: Offshore–inshore flux of marine and estuarine species. Cont. Shelf Res. 2011, 31, 1599–1610. [Google Scholar] [CrossRef]
- Muelbert, J.H.; Weiss, G. Abundance and distribution of fish larvae in the channel area of the Patos Lagoon Estuary, Brazil. NOAA Tech. Rep. NMFS. 1991, 95, 43–54. [Google Scholar]
- Ekau, W.; Westhaus-Ekau, P.; de Macedo, S.J.; Von Dorrien, C. The larval fish fauna of the “canal de Santa Cruz” estuary in northeast Brazil. Trop. Oceanogr. 2001, 29, 117–128. [Google Scholar] [CrossRef][Green Version]
- Osório, F.M.; Godinho, W.O.; Lotufo, T.M.D.C. Ictiofauna associada às raízes de mangue do estuário do Rio Pacoti-CE, Brasil. Biota Neotrop. 2011, 11, 415–420. [Google Scholar] [CrossRef]
- Ré, P.; Azeiteiro, U.; Morgado, F. Ecologia do Plâncton Marinho e Estuarino; Edições Afrontamento: Porto, Portugal, 2005; p. 144. [Google Scholar]
- Lacerda, C.H.F.; Barletta, M.; Dantas, D.V. Temporal patterns in the intertidal faunal community at the mouth of a tropical estuary. J. Fish Biol. 2014, 85, 1571–1602. [Google Scholar] [CrossRef]
- Do, A.N.T.; Tran, H.D.; Nguyen, T.T. Spatial distribution of fish larvae in relation to salinity and habitat structure in a tropical estuary: A fuzzy logic approach. Aquat. Ecol. 2024, 58, 983–998. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Houde, E.D.; Neira, F.J.; Potter, I.C. Importance of marine–estuarine–riverine connectivity to larvae and early juveniles of estuary-associated fish taxa. Environ. Biol. Fishes 2023, 106, 1983–2009. [Google Scholar] [CrossRef]
- Arévalo, E.; Cabral, H.N.; Villeneuve, B.; Possémé, C.; Lepage, M. Fish larvae dynamics in temperate estuaries: A review on processes, patterns and factors that determine recruitment. Fish Fish. 2023, 24, 466–487. [Google Scholar] [CrossRef]
- Castillo-Rivera, M.; Zárate, R. Temporal and spatial variation in fish larval assemblages in a tropical estuarine lagoon: Diversity metrics and environmental drivers. Reg. Stud. Mar. Sci. 2025, 65, 102923. [Google Scholar]
