Characterization and Prevalence of Different Isolates of Pseudomonas savastanoi and Pathogenicity Properties on Olive and Oleander Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey and Sampling Procedures
2.2. Calculation of Disease Severity, Morbidity, and Prevalence Rates
2.3. Isolation of Bacteria
2.4. Pathogenicity and Phenotypic Characterization
2.5. DNA Extraction of Bacteria
2.6. Species-Specific PCR Targeting IaaL (IAALF/IAALR)
2.7. Virulence Tests: Calculation of Knot and Lesion Areas
3. Results
3.1. Bacterial Isolation and Morphological Characterization
3.2. Biochemical and Molecular Identification
3.3. Pathogenicity and Virulence Tests
3.4. Field Survey Results
4. Discussion
4.1. Morphological and Biochemical Characterization in Context
4.2. Molecular Identification and Genetic Insights
4.3. Pathogenicity Assessment and Comparative Host Interaction
4.4. Distributional Heterogeneity and Regional Epidemiology of Olive Knot (Pseudomonas savastanoi) in Southeastern Anatolia, Turkey
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öztürk, İ.; İkinci, A.; Bolat, İ. The current status of olive cultivation in the Southeastern Anatolia Region and the problems encountered. In Proceedings of the 1st International Harran Multidisciplinary Studies Congress, Urfa, Turkey, 8–10 March 2019; p. 578. [Google Scholar]
- FAO. The Food and Agriculture Organization Corporate Statistical Database, Crops and Livestock Products. 2020. Available online: https://bit.ly/3OD0Bpt (accessed on 25 March 2022).
- FAO. The Food and Agriculture Organization Corporate Statistical Database, Crops and Livestock Products. 2021. Available online: https://bit.ly/3OD0Bpt (accessed on 25 March 2022).
- TUIK. Turkish Statistical Institute, Crop Production Statistics. 2021. Available online: https://bit.ly/3VuQWTM (accessed on 25 March 2022).
- Gardan, L.; Bollet, C.; Abu Ghorrah, M.; Grımont, F.; Grımont, P.A.D. DNA relatedness among the pathovar strains of Pseudomonas syringae subsp. savastanoi Janse (1982) and proposal of Pseudomonas savastanoi sp. nov. Int. J. Syst. Evol. Microbiol. 1992, 42, 606. [Google Scholar] [CrossRef]
- Ramos, C.; Matas, I.M.; Bardaji, L.; Aragón, I.M.; Murillo, J. Pseudomonas savastanoi pv. savastanoi: Some like it knot. Mol. Plant Pathol. 2012, 13, 998–1009. [Google Scholar] [CrossRef]
- Savastano, L. Les maladies de l’olivier, et la tuberculose en particulier. CR Séance Académie D’agriculture De Fr. 1886, 103, 1144. [Google Scholar]
- Savastano, L. Il bacillo della tubercolosi dell’olivo. Rend. Della Regia Accad. Dei Lincei 1889, 5, 92–94. [Google Scholar]
- Smith, E.R.; Rorer, J.B. The olive tubercle. Science 1904, 19, 416. [Google Scholar]
- Surico, G.; Iacobellis, N.S.; Sısto, A. Studies on the role of indole-3-acetic acid and cytokinins in the formation of knots on olive and oleander plants by Pseudomonas syringae pv. savastanoi. Physiol. Plant Pathol. 1985, 26, 309. [Google Scholar] [CrossRef]
- Bozkurt, I.A.; Soylu, S.; Mirik, M.; Ulubas Serce, C.; Baysal, Ö. Characterization of bacterial knot disease caused by Pseudomonas savastanoi pv. savastanoi on pomegranate (Punica granatum L.) trees: A new host of the pathogen. Lett. Appl. Microbiol. 2014, 59, 520. [Google Scholar] [CrossRef]
- Eltlbany, N.; Prokscha, Z.Z.; Castañeda-Ojeda, M.P.; Krogerrecklenfort, E.; Heuer, H.; Wohanka, W.; Ramos, C.; Smalla, K. A new bacterial disease on Mandevilla sanderi, caused by Pseudomonas savastanoi: Lessons learned for bacterial diversity studies. Appl. Environ. Microbiol. 2012, 78, 8492. [Google Scholar] [CrossRef]
