Asymmetric Distribution of Fish Diversity in Inflows of the Black Irtysh River (Central Asia, Kazakhstan)
Abstract
1. Introduction
2. Materials and Methods
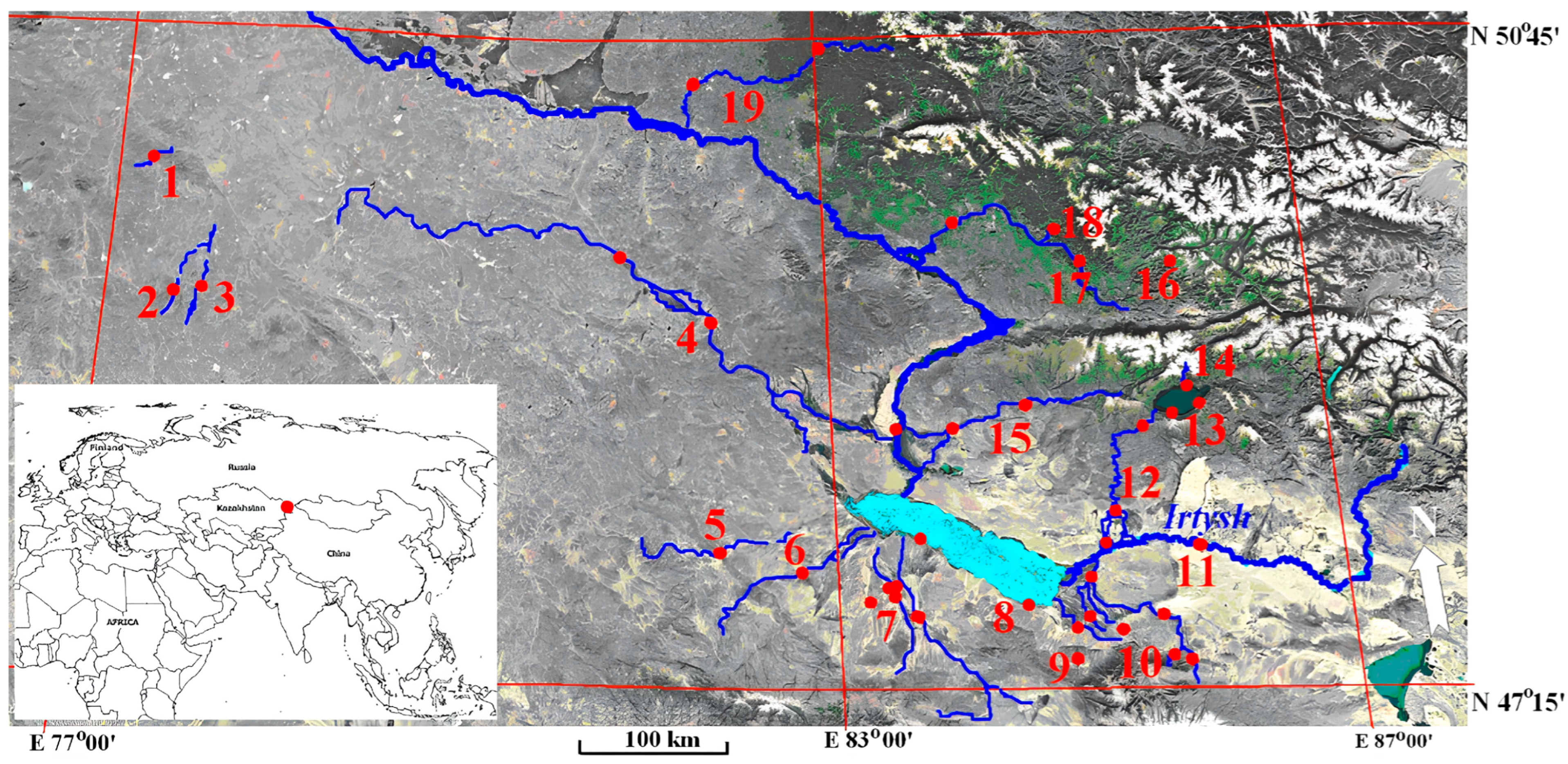
2.1. Study Area and Sampling
2.2. Descriptive Statistics
3. Results
4. Discussion
5. Conclusions
- The fish diversity of the right-bank and left-bank tributaries of the Black Irtysh River significantly differ in species composition. Seven fish species were found only in the right tributaries and only eight species in the left tributaries. The size of reservoirs and the speed of the current are the main abiotic factors affecting the distribution of fish in the Black Irtysh basin. Thus, the presence of a hydrological connection of the water bodies by itself does not guarantee the distribution of species throughout the basin.