- Carvalho, D.C. Ichthyoplankton DNA metabarcoding: Challenges and perspectives. Mol. Ecol. 2022, 31, 1612–1614. [Google Scholar] [CrossRef]
- Ferreira, A.O.; Azevedo, O.M.; Barroso, C.; Duarte, S.; Egas, C.; Fontes, J.T.; Ré, P.; Santos, A.M.P.; Costa, F.O. Multi-marker DNA metabarcoding for precise species identification in ichthyoplankton samples. Sci. Rep. 2024, 14, 19772. [Google Scholar] [CrossRef]
- Nama, S.; Shanmughan, A.; Akter, S.; Bhushan, S.; Ramteke, K.; Deshmukhe, G.; Jaiswar, A.; Kumar, A.; Nayak, B.B. DNA barcoding reveals the temporal variation of ichthyoplankton assemblages and their relationship with environmental variables in a tropical mangrove estuary. Environ. Monit. Assess. 2025, 197, 1060. [Google Scholar] [CrossRef] [PubMed]

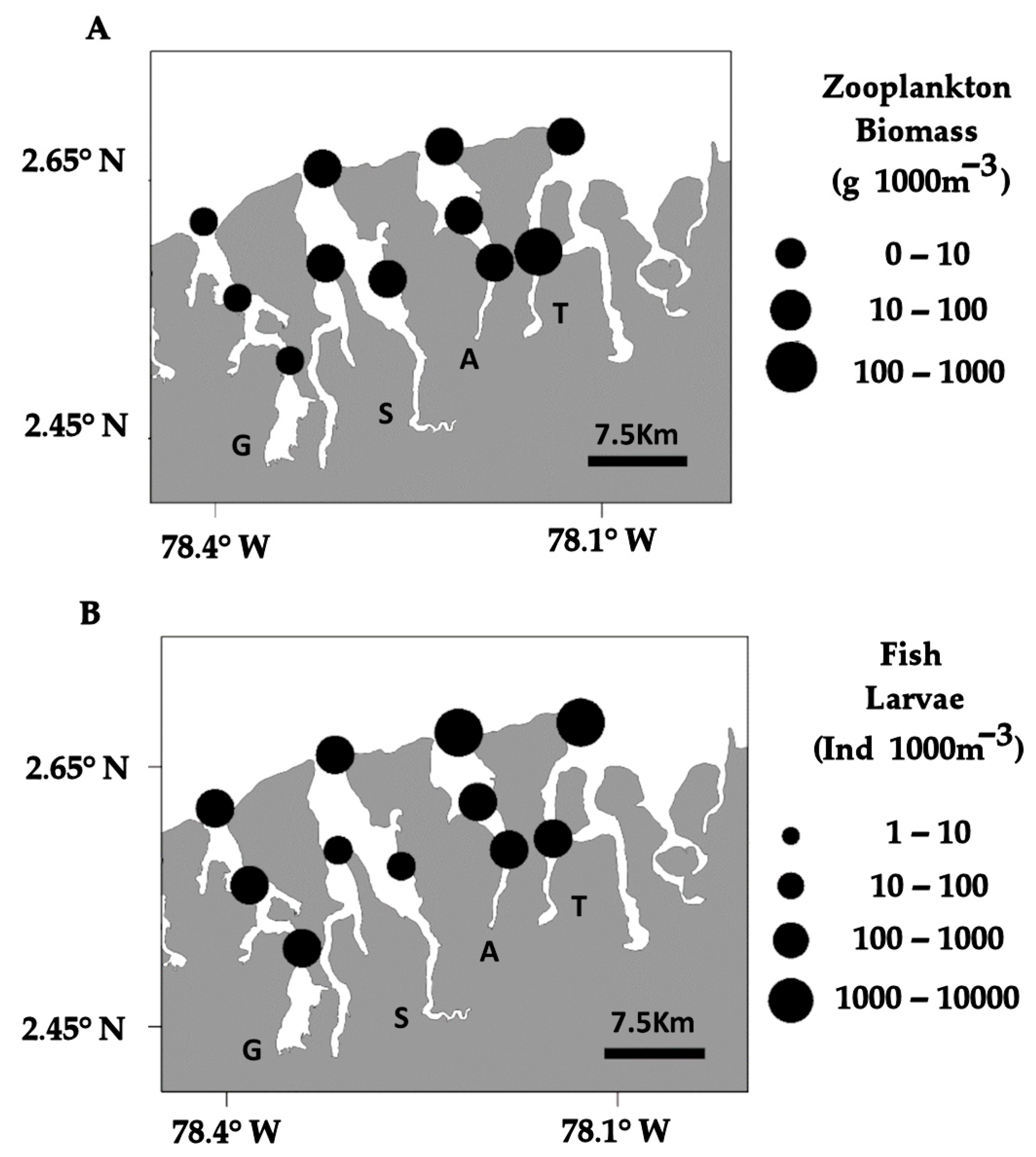
| Family/Specie | % | |
|---|---|---|
| Clupeidae | 0.84 | |
| Opisthonema sp. | 0.84 | |
| Engraulidae | 39.50 | |
| Anchoa sp. | 1.96 | |
| Cetengraulis mysticetus | 37.54 | |
| Hemiramphidae | 0.28 | |
| Hemiramphus sp. | 0.28 | |
| Carangidae | 15.12 | |
| Caranx sp. | 4.76 | |
| Chloroscombrus orqueta | 5.60 | |
| Oligoplites saurus | 4.48 | |
| Seriola sp. | 0.28 | |
| Haemulidae | 1.40 | |
| Anisotremus sp. | 1.40 | |
| Sciaenidae | 14.00 | |
| Bairdiella sp. | 8.96 | |
| Menticirrhus sp. 1 | 1.40 | |
| Menticirrhus sp. 2 | 3.64 | |
| Gobiosocidae | 0.28 | |
| Gobiesox sp. | 0.28 | |
| Gobiidae | 26.61 | |
| Gobulus crescentalis | 1.40 | |
| Microgobius sp. | 0.57 | |
| Gobiidae sp. 1 | 12.04 | |
| Gobiidae sp. 2 | 0.28 | |
| Gobiidae sp. 3 | 0.28 | |
| Gobiidae sp. 4 | 12.04 | |
| Ephippidae | 0.28 | |
| Chaetodipterus zonatus | 0.28 | |
| Pomacentridae | 1.13 | |
| Stegastes sp. | 0.29 | |
| Chromis sp. | 0.84 | |
| Achiridae | 0.56 | |
| Achirus sp. | 0.56 |
| Diversity Metrics | G | S | A | T | ||