- Morettı, C.; Vınatzer, B.A.; Onofrı, A.; Valentını, F.; Buonaurıo, R. Genetic and phenotypic diversity of Mediterranean populations of the olive knot pathogen, Pseudomonas savastanoi pv. savastanoi. Plant Pathol. 2017, 66, 595. [Google Scholar] [CrossRef]
- Mirik, M.; Aysan, Y.; Sahin, F. Characterization of Pseudomonas savastanoi pv. savastanoi strains isolated from several host plants in Turkey and report of fontanesia as a new host. J. Plant Pathol. 2011, 93, 263–270. [Google Scholar]
- Kavak, H.; Üstün, N. Oleander knot caused by Pseudomonas savastanoi pv. nerii in Turkey. J. Plant Pathol. 2009, 91, 701–703. [Google Scholar]
- Lazarov, A.; Grigorov, P. Karantina na Rastenijata; Zemizdat: Sofia, Bulgaria, 1961; p. 258. [Google Scholar]
- Kıpçak, C. Detection of Fire Blight Disease Caused by Erwinia amylovora and the Disease Incidence in Apple Trees in Lake Van Basin. Master’s Thesis, Van Yüzüncü Yıl University, Institute of Science, Van, Turkey, 2016. [Google Scholar]
- Bora, T.; Karaca, İ. Measurement of Disease and Damage in Crop Plants; Ege University Supplementary Textbook, Publication No: 167; Ege University Press: Bornova-İzmir, Turkey, 1970; p. 43. [Google Scholar]
- Popovıć, T.; Menkovıć, J.; Prokıć, A.; Zlatkovıć, N.; Obradovıć, A. Isolation and characterization of Pseudomonas syringae isolates affecting stone fruits and almond in Montenegro. J. Plant Dis. Prot. 2021, 128, 391. [Google Scholar] [CrossRef]
- Penyalver, R.; García, A.; Ferrer, A.; Bertolini, E.; López, M.M. Detection of Pseudomonas savastanoi pv. savastanoi in olive plants by enrichment and PCR. Appl. Environ. Microbiol. 2000, 66, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Matas, I.M.; Lambertsen, L.; Rodríguez-Moreno, L.; Ramos, C. Identification of novel virulence genes and metabolic pathways required for full fitness of Pseudomonas savastanoi pv. savastanoi in olive (Olea europaea) knots. New Phytol. 2012, 196, 1182–1196. [Google Scholar] [CrossRef] [PubMed]
- Doksöz, S.F.; Bozkurt, İ.A. A new and simple pathogenicity test using carrot slices for Pseudomonas savastanoi pv. savastanoi, causal disease agent of olive knot. J. Plant Pathol. 2020, 102, 1173. [Google Scholar] [CrossRef]
- Penyalver, R.; García, A.; Ferrer, A.; Bertolini, E.; Quesada, J.M.; Salcedo, C.I.; Piquer, J.; Pérez-Panadés, J.; Carbonell, E.A.; del Río, C.; et al. Factors affecting Pseudomonas savastanoi pv. savastanoi plant inoculations and their use for evaluation of olive cultivar susceptibility. Phytopathology 2006, 96, 313–319. [Google Scholar] [CrossRef]
- Üstün, N.; Güven, N. Virulence and indole-3-acetic acid (IAA) biosynthesis ability of Turkish Pseudomonas savastanoi pv. savastanoi isolates and susceptibility of some native olive genotypes. Span. J. Agric. Res. 2021, 19, e1003. [Google Scholar] [CrossRef]
- Schaad, N.W. (Ed.) Initial identification of common genera. In A Laboratory Guide for Identification of Plant Pathogenic Bacteria; American Phytopathological Society Press: St Paul, MN, USA, 1988. [Google Scholar]
- Moretti, C.; Trabalza, S.; Granierı, L.; Caballo-Ponce, E.; Devescovi, G.; Del Pino, A.M.; Ramos, C.; Venturi, V.; Van Den Burg, H.A.; Buonaurio, R.; et al. A Na+/Ca2+ exchanger of the olive pathogen Pseudomonas savastanoi pv. savastanoi is critical for its virulence. Mol. Plant Pathol. 2019, 20, 716. [Google Scholar] [CrossRef]