- A rare species like the taimen H. taimen and a local endemic as B. savinovi are still preserved in right-bank tributaries and Markakol Lake.
- Fish species new to the Kazakh section of the Black Irtysh River have been discovered: Tibetan stone loach T. stolickai and Severtsov’s loach T. sewerzowi and alien fish species including asp L. aspius, Chinese false gudgeon A. rivularis, sunbleak L. delinetaus, and Misgurnus sp., whereas Misgurnus sp. Are related to the first functional group (I) and others to the fifth (V) and sixth (VI) groups. The appearance of new alien species indicates unfavorable changes in the ecosystems of the Black Irtysh.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jablonski, D.; Flessa, K.W.; Valentine, J.W. Biogeography and paleobiology. Paleobiology 1985, 11, 75–90. [Google Scholar] [CrossRef]
- Holloway, J.D.; Hall, R. SE Asian geology and biogeography: An introduction. In Biogeography and Geological Evolution of SE Asia; Backbuys Publishers: Leiden, The Netherlands, 1998; pp. 1–23. [Google Scholar]
- Flynn, D.F.; Mirotchnick, N.; Jain, M.; Palmer, M.I.; Naeem, S. Functional and phylogenetic diversity as predictors of biodiversity–ecosystem-function relationships. Ecology 2011, 92, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E.; Jenkins, D.G. Biogeography and ecology: Towards the integration of two disciplines. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2438–2448. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, B.; Wang, S.; Zhao, W. Global ecological regionalization: From biogeography to ecosystem services. Curr. Opin. Environ. Sustain. 2018, 33, 1–8. [Google Scholar] [CrossRef]
- Ziarati, M.; Zorriehzahra, M.J.; Hassantabar, F.; Mehrabi, Z.; Dhawan, M.; Sharun, K.; Emran, T.B.; Dhama, K.; Chaicumpa, W.; Shamsi, S. Zoonotic diseases of fish and their prevention and control. Vet. Q. 2022, 42, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Levêque, C.; Oberdorff, T.; PAUGy, D.; Stiassny, M.L.J.; Tedesco, P.A. Global diversity of fish (Pisces) in freshwater. Hydrobiologia 2008, 595, 545–567. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Cowx, I.G.; Portocarrero Aya, M. Paradigm shifts in fish conservation: Moving to the ecosystem services concept. J. Fish Biol. 2011, 79, 1663–1680. [Google Scholar] [CrossRef]
- McIntyre, P.B.; Reidy Liermann, C.A.; Revenga, C. Linking freshwater fishery management to global food security and biodiversity conservation. Proc. Natl. Acad. Sci. USA 2016, 113, 12880–12885. [Google Scholar] [CrossRef]
- Su, G.; Logez, M.; Xu, J.; Tao, S.; Villéger, S.; Brosse, S. Human impacts on global freshwater fish biodiversity. Science 2021, 371, 835–838. [Google Scholar] [CrossRef]
- Berra, T.M. Freshwater Fish Distribution, 2001, 2nd ed.; The University of Chicago Press: Chicago, IL, USA; London, UK, 2007; 599p. [Google Scholar]
- Leroy, B.; Dias, M.S.; Giraud, E.; Hugueny, B.; Jézéquel, C.; Leprieur, F.; Thierry, O.; Tedesco, P.A. Global biogeographical regions of freshwater fish species. J. Biogeogr. 2019, 46, 2407–2419. [Google Scholar] [CrossRef]
- Pinkert, S.; Sica, Y.V.; Winner, K.; Jetz, W. The potential of ecoregional range maps for boosting taxonomic coverage in ecology and conservation. Ecography 2023, 12, e06794. [Google Scholar] [CrossRef]
- Davydov, L.K. Hydrography of the USSR. Part 2; Leningrad State University Publishing House: Leningrad, Russia, 1955; 599p. [Google Scholar]
- Karasev, G.L.; Pavlov, D.S.; Mochek, A.D.; Rodin, V.M. Reservoirs of the Upper Irtysh basin and the steppe zone of Kazakhstan. In Ecology of Fish of the Ob-Irtysh Basin; Pavlov, D.S., Mochek, A.D., Eds.; Association of Scientific Publications of the KMC: Moscow, Russia, 2006; pp. 80–82. [Google Scholar]