|---|---|---|---|---|---|---|
| Taxa (S) | 10 | 8 | 15 | 13 | ||
| Abundance (N) | 199 | 85 | 696 | 811 | ||
| Shannon (H’) | 1.99 | 1.77 | 1.63 | 2.02 | ||
| Evenness (J’) | 0.87 | 0.85 | 0.60 | 0.79 | ||
| Comparisons | G-S | G-A | G-T | S-A | S-T | A-T |
| Whittaker (βw) | 0.33 | 0.36 | 0.48 | 0.48 | 0.33 | 0.50 |
| Permutation H’ (p value) | 0.0575 | 0.0007 * | 0.8073 | 0.4322 | 0.0494 * | 0.0001 * |
| Permutation J’ (p value) | 0.6766 | 0.0001 * | 0.0023 * | 0.0001 * | 0.2412 | 0.0001 * |
| Variable | rs | p Level |
|---|---|---|
| Transparency | 0.45 | 0.161 |
| Sea Surface Temperature | −0.03 | 0.937 |
| Sea Surface Salinity | 0.81 | 0.003 * |
| Dissolved Oxygen Saturation | 0.68 | 0.021 * |
| Dissolved Oxygen Concentration | 0.69 | 0.019 * |
| Phytoplankton Biomass (Chlorophyll-a) | 0.29 | 0.393 |
| Turbidity | −0.20 | 0.555 |
| Zooplankton Biomass | 0.35 | 0.285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego-Zerrato, J.J.; Cuellar, A.; Giraldo, A. Ichthyoplankton Composition and Environmental Drivers in the Sanquianga Tapaje Estuarine System, Eastern Tropical Pacific. Diversity 2025, 17, 649. https://doi.org/10.3390/d17090649
Gallego-Zerrato JJ, Cuellar A, Giraldo A. Ichthyoplankton Composition and Environmental Drivers in the Sanquianga Tapaje Estuarine System, Eastern Tropical Pacific. Diversity. 2025; 17(9):649. https://doi.org/10.3390/d17090649
Chicago/Turabian StyleGallego-Zerrato, Juan José, Andrés Cuellar, and Alan Giraldo. 2025. "Ichthyoplankton Composition and Environmental Drivers in the Sanquianga Tapaje Estuarine System, Eastern Tropical Pacific" Diversity 17, no. 9: 649. https://doi.org/10.3390/d17090649
APA StyleGallego-Zerrato, J. J., Cuellar, A., & Giraldo, A. (2025). Ichthyoplankton Composition and Environmental Drivers in the Sanquianga Tapaje Estuarine System, Eastern Tropical Pacific. Diversity, 17(9), 649. https://doi.org/10.3390/d17090649