- Košćak, L.; Lamovšek, J.; Đermić, E.; Tegli, S.; Gruntar, I.; Godena, S. Identification and characterisation of Pseudomonas savastanoi pv. savastanoi as the causal agent of olive knot disease in Croatian, Slovenian and Portuguese olive (Olea europaea L.) orchards. Plants 2023, 12, 307. [Google Scholar] [CrossRef]
- Mitro, S.; Zanane, C.; Hakim, T.; Mazigh, D.; Lekchiri, S.; El Louali, M.; Latrache, H.; Zahir, H. Prediction of olive tuberculosis through physicochemical characterisation of Pseudomonas savastanoi and surfaces of different olive tree parts. Int. J. Environ. Stud. 2024, 82, 223–239. [Google Scholar] [CrossRef]
- Janse, J.D. The bacterial disease of ash (Fraxinus excelsior), caused by Pseudomonas syringae subsp. savastanoi pv. fraxini II. Etiology and taxonomic considerations. Eur. J. For. Pathol. 1981, 11, 425–438. [Google Scholar] [CrossRef]
- Al-Dabagh, R.A.; Gergees, R.N. Antimicrobial & antioxidant activity of a novel exopolysaccharide production by Pseudomonas savastanoi pv. savastanoi bacterium isolated from olive knot. Sumer J. Pure Sci. 2024, 1, 158–174. [Google Scholar]
- Salman, M.; Greenhut, R.; Preece, J.; Ferguson, L.; Kluepfel, D. Field evaluation of olive (Olea europaea) genotypes for resistance to Pseudomonas savastanoi pv. savastanoi. J. Plant Pathol. 2020, 102, 663. [Google Scholar] [CrossRef]
- Bitgen, E.; Mirik, M. Identification of bacterial knot disease agent Pseudomonas savastanoi pv. savastanoi in Tekirdag province and their biological control by using antagonistic bacterial isolates. Mustafa Kemal Univ. J. Agric. Sci. 2021, 26, 326. [Google Scholar]
- Basım, H.; Basım, E.; Ersoy, A. Phenotypic and genotypic characterization of Pseudomonas savastanoi pv. savastanoi causing olive knot disease in Turkey. Appl. Ecol. Environ. Res. 2019, 17, 14927–14944. [Google Scholar] [CrossRef]
- Tsuji, M.; Ohta, K.; Tanaka, K.; Takikawa, Y. Comparison among Japanese isolates of Pseudomonas savastanoi pv. savastanoi, causal agent of olive knot disease. J. Gen. Plant Pathol. 2017, 83, 152. [Google Scholar] [CrossRef]
- Hall, B.H.; Cother, E.J.; Whattam, M.; Noble, D.; Luck, J.; Cartwright, D. First report of olive knot caused by Pseudomonas savastanoi pv. savastanoi on olives (Olea europaea) in Australia. Australas. Plant Pathol. 2004, 33, 433. [Google Scholar] [CrossRef]
- Young, J.M.; Wilkie, J.P.; Fletcher, M.J.; Park, D.C.; Pennycook, S.R.; Triggs, C.M.; Watson, D.R.W. Relative tolerance of nine olive cultivars to Pseudomonas savastanoi causing bacterial knot disease. Phytopathol. Mediterr. 2004, 43, 395. [Google Scholar]
- Turco, S.; Drais, M.I.; Rossini, L.; Chaboteaux, E.; Rahi, Y.J.; Balestra, G.M.; Iacobellis, N.S.; Mazzaglia, A. Complete genome assembly of the levan-positive strain PVFi1 of Pseudomonas savastanoi pv. savastanoi isolated from olive knots in Central Italy. Environ. Microbiol. Rep. 2022, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Rahi, Y.J.; Turco, S.; Taratufolo, M.C.; Tatì, M.; Cerboneschi, M.; Tegli, S.; Valentini, F.; D’onghia, A.; Iacobellis, N.S.; Balestra, G.M.; et al. Genetic diversity and population structure of Pseudomonas savastanoi, an endemic pathogen of the Mediterranean area, revealed up to strain level by the MLVA assay. J. Plant Pathol. 2020, 102, 1064. [Google Scholar] [CrossRef]
- Iacobellis, N.S.; Sisto, A.; Surico, G. Occurrence of unusual strains of Pseudomonas syringae subsp. savastanoi on olive in central Italy 1. EPPO (Eur. Mediterr. Plant Prot. Organ.) Bull. 1993, 23, 429. [Google Scholar]