- Belyanin, N.I.; Berezovikov, N.N.; Ugorina, A.V.; Kan, N.S.; Lukhtanov, A.G.; Zinchenko, Y.K.; Zinchenko, E.S.; Zinchenko, V.K.; Prokopov, K.P.; Starikov, S.V.; et al. Geographical encyclopedia. East Kazakhstan. Part 3; KASU Publishing House: Ust-Kamenogorsk, Kazakhstan, 2013; 264p. [Google Scholar]
- Vasilenko, V.A. The Ob-Irtysh basin: Socio-economic problems. Reg. Res. Russ. 2014, 4, 198–205. [Google Scholar] [CrossRef]
- Krasnoyarova, B.; Vinokurov, Y.; Antyufeeva, T. International water development problems in the transboundary Irtysh River basin: “new” solutions to old problems. IOP Conf. Ser. Earth Environ. Sci. 2019, 381, 012049. [Google Scholar] [CrossRef]
- Zinoviev, A.T.; Kosheleva, E.D.; Galakhov, V.P.; Golubeva, A.B.; Rybkina, I.D.; Stoyashcheva, N.V.; Kurepina, N.Y. Current State of Water Resources and Problems of Their Use in Border Regions of Russia (The Ob-Irtysh Basin as a Case Study). In Water Resources Management in Central Asia. The Handbook of Environmental Chemistry; Zonn, I., Zhiltsov, S., Kostianoy, A., Semenov, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 105, pp. 163–188. [Google Scholar] [CrossRef]
- Radelyuk, I.; Zhang, L.; Assanov, D.; Maratova, G.; Tussupova, K. A state-of-the-art and future perspectives of transboundary rivers in the cold climate—A systematic review of Irtysh River. J. Hydrol. Reg. Stud. 2022, 42, 101173. [Google Scholar] [CrossRef]
- Berendeev, S.F.; Bogdanov, D.D.; Bogdanov, N.M.; Bocharova, T.M.; Vizer, A.M.; Vizer, L.S.; Voskoboynikov, V.A.; Golubtsov, A.S.; Ghoskova, O.A.; Egorov, E.V.; et al. Ecology of Fishes of the Ob-Irtysh Basin; Pavlov, D.S., Mochek, A.D., Eds.; Association of Scientific Publications of the KMC: Moscow, Russia, 2006; 596p. [Google Scholar]
- Korzun, A.S.; Kassal, B.Y. Distribution of alien fish species in reservoirs of Omsk region. Russ. J. Biol. Invasions 2012, 4, 57–66. [Google Scholar]
- Prokopov, K.P.; Starikov, S.V.; Bratash, I.V. Vertebrates of Eastern Kazakhstan; Sarsen Amanzholov East Kazakhstan University: Ust-Kamenogorsk, Kazakhstan, 2000; 208p. [Google Scholar]
- Prokopov, K.P.; Fedotova, L.A.; Kulikov, E.V.; Kirichenko, O.I. Ichthyofauna of Eastern Kazakhstan; Media Alliance: Ust-Kamenogorsk, Kazakhstan, 2006; 132p. [Google Scholar]
- Baymukanov, M.T.; Zinchenko, V.K.; Berezovikov, N.N.; Zinchenko, Y.K. Fauna of Vertebrates of the Markakolsky Reserve. Fish, Amphibians, Reptiles, Birds, Mammals (Annotated Lists); Bastau: Almaty, Kazakhstan, 2008; 86p. [Google Scholar]
- Starikov, S.V. Ichthyofauna of the Katon-Karagai National Park. In Notes of the Ust-Kamenogorsk branch of the Kazakh Geographical Society; ARGO: Ust-Kamenogorsk, Kazakhstan, 2012; Volume 6, pp. 66–70. [Google Scholar]
- Prokopov, K.P.; Tagaev, D.A. Fishes of East Kazakhstan; VKPK ARGO LLP: Ust-Kamenogorsk, Kazakhstan, 2017; 114p. [Google Scholar]
- Evseeva, A.A.; Kirichenko, O.I. An annotated list of the fish-like and fishes of the ponds and streams of the basin of the Upper Irtysh in Eastern Kazakhstan, with comments on their taxonomy and zoogeography of the region. In The State of Aquatic Biological Resources and Aquaculture in Kazakhstan and Neighboring Countries; Kazak University: Almaty, Kazakhstan, 2019; pp. 236–246. [Google Scholar]
- Berg, L.S. Division of Paleoarctic and Amur District into Zoogeographical Regions on the Basis of Freshwater Fish Distribution. In Selected Works; Akad. Nauk USSR: Leningrad, USSR, 1962; Volume 5, pp. 320–363. [Google Scholar]