- Lelliott, R.A.; Billing, E.; Hayward, A.C. A determinative scheme for the fluorescent plant pathogenic Pseudomonads. J. Appl. Bacteriol. 1966, 29, 470. [Google Scholar] [CrossRef]
- Marchi, G.; Viti, C.; Giovannetti, L.; Surico, G. Spread of levan-positive populations of Pseudomonas savastanoi pv. savastanoi, the causal agent of olive knot, in central Italy. Eur. J. Plant Pathol. 2005, 112, 101. [Google Scholar] [CrossRef]
- Lavado-Benito, C.; Murillo, J.; Martínez-Gil, M.; Ramos, C.; Rodríguez-Moreno, L. GacA reduces virulence and increases competitiveness in planta in the tumorigenic olive pathogen Pseudomonas savastanoi pv. savastanoi. Front. Plant Sci. 2024, 15, 1347982. [Google Scholar] [CrossRef] [PubMed]
- Mina, D.; Pereira, J.A.; Lino-Neto, T.; Baptista, P. Screening of potential biocontrol bacterial against Pseudomonas savastanoi pv. savastanoi and elucidation of their mode of action. In Proceedings of the 15th Congress of the Mediterranean Phytopathological Union, Plant Health Sustaining Mediterranean Ecosystems, Cordoba, Spain, 20–23 June 2017. [Google Scholar]
- Mina, D.; Pereira, J.A.; Lino-Neto, T.; Baptista, P. Screening the olive tree phyllosphere: Search and find potential antagonists against Pseudomonas savastanoi pv. savastanoi. Front. Microbiol. 2020, 11, 2051. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, A.; Ramos, C.; Rodríguez-Moreno, L. HrpL regulon of bacterial pathogen of woody host Pseudomonas savastanoi pv. savastanoi NCPPB 3335. Microorganisms 2021, 9, 1447. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.; Rezzonico, F.; Orfei, B.; Cortese, C.; Moreno-Pérez, A.; Van Den Burg, H.A.; Onofri, A.; Firrao, G.; Ramos, C.; Smits, T.H.M.; et al. Synergistic interaction between the type III secretion system of the endophytic bacterium Pantoea agglomerans DAPP-PG 734 and the virulence of the causal agent of olive knot Pseudomonas savastanoi pv. savastanoi DAPP-PG 722. Mol. Plant Pathol. 2021, 22, 1209. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Forster, H.; Adaskaveg, J.E. Genetic diversity of Pseudomonas savastanoi pv. savastanoi in California and characterization of epidemiological factors for olive knot development. Plant Dis. 2018, 102, 1718. [Google Scholar] [CrossRef]
- Tegli, S.; Bini, L.; Calamai, S.; Cerboneschi, M.; Biancalani, C. A MATE transporter is involved in pathogenicity and IAA homeostasis in the hyperplastic plant pathogen Pseudomonas savastanoi pv. nerii. Microorganisms 2020, 8, 156. [Google Scholar] [CrossRef]
- Caballo-Ponce, E.; Meng, X.; Uzelac, G.; Halliday, N.; Cámara, M.; Licastro, D.; Da Silva, D.P.; Ramos, C.; Venturi, V. Quorum sensing in Pseudomonas savastanoi pv. savastanoi and Erwinia toletana: Role in virulence and interspecies interactions in the olive knot. Appl. Environ. Microbiol. 2018, 84, e00950-18. [Google Scholar] [CrossRef]
- Castañeda-Ojeda, M.P.; López-Solanilla, E.; Ramos, C. Differential modulation of plant immune responses by diverse members of the Pseudomonas savastanoi pv. savastanoi HopAF type III effector family. Mol. Plant Pathol. 2017, 18, 625. [Google Scholar] [CrossRef]
- Castañeda-Ojeda, M.P.; Moreno-Pérez, A.; Ramos, C.; López-Solanilla, E. Suppression of plant immune responses by the Pseudomonas savastanoi pv. savastanoi NCPPB 3335 type III effector tyrosine phosphatases HopAO1 and HopAO2. Front. Plant Sci. 2017, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Filiz Doksöz, S.; Bozkurt, I.A. Biological control of Pseudomonas savastanoi pv. savastanoi causing the olive knot disease with epiphytic and endophytic bacteria. J. Plant Pathol. 2022, 104, 65. [Google Scholar] [CrossRef]