- Mitrofanov, V.P. Formation of modern ichthyofauna in Kazakhstan and ichthyogeographic zoning. In Fish of Kazakhstan; Nauka: Alma-Ata, Kazakhstan, 1986; Volume 1, pp. 20–40. [Google Scholar]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Johanzen, B.G. Studies on the Geography and Genesis of the Ichthyofauna of Siberia; Scientific Notes of Tomsk University; Tomsk University: Tomsk, Russia, 1946; Volume 1, pp. 23–24. [Google Scholar]
- Johanzen, B.G. Studies on the Geography and Genesis of the Ichthyofauna of Siberia; Scientific Notes of Tomsk University; Tomsk University: Tomsk, Russia, 1947; Volume 3, pp. 43–60. [Google Scholar]
- Johanzen, B.G. Studies on the Geography and Genesis of the Ichthyofauna of Siberia; Scientific Notes of Tomsk University; Tomsk University: Tomsk, Russia, 1948; Volume 8, pp. 8–31. [Google Scholar]
- Starobogatov, Y.I. Mollusk Fauna and Zoogeographic Zoning of the Continental Reservoirs of the Globe; Science: Leningrad, Russia, 1970; 370p. [Google Scholar]
- Karasev, G.L. Zoogeographic zoning of the territory of the West Siberian region by fish fauna. In Ecology of Fishes of the Ob-Irtysh Basin; Pavlov, D.S., Mochek, A.D., Eds.; Association of Scientific Publications of the KMC: Moscow, Russia, 2006; pp. 37–70. [Google Scholar]
- Johanzen, B.G.; Petkevich, A.N. Results and prospects of fish acclimatization in reservoirs of Western Siberia. In Acclimatization of Fish and Invertebrates in the USSR; Karpevich, A.F., Ed.; Nauka: Moscow, Russia, 1968; pp. 208–216. [Google Scholar]
- Dukravets, G.M.; Mitrofanov, V.P. History of acclimatization of fishes in Kazakhstan. In Fish of Kazakhstan; Gylym: Alma-Ata, Kazakhstan, 1992; Volume 5, pp. 6–44. [Google Scholar]
- Dudgeon, D.; Arthington, A.; Gessner, M.; Kawabata, Z.I.; Knowler, D.; Leveque, C.; Naiman, R.; Prieur-Richard, A.H.; Soto, D.; Stianssy, M.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Darwall, W.R.T.; Freyhof, Y. Lost fishes, who is counting? The extent of the threat to freshwater fish diversity. In Conservation of Freshwater Fishes; Cambridge University Press: Cambridge, UK, 2016; pp. 1–36. [Google Scholar]
- Leprieur, F.; Beauchard, O.; Blanchet, S.; Oberdorff, T.; Brosse, S. Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PLoS Biol. 2008, 6, e28. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55 (Suppl. 1), 152–174. [Google Scholar] [CrossRef]
- Ubaskin, A.V.; Kalieva, A.B.; Bitkeeva, A.A.; Dyusembaeva, A.T. Materials for the creation of the “Black Book of the fauna of Pavlodar region”. Alien fish species in the ecosystems of the Middle Irtysh. Vestn. Karagand. Univ. Ser. Biol. Meditsina. Geogr. 2019, 4, 57–62. [Google Scholar]
- Ubaskin, A.V.; Akhmetov, K.K.; Kolpakova, V.P.; Shaimardanov, Z.K.; Arynova, S.Z.; Azhaev, G.S. To the question about the current state and problems of formation of invasive ichthyofauna of the Middle Irtysh basin. Bull. Natl. Nucl. Cent. Repub. Kazakhstan 2024, 4, 79–87. [Google Scholar] [CrossRef]
- Kolesnikov, I.P.; Zhigileva, O.N. Features of distribution and genetic potential of the invasive species of sleeper in Southern Siberia and Northern Kazakhstan. In Water Resources—The Basis of Global and Regional Projects Development of Russia, Siberia and the Arctic in the 21st Century; Collection of Articles from the National Scientific and Practical Conference with International Participation. In 2 volumes, Tyumen, 21–22 March 2024; Tyumen Industrial University: Tyumen, Russia, 2024; pp. 243–248. [Google Scholar]
- Zhigileva, O.N.; Melnichuk, A.D.; Mogilnikova, E.N.; Kulikova, A.A. Spatio-temporal dynamics of genetic polymorphism indices in fish of the Ob-Irtysh basin. In Biodiversity and Ecology of Populations and Communities of Aquatic and Semi-Aquatic Organisms in the Middle and Lower Ob Basin; Periscope-Volga: Volgograd, Russia, 2024; pp. 220–282. [Google Scholar]