- Salman, M.; Mcclean, A.; Kluepfel, D. An in vitro bioassay for evaluating the virulence of Pseudomonas savastanoi pv savastanoi isolates on olive. Australas. Plant Dis. Notes 2022, 17, 1. [Google Scholar] [CrossRef]
- Wreikat, B.I. Genetic characterization of Pseudomonas savastanoi pv. savastanoi strains isolated from different olive cultivars grown in jordan by PCR-RFLP and AFLP. Fresenius Environ. Bull. 2021, 30, 8292. [Google Scholar]
- Doksöz, S.F.; Bozkurt, İ.A. Determination of Olive Knot Disease (Pseudomonas savastanoi pv. savastanoi) in Olive Production Areas of Hatay Province. Turk. J. Agric. Nat. Sci. 2020, 7, 96. [Google Scholar]
- Licciardello, G.; Mosca, A.; Di Silvestro, S.; Puglisi, D.; Russo, M.P.; Catara, V.; Caruso, P. Cultivar susceptibility to olive knot disease and association with endophytic microbiota community. Agronomy 2023, 13, 468. [Google Scholar] [CrossRef]
- Glickmann, E.; Gardan, L.; Jacquet, S.; Hussain, S.; Elasri, M.; Petit, A.; Dessaux, Y. Auxin production is a common feature of most pathovars of Pseudomonas syringae. Mol. Plant-Microbe Interact. 1998, 11, 156–162. [Google Scholar] [CrossRef]
- Mougou, I.; Rhouma, A. Differential susceptibility of olive cultivars to olive knot disease and possible involvement of phenolic compounds in disease tolerance. Arab Gulf J. Sci. Res. 2021, 39, 261. [Google Scholar] [CrossRef]
- Fodor, A.; Juhasz, Á.; Vitári, V.; Anita, K.V. Carrot slice test: A reliable method for evaluating the tumorigenicity of Pseudomonas savastanoi pv. nerii. Open J. Bacteriol. 2024, 8, 1. [Google Scholar] [CrossRef]
- Abuamsha, R.; Kluepfel, D.; Mcclean, A.; Salman, M. Evaluation of Commercial Olive Accessions for Resistance to the Olive Knot Disease Caused by Pseudomonas savastanoi pv. savastanoi. Arab. J. Sci. Eng. 2024, 49, 87. [Google Scholar] [CrossRef]
- Bouaichi, A.; Benkirane, R.; El-Kinany, S.; Habbadi, K.; Lougraimzi, H.; Sadık, S.; Benbouazza, A.; Achbani, E.H. Potential effect of antagonistic bacteria in the management of olive knot disease caused by Pseudomonas savastanoi pv. savastanoi. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1035. [Google Scholar] [CrossRef]
- Caballo-Ponce, E.; Van Dillewijn, P.; Wittich, R.M.; Ramos, C. WHOP, a genomic region associated with woody hosts in the Pseudomonas syringae complex contributes to the virulence and fitness of Pseudomonas savastanoi pv. savastanoi in olive plants. Mol. Plant-Microbe Interact. 2017, 30, 113. [Google Scholar] [CrossRef]
- Košćak, L.; Lamovšek, J.; Lukić, M.; Kovačević, T.K.; Đermić, E.; Goreta Ban, S.; Major, N.; Godena, S. Varietal Susceptibility of Olive to Pseudomonas savastanoi pv. savastanoi and the Antibacterial Potential of Plant-Based Agents. Microorganisms 2024, 12, 1301. [Google Scholar] [CrossRef] [PubMed]
- Wreikat, B.; Khlaif, H. Epiphytic Population Dynamics Of Olive Knot Pathogen Pseudomonas savastanoi pv. savastanoi. Jordan J. Agric. Sci. 2017, 13, 977–986. [Google Scholar]
- Rhimini, Y.; Bouaichi, A.; Chliyeh, M.; Msairi, S.; Touhami, A.O.; Benkirane, R.; Achbani, E.H.; Douira, A. Influence of variations in climatic factors and some cultural practices on knot disease development on oleaster and olive tree (Olea europaea L.) Northwest of Morocco. Annu. Res. Rev. Biol. 2018, 24, 9. [Google Scholar] [CrossRef]