- Krupa, E.; Romanova, S.; Serikova, A.; Shakhvorostova, L. A Comprehensive Assessment of the Ecological State of the Transboundary Irtysh River (Kazakhstan, Central Asia). Water 2024, 16, 973. [Google Scholar] [CrossRef]
- Trigal, C.; Degerman, E. Multiple factors and thresholds explaining fish species distributions in lowland streams. Glob. Ecol. Conserv. 2015, 4, 589–601. [Google Scholar] [CrossRef]
- Crane, D.P.; Kapuscinski, K.L. Capture efficiency of a fine mesh seine in a large river: Implications for abundance, richness, and diversity analyses. Fish. Res. 2018, 205, 149–157. [Google Scholar] [CrossRef]
- French, B.; Wilson, S.; Holmes, T.; Kendrick, A.; Rule, M.; Ryan, N. Comparing five methods for quantifying abundance and diversity of fish assemblages in seagrass habitat. Ecol. Indic. 2021, 124, 107415. [Google Scholar] [CrossRef]
- Deacon, A.E.; Mahabir, R.; Inderlall, D.; Ramnarine, I.W.; Magurran, A.E. Evaluating detectability of freshwater fish assemblages in tropical streams: Is hand-seining sufficient? Environ. Biol. Fishes 2017, 100, 839–849. [Google Scholar] [CrossRef]
- Bonar, S.A.; Hubert, W.A. Standard Sampling of Inland Fish: Benefits, Challenges, and a Call for Action. Fisheries 2020, 27, 10–16. [Google Scholar] [CrossRef]
- Berg, L.S. Fishes of Freshwaters of USSR and Adjacent Countries; Print House of Academy of Science USSR: Moscow/Leningrad, USSR, 1948; Volume 1, pp. 1–467. [Google Scholar]
- Berg, L.S. Fishes of Freshwaters of USSR and Adjacent Countries; Print House of Academy of Science USSR: Moscow/Leningrad, USSR, 1949; Volume 2, pp. 468–926. [Google Scholar]
- Berg, L.S. Fishes of Freshwaters of USSR and Adjacent Countries; Print House of Academy of Science USSR: Moscow/Leningrad, USSR, 1949; Volume 3, pp. 927–1383. [Google Scholar]
- Mitrofanov, V.P. Distribution and systematics of species of the genus Phoxinus in Kazakhstan. In Biological Sciences; Kazakh State University: Alma-Ata, Kazakhstan, 1973; Issue 5; pp. 144–151. [Google Scholar]
- Mitrofanov, V.P.; Mitrofanov, I.V. Genus Phoxinus Agassiz, 1835—Minnow. In Fishes of Kazakhstan; Science: Alma-Ata, Kazakhstan, 1987; Volume 2, pp. 123–145. [Google Scholar]
- Mitrofanov, V.P. Genus Gobio Cuvier, 1817—Gudgeon. In Fish of Kazakhstan; Science: Alma-Ata, Kazakhstan, 1988; Volume 3, pp. 5–23. [Google Scholar]
- Mitrofanov, V.P. Genus Noemacheilus Van Hasselt, 1823–Stone loaches. In Fishes of Kazkahstan; Nauka: Alma-Ata, Kazakhstan, 1989; Volume 4, pp. 6–63. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland; Berlin, Germany, 2007; pp. 1–646. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. (Eds.) ESCHMEYER’S CATALOG OF FISHES: GENERA, SPECIES, REFERENCES. 2025. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 14 July 2025).
- Jost, L.; Chao, A.; Chazdon, R.L. Compositional similarity and β (beta) Diversity. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 66–84. [Google Scholar]
- Cruz, L.C.; Pompeu, P.S. Drivers of fish assemblage structures in a Neotropical urban watershed. Urban Ecosyst. 2020, 23, 819–829. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd English ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2012; 989p. [Google Scholar]
- Braak, C.J.E.; Verdonschot, P.E.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Hogan, Z.; Jensen, O. Hucho taimen. The IUCN Red List of Threatened Species 2013: E.T188631A22605180. 2013. Available online: https://www.iucnredlist.org/species/188631/22605180 (accessed on 10 June 2025).
- Mamilov, N. Brachymystax savinovi. The IUCN Red List of Threatened Species 2020: E.T156767157A156767203. 2020. Available online: https://www.iucnredlist.org/species/156767157/156767203 (accessed on 10 June 2025).