- Kluchevich, M.M.; Chumak, P.Y.; Vigera, S.M. New and dangerous bacterial disease of oleander Pseudomonas savastanoi pv. nerii in greenhouses of Ukraine. Mod. Phytomorphol. 2018, 12, 134. [Google Scholar]
- Mohammed, J.M.; Hassan, W.A.; Aziz, F.F.A. Identification and phylogenetic analysis of Pseudomonas savastanoi pv. savastanoi from two provinces in Iraq. Trop. Plant Pathol. 2024, 49, 232. [Google Scholar] [CrossRef]
- Tarakanov, R.I.; Lukianova, A.A.; Evseev, P.V.; Toshchakov, S.V.; Kulikov, E.E.; Ignatov, A.N.; Miroshnikov, K.A.; Dzhalilov, F.S.U. Bacteriophage Control of Pseudomonas savastanoi pv. glycinea in Soybean. Plants 2022, 11, 938. [Google Scholar] [CrossRef]
- Khezri, M.; Ghasemi, A.; Ahangaran, A. Detection and characterization of endophytic bacteria causing knot in young olive trees. Acta Agric. Slov. 2019, 113, 119. [Google Scholar] [CrossRef]
- Vinatzer, B.A.; Monteil, C.L.; Clarke, C.R. Harnessing population genomics to understand how bacterial pathogens emerge, adapt to crop hosts, and disseminate. Annu. Rev. Phytopathol. 2014, 52, 19. [Google Scholar] [CrossRef]
- Young, J.M.; Bradbury, J.F.; Davis, R.E.; Dickey, R.S.; Ercolani, G.L.; Hayward, A.C.; Vidaver, A.K. Nomenclatural revisions of plant pathogenic bacteria and list of names 1980-1988. Rev. Plant Pathol. 1991, 70, 211. [Google Scholar]
- Mirik, M.; Ayvaz, C. Determination of Pseudomonas savastanoi from oleander in Tekirdag of Turkey. In Proceedings of the IX International Agricultural Symposium “Agrosym 2018”, Jahorina mountain, Bosnia and Herzegovina, 4–7 October 2018; p. 1124. [Google Scholar]
- Azeri, T. Research on olive leaf spot, olive knot and verticillium wilt of olive in Turkey. EPPO Bull. 1993, 23, 437–440. [Google Scholar] [CrossRef]
- Basim, H.; Ersoy, A. Identification and spread on Pseudomonas savastanoi pv. savastanoi caused by knot disease on olive in western Mediterranean region. In Proceedings of the Turkish First Olive Symposium, Bursa, Turkey, 10–15 March 2000. [Google Scholar]
- Tatli, B.; Benlioglu, K. Study on olive knot disease (Pseudomonas savastanoi pv. savastanoi) occurring olive areas of Aydin and Mugla Provinces. In Proceedings of the First Plant Protection Congress of Turkey, Samsun, Turkey, 8–11 September 2004; p. 147. [Google Scholar]
- Servi, D. The Determination of Prevalence and Identification by Pcr Tecniques of Olive Knot Disease (Pseudomas savastanoi pv. savastanoi) in Aydin Province. Master’s Thesis, Selcuk University, Institute of Science, Konya, Turkey, 2009. [Google Scholar]
- Mirik, M.; Aysan, Y. Phenotypic and Genotypic Characterization of Pseudomonas savastanoi pv. savastanoi Isolates and Disease Prevalence of Olive Knot Disease in Marmara Region of Turkey. J. Agric. Sci. 2011, 17, 279. [Google Scholar]
- Sivri, N. Identification of Olive Knot Diseases Pseudomonas savastanoi pv. savastanoi in Kahramanmaraş, Gaziantep and Kilis Olive Grown Areas. Master’s Thesis, Kahramanmaraş Sütçü İmam University, Institute of Science, Onikişubat, Turkey, 2012. [Google Scholar]
- Quesada, J.M.; Penyalver, R.; López, M.M. Epidemiology and control of plant diseases caused by phytopathogenic bacteria: The case of olive knot disease caused by Pseudomonas savastanoi pv. savastanoi. Plant Pathol. 2012, 299–326. [Google Scholar] [CrossRef]
- ECA&D. European Climate Assessment and Dataset, Climate Data for Adiyaman, Turkey. 2025. Available online: https://www.ecad.eu/ (accessed on 15 August 2025).