- Cao, L.; Causse, R.; Zhang, E. Revision of the loach species Barbatula nuda (Bleeker 1865) (Pisces: Balitoridae) from North China, with a description of a new species from Inner Mongolia. Zootaxa 2012, 3586, 236–248. [Google Scholar] [CrossRef]
- Prokofiev, A.M. Loaches of the Nemacheilinae Subfamily of the World Fauna; Filigran: Yaroslavl, Russia, 2017. [Google Scholar]
- Martynova, A.L.; Vasil’eva, E.D. Problems of Taxonomy and Diagnostics of Gudgeons of the Genus Gobio (Cyprinidae) from Ural, Siberia, Kazakhstan and the Amur River Basin. J. Ichthyol. 2021, 61, 685–700. [Google Scholar] [CrossRef]
- Vasil’eva, E.D.; Mamilov, N.S.; Sharakhmetov, S.E. Gudgeon from the Emel River and Problems of the Gudgeon Taxonomy (Genus Gobio, Cyprinidae) in Kazakhstan and Siberia. J. Ichthyol. 2023, 63, 849–863. [Google Scholar] [CrossRef]
- Dyldin, Y.V.; Orlov, A.M.; Hanel, L.; Romanov, V.I.; Fricke, R.; Vasil’eva, E.D. Ichthyofauna of the fresh and brackish waters of Russia and adjacent areas: Annotated list with taxonomic comments. 2. Order Cypriniformes, suborders Catostomoidei, Cobitoidei and Cyprinoidei. J. Ichthyol. 2023, 63, 636–686. [Google Scholar] [CrossRef]
- Mitrofanov, I.V.; Matmuratov, S.A. Variability and condition of minnows (Cyprinidae; Cypriniformes) on Semipalatinsk range and out of its influence. Tethys Aqua Zool. Res. 2007, 3, 65–76. [Google Scholar]
- Mitrofanov, I.V. Loaches of the Shagan River (Irtysh basin). Selevinia 1994, 2, 24–28. [Google Scholar]
- Matmuratov, S.A.; Mitrofanov, I.V. Morphological and ecological variability of loaches (Balitoridae, Nemacheilus) in the conditions of the zone of influence of the Semipalatinsk test site. Bull. Natl. Nucl. Cent. Repub. Kazakhstan 2002, 3, 85–89. [Google Scholar]
- Xie, P.; Zhao, G.; Niu, J.G.; Wang, J.; Zhou, Q.; Guo, Y.; Ma, X.F. Comprehensive analysis of population genetics of Phoxinus phoxinus ujmonensis in the Irtysh River: Abiotic and biotic factors. Ecol. Evol. 2019, 9, 7997–8012. [Google Scholar] [CrossRef]
- Tian, C.; Fang, L.; Li, X.; Li, Y.; Song, T.; Chang, J.; Liu, C.; Zhao, Y. Non-native fish of the Upper Irtysh and the Ulungur Rivers in China. Biodivers. Data J. 2023, 11, e97884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Xie, C.X.; Ding, H.P.; Liu, C.J.; Xie, P.; Guo, Y. Length–weight and length–length relationships of seven fish species from Irtysh River and its tributaries, northwest China. J. Appl. Ichthyol. 2016, 32, 986–988. [Google Scholar] [CrossRef]
- Yang, T.; Meng, W.; Zhang, R.; Gao, T.; Cai, L.; Hai, S.; Zhou, Q. DNA Barcoding of Fishes in Irtysh River China. Russ. J. Genet. 2016, 52, 969–976. [Google Scholar] [CrossRef]
- Reshetnikov, A.N.; Chibilev, E.A. Distribution of the fish rotan (Perccottus glenii Dybowski, 1877) in the Irtysh River basin and analysis of possible consequences for environment and people. Contemp. Probl. Ecol. 2009, 2, 224–228. [Google Scholar] [CrossRef]
- Reshetnikov, A.N.; Golubtsov, A.S.; Zhuravlev, V.B.; Lomakin, S.L.; Rezvyi, A.S. Range expansion of rotan Perccottus glenii, sunbleak Leucaspius delineatus, and bleak Alburnus alburnus in the Ob River basin. Contemp. Probl. Ecol. 2017, 10, 612–620. [Google Scholar] [CrossRef]
- Reshetnikov, A.N.; Interesova, E.A.; Borovikova, E.A. Using a combined approach to analyse the invasion history of the fish rotan, widespread in northern Eurasia. Biol. Invasions 2025, 27, 84. [Google Scholar] [CrossRef]
- Interesova, E.A.; Yadrenkina, E.N.; Vasil’eva, E.D. The first record of Misgurnus nikolskyi (Cobitidae) in the South of Western Siberia. J. Ichthyol. 2010, 50, 281–284. [Google Scholar] [CrossRef]
- van der Sleen, P.; Albert, J.S. Patterns in freshwater fish diversity. Ref. Modul. Earth Syst. Environ. Sci. 2022, 26, 894–907. [Google Scholar]






| Water Bodies | Abbreviation | N | E | Above Sea Level, m |
|---|---|---|---|---|
| Irtysh River | IRT | 47°55′29″ | 84°59′50″ | 408 |
| 47°52′35″ | 84°46′49″ | 394 | ||
| 49°07′56″ | 84°06′43″ | 390 | ||
| Zaisan Lake | ZAI | 47°40′22″ | 84°27′05″ | 437 |
| 47°52′14″ | 84°46′49″ | 437 | ||
| Right (North) bank: | ||||
| Shanghin Lake (=Bukhtarminskoe) | LBU | 49°17′44″ | 83°53′07″ | 2068 |
| Markakol Lake | LMA | 48°47′32″ | 86°01′25″ | 1451 |
| 49°39′39″ | 85°42′51″ | 1450 | ||
| Kalzhyr River | Kal | 48°00′39” | 85°11′01” | 411 |
| 48°29′52” | 84°13′49” | 862 | ||
| Sarymsakty River | Sar | 49°10′01″ | 85°36′35″ | 1058 |
| Bukhtarma River | Buk | 49°15′15″ | 85°22′13″ | 739 |
| 49°27′31″ | 85°03′59″ | 591 | ||
| 49°47′44″ | 84°08′24″ | 407 | ||
| Belaya River | Bel | 49°18′06″ | 85°18′43″ | 685 |
| 49°16′59” | 85°17′56” | 677 | ||
| Kara Kaba River | Kab | 49°01′14″ | 85°51′23″ | 2140 |
| Kurchum River | Kur | 48°37′20″ | 83°54′48″ | 476 |
| Topolevka River | Top | 48°54′45″ | 85°50′18” | 2019 |
| Tikhaya River | Tik | 50°24′36″ | 83°30′36″ | 749 |
| Left (South) bank: | ||||
| Kendyrlik River | Ken | 47°37′27″ | 84°57′13″ | 450 |
| 47°79′58″ | 84°79′60″ | 390 | ||
| Saryeshki River | Sae | 47°37′01″ | 85°00′30″ | 453 |
| Karatuma River | Kar | 47°30′50″ | 84°56′59″ | 489 |
| Kogeady River | Kgd | 47°34′41″ | 85°05′36″ | 507 |
| Shar River | SHR | 49°14′35″ | 81°49′20″ | 602 |
| Shagan River | Sha | 49°19′23” | 78°24′17” | 681 |
| 49°47′34” | 78°45′52” | 367 | ||
| Ashysu River | Ash | 49°43′31” | 78°52′58” | 403 |
| Uzunbulak brook | Uzb | 49°45′51” | 78°5′54” | 794 |
| Karasu River | Ksu | 47°33′38″ | 83°38′58″ | 647 |
| Shet-Karasu River | KKS | 47°33′49″ | 83°39′05″ | 645 |
| Shet-Kandysu River | Ska | 47°12′24″ | 84°27′59″ | 730 |
| Bogaz River | Bog | 47°87′11″ | 82°00′21″ | 764 |
| Kargyba River | Kgb | 47°57′40″ | 82°57′30″ | 533 |
| Beiyttibulak River | BBk | 47°29′31″ | 85°10′17″ | 722 |
| Ujdene Reservior | Ujd | 47°22′34″ | 84°47′27″ | 805 |
| Zhineshkesu River | Zhy | 47°38′09″ | 85°06′31″ | 476 |
| Species | Common Name | Abbreviation | Irtysh and Zaisan | Right Side Inflows | Left Side Inflows |
|---|---|---|---|---|---|
| Indigenous: | |||||
| Lethenteron sp. | Siberian brook lamprey or Arctic | Let | 0 | + | 0 |
| Hucho taimen | Siberian Taimen | Huc | 0 | + | 0 |
| Brachymystax lenok | Lenok | Brl | + | + | 0 |
| Brachymystax savinovi | Markakol Lenok | Brs | 0 | + | 0 |
| Thymallus arcticus | Arctic Grayling | Thy | 0 | + | 0 |
| Esox lucius | Northern Pike | Eso | + | + | + |
| Leuciscus baicalensis | Siberian Dace | Lba | + | + | + |
| Leuciscus idus | Ide | Lid | + | + | 0 |
| Leuciscus aspius | Asp | Las | + | 0 | 0 |