- Tatulli, G.; Pucci, N.; Santilli, E.; Scala, V.; Loreti, S. Droplet Digital PCR for the Detection of Pseudomonas savastanoi pv. savastanoi in Asymptomatic Olive Plant Material. Plants 2025, 14, 1831. [Google Scholar] [CrossRef] [PubMed]

| Isolate | Orchard Location | Plant Part/Symptom | Year of Recovery | Fluorescence on King’s B | LOPAT Group | Pathogenicity |
|---|---|---|---|---|---|---|
| Olive 1 | Derik (Mardin Province) | Shoot/Knot | 2020 | − | 1a | + |
| Olive 2 | Derik (Mardin Province) | Shoot/Knot | 2020 | − | 1a | + |
| Olive 3 | Derik (Mardin Province) | Shoot/Knot | 2020 | + | 1b | + |
| Olive 4 | Derik (Mardin Province) | Shoot/Knot | 2020 | + | 1b | + |
| Plant | Isolate | Knot Length (mm) | Knot Width (mm) | Knot Volume (mm3) | Percentage of Infection Area (%) |
|---|---|---|---|---|---|
| Oleander (Pink) | Olive 1 | 11.2 ± 4.4 a | 7.2 ± 2.2 a | 290 ± 82 a | 22.86 ± 1.84 b |
| Olive 2 | 9.9 ± 3.8 ab | 6.5 ± 1.9 ab | 265 ± 76 ab | 20.75 ± 2.91 c | |
| Olive 3 | 9.7 ± 3.2 ab | 6.4 ± 1.9 ab | 260 ± 69 ab | 21.19 ± 2.21 c | |
| Olive 4 | 13.5 ± 4.7 b | 8.1 ± 2.8 b | 340 ± 92 b | 23.58 ± 1.64 a | |
| Oleander (White) | Olive 1 | 10.4 ± 3.5 a | 6.8 ± 1.9 a | 275 ± 73 a | 20.17 ± 1.89 b |
| Olive 2 | 9.2 ± 2.8 a | 6.1 ± 1.6 a | 250 ± 63 a | 19.30 ± 2.28 c | |
| Olive 3 | 9.3 ± 2.5 a | 6.2 ± 1.6 a | 252 ± 66 a | 19.66 ± 3.41 bc | |
| Olive 4 | 12.8 ± 3.8 b | 7.9 ± 2.5 b | 330 ± 85 b | 21.66 ± 2.18 a | |
| Olive | Olive 1 | 12.5 ± 3.8 a | 7.5 ± 2.5 a | 310 ± 79 a | 18.32 ± 2.06 b |
| Olive 2 | 10.8 ± 3.2 b | 6.9 ± 2.2 b | 280 ± 69 b | 17.66 ± 1.77 c | |
| Olive 3 | 10.6 ± 3.5 b | 6.8 ± 1.9 b | 278 ± 66 b | 17.78 ± 1.86 c | |
| Olive 4 | 14.7 ± 4.1 c | 8.6 ± 2.8 c | 360 ± 88 c | 19.41 ± 3.03 a |
| Trait | Plant | Source | df | F | p-Value |
|---|---|---|---|---|---|
| Knot Length | Oleander (Pink) | Isolate | 3 | 124.88 | 4.38 × 10−19 |
| Oleander (White) | Isolate | 3 | 70.80 | 3.61 × 10−15 | |
| Olive | Isolate | 3 | 118.54 | 1.03 × 10−18 | |