| Rutilus lacustris | Roach | Rla | + | + | + |
| Carassius carassius | Crucian Carp | Car | + | 0 | + |
| Rhynchocypris percnurus | Lake Minnow | Pho | 0 | 0 | + |
| Phoxinus poljakowi | Balkhash Minnow | Rhy | 0 | 0 | + |
| Phoxinus ujmonensis | Phu | + | + | + | |
| Tinca tinca | Tench | Tin | + | 0 | + |
| Gobio sibiricus | Siberian gudgeon | Gos | + | + | + |
| Gobio acutipinnatus | Goa | 0 | + | 0 | |
| Gymnodiptychus dybowskii | Naked osman | Gym | 0 | 0 | + |
| Triplophysa dorsalis | Gray loach | Tdo | 0 | 0 | + |
| Triplophysa strauchii | Spotted thicklip loach | Tst | + | + | + |
| Triplophysa stolickai | Tibetan stone loach | Tsz | 0 | 0 | + |
| Triplophysa sewerzowii | Severtsov’s loach | Tsw | 0 | 0 | + |
| Triplophysa sp. | Tsp | 0 | 0 | + | |
| Barbatula toni | Bar | 0 | + | + | |
| Cobitis melanoleuca | Spined loach | Cob | + | + | + |
| Lota lota | Burbot | Lot | + | + | 0 |
| Perca fluviatilis | European perch | Per | + | + | + |
| Gymnocephalus cernuus | Ruffe | Cer | + | + | 0 |
| Cottus sibiricus | Siberian sculpin | Cot | 0 | + | 0 |
| Cottus dzungaricus | Cot | 0 | + | 0 | |
| Alien: | |||||
| Coregonus albula | Vendace | Coa | + | 0 | + |
| Coregonus lavaretus | European whitefish | Cor | + | 0 | + |
| Carassius gibelio | Prussian carp | Cag | + | + | + |
| Abramis brama | Freshwater bream | Abr | + | + | + |
| Alburnus alburnus | Bleak | Alb | + | + | + |
| Leucaspius delinetaus | Sunbleak (=belica) | Leu | 0 | 0 | + |
| Cyprinus carpio | Common carp | Cyp | + | + | + |
| Pseudorasbora parva | Topmouth gudgeon | Pse | 0 | + | + |
| Abbottina rivularis | Chinese false gudgeon | Abb | 0 | 0 | + |
| Misgurnus sp. | Pond loach | Mis | 0 | + | + |
| Sander lucioperca | Pike-perch | San | + | 0 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamilov, N.S.; Sapargaliyeva, N.S.; Kegenov, E.; Kegenova, G.B.; Magda, I.N.; Lopatin, O.E.; Barinova, G.; Sharakhmetov, S.E.; Gabdullina, A.; Bolbotov, G.; et al. Asymmetric Distribution of Fish Diversity in Inflows of the Black Irtysh River (Central Asia, Kazakhstan). Diversity 2025, 17, 648. https://doi.org/10.3390/d17090648
Mamilov NS, Sapargaliyeva NS, Kegenov E, Kegenova GB, Magda IN, Lopatin OE, Barinova G, Sharakhmetov SE, Gabdullina A, Bolbotov G, et al. Asymmetric Distribution of Fish Diversity in Inflows of the Black Irtysh River (Central Asia, Kazakhstan). Diversity. 2025; 17(9):648. https://doi.org/10.3390/d17090648
Chicago/Turabian StyleMamilov, Nadir Shamilevich, Nazym Sapargaliyevna Sapargaliyeva, Erlan Kegenov, Gulnar Bolatovna Kegenova, Igor Nikolaevich Magda, Oleg Efimovich Lopatin, Gulnaz Barinova, Sayat Ermukhanbetovich Sharakhmetov, Aliya Gabdullina, Gleb Bolbotov, and et al. 2025. "Asymmetric Distribution of Fish Diversity in Inflows of the Black Irtysh River (Central Asia, Kazakhstan)" Diversity 17, no. 9: 648. https://doi.org/10.3390/d17090648
APA StyleMamilov, N. S., Sapargaliyeva, N. S., Kegenov, E., Kegenova, G. B., Magda, I. N., Lopatin, O. E., Barinova, G., Sharakhmetov, S. E., Gabdullina, A., Bolbotov, G., Rudoi, V., & Vorobyov, V. (2025). Asymmetric Distribution of Fish Diversity in Inflows of the Black Irtysh River (Central Asia, Kazakhstan). Diversity, 17(9), 648. https://doi.org/10.3390/d17090648