| Knot Width | Oleander (Pink) | Isolate | 3 | 46.89 | 1.61 × 10−12 |
| Oleander (White) | Isolate | 3 | 44.35 | 3.54 × 10−12 | |
| Olive | Isolate | 3 | 51.26 | 4.48 × 10−13 | |
| Knot Volume | Oleander (Pink) | Isolate | 3 | 84.43 | 2.35 × 10−16 |
| Oleander (White) | Isolate | 3 | 66.23 | 9.99 × 10−15 | |
| Olive | Isolate | 3 | 94.09 | 4.24 × 10−17 | |
| Infection area (%) | Combined analysis across hosts | Plant | 2 | 208.45 | 8.34 × 10−38 |
| Isolate | 3 | 58.35 | 1.68 × 10−22 | ||
| Plant × Isolate | 6 | 4.34 | 0.00058 |
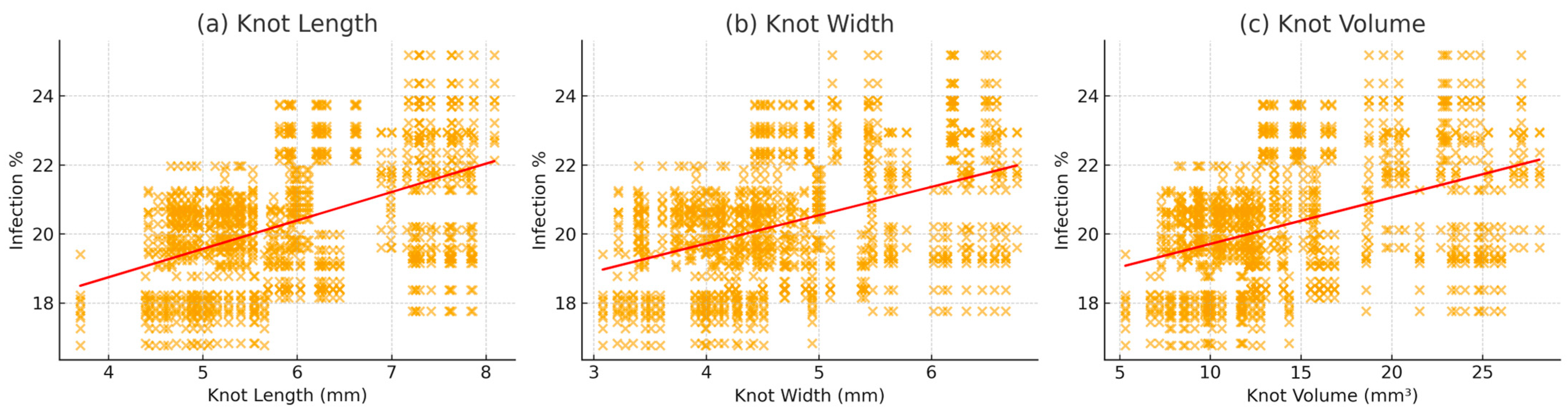
| Variable | r | R2 | p-Value |
|---|---|---|---|
| Knot Length (mm) | 0.48 | 0.23 | <0.001 |
| Knot Width (mm) | 0.43 | 0.18 | <0.001 |
| Knot Volume (mm3) | 0.43 | 0.18 | <0.001 |
| Province | District | Disease Incidence (%) | Prevalence Rate (%) |
|---|---|---|---|
| Adıyaman | Besni | 0.02 | 50 |
| Kahta | 0.01 | 50 | |
| Mean (Adıyaman) | - | 0.017 | 50 |
| Mardin | Derik | 50.37 | 100 |
| Mardin Center | 0.15 | 50 | |
| Mean (Mardin) | - | 33.28 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayman, S.; Kavak, H. Characterization and Prevalence of Different Isolates of Pseudomonas savastanoi and Pathogenicity Properties on Olive and Oleander Plants. Diversity 2025, 17, 646. https://doi.org/10.3390/d17090646
Bayman S, Kavak H. Characterization and Prevalence of Different Isolates of Pseudomonas savastanoi and Pathogenicity Properties on Olive and Oleander Plants. Diversity. 2025; 17(9):646. https://doi.org/10.3390/d17090646
Chicago/Turabian StyleBayman, Serkan, and Hamit Kavak. 2025. "Characterization and Prevalence of Different Isolates of Pseudomonas savastanoi and Pathogenicity Properties on Olive and Oleander Plants" Diversity 17, no. 9: 646. https://doi.org/10.3390/d17090646
APA StyleBayman, S., & Kavak, H. (2025). Characterization and Prevalence of Different Isolates of Pseudomonas savastanoi and Pathogenicity Properties on Olive and Oleander Plants. Diversity, 17(9), 646. https://doi.org/10.3390/d17090646






