Thermal Ecology of Hermann’s Tortoise, Testudo hermanni and Glass Lizard Pseudopus apodus in a Seasonal Environment
Abstract
1. Introduction
- 1.
- What are the environmental temperatures that T. hermanni and P. apodus experience during a complete year in a temperate zone, and how do the changes impact their activity? To answer this question, we use information from null models and relate it to above-ground activity and inactivity.
- 2.
- Do both species show microhabitat matching and, if so, to what extent? To answer this question, we compare monthly changes in microhabitat selection during the full activity season.
- 3.
- Theory predicts that herbivorous reptiles require higher Tb’s than carnivorous forms due to a greater food passage time for herbivores to digest plant material. This predicts that the herbivorous T. hermanni should maintain higher Tb levels than the carnivorous P. apodus [38]. We answer this question by comparing the Tb’s of both species throughout the annual activity period.
- 4.
- How efficient are T. hermanni and P. apodus in regulating Tb’s to within their respective Tset ranges, and how frequently do they exceed or are unable to achieve Tset? This is an important question, because an inability to reach Tset’s may impact the efficiency of physiological process, whilst Tb’s exceeding critical thermal maximum temperatures risk overheating. To answer this question, we used the thermoregulatory efficiency indexes to compare the frequencies of Tb’s within Tsets.
- 5.
- Are there interspecific differences in terms of the efficiency of thermoregulation? This is a key question because of the dietary and major differences in body morphology, specifically in skin surface areas to body mass geometry and lifestyle—predator versus herbivore. To answer this question, we calculated monthly thermoregulatory efficiency E-values for both species to enable direct comparisons.
2. Methods
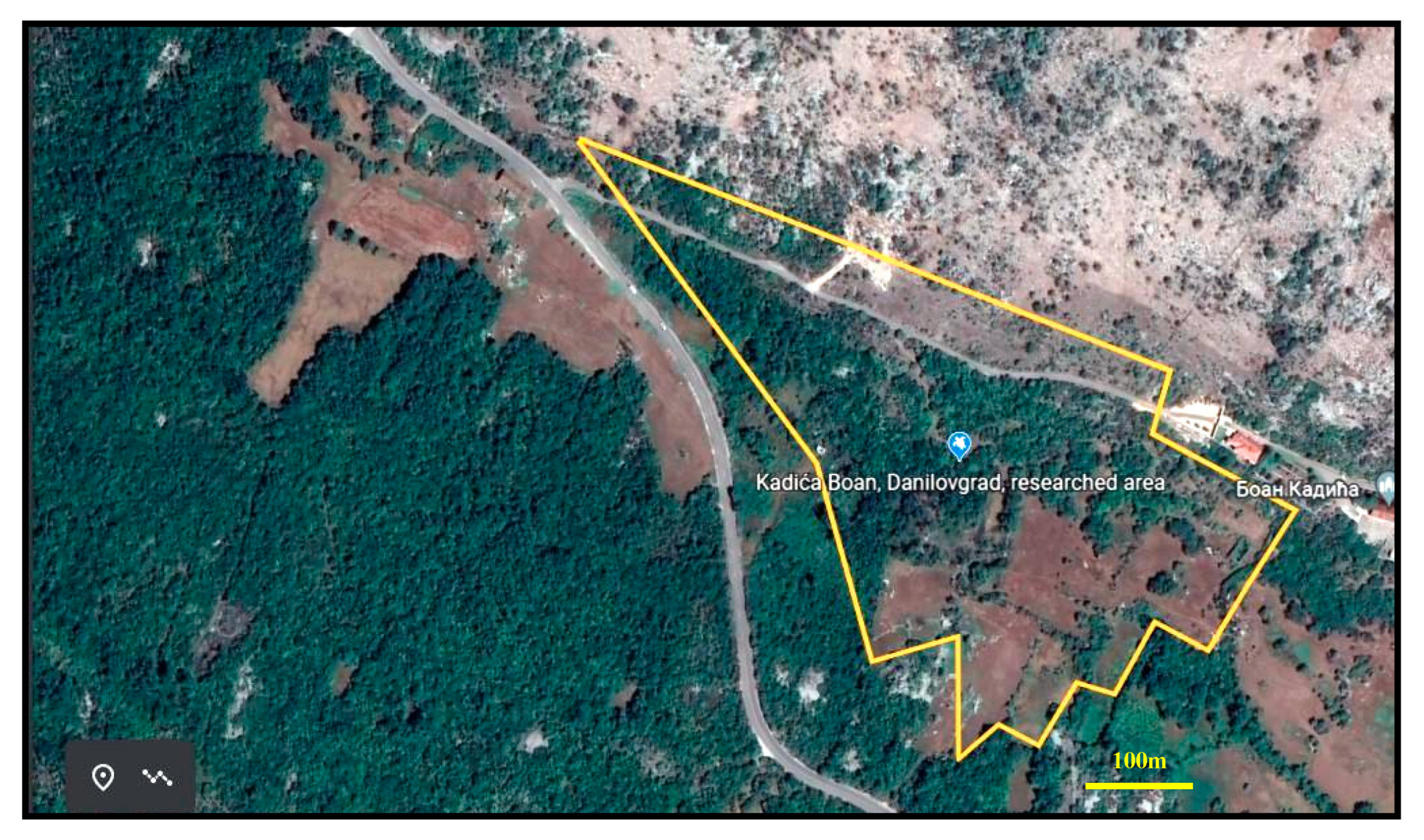
2.1. Study Area
2.2. Null Models
2.3. Statistical Analysis
3. Results
3.1. General Considerations
3.2. The Thermal Environment
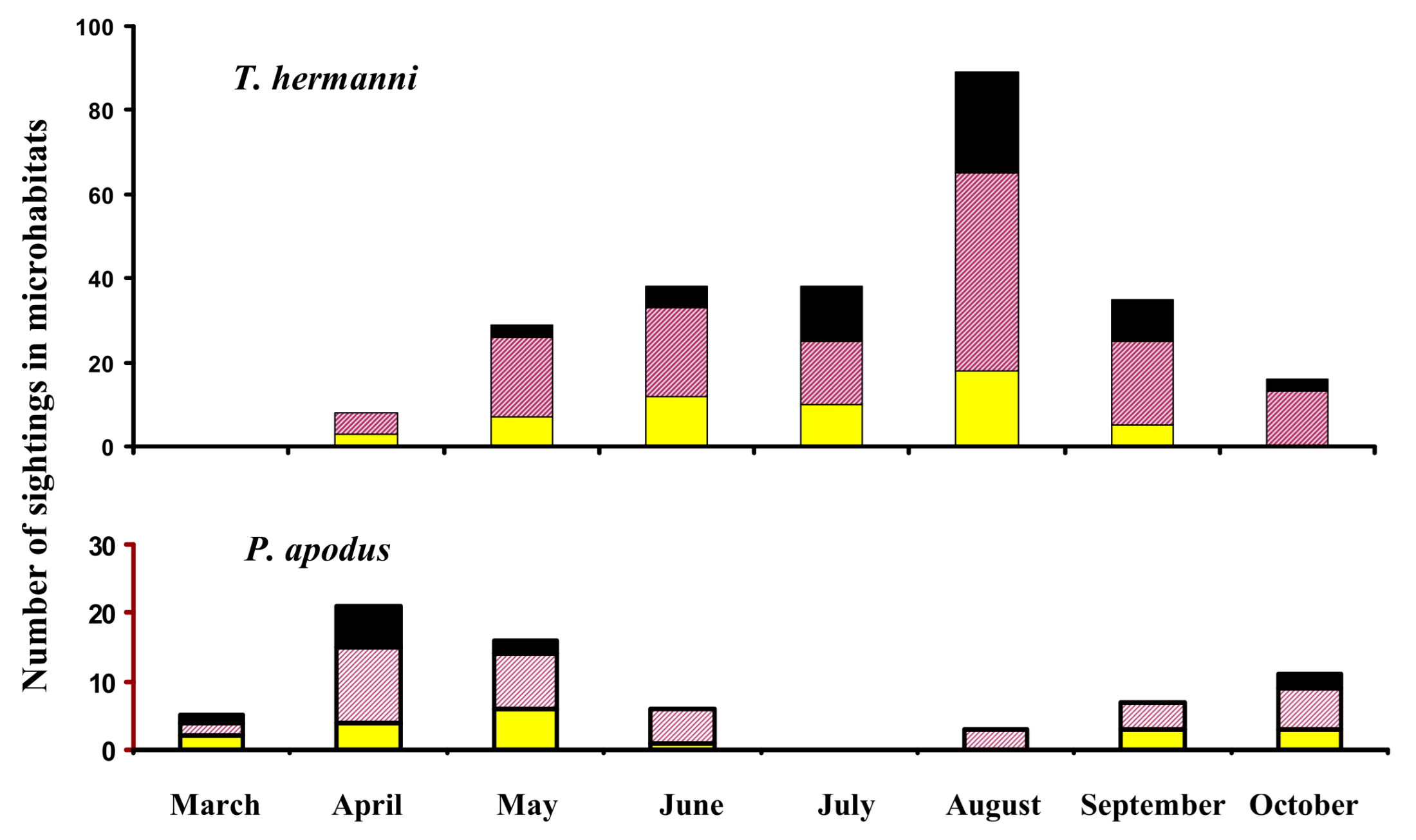
3.3. Microhabitat Selection
3.4. Heating Rates of Adult Male and Female T. hermanni
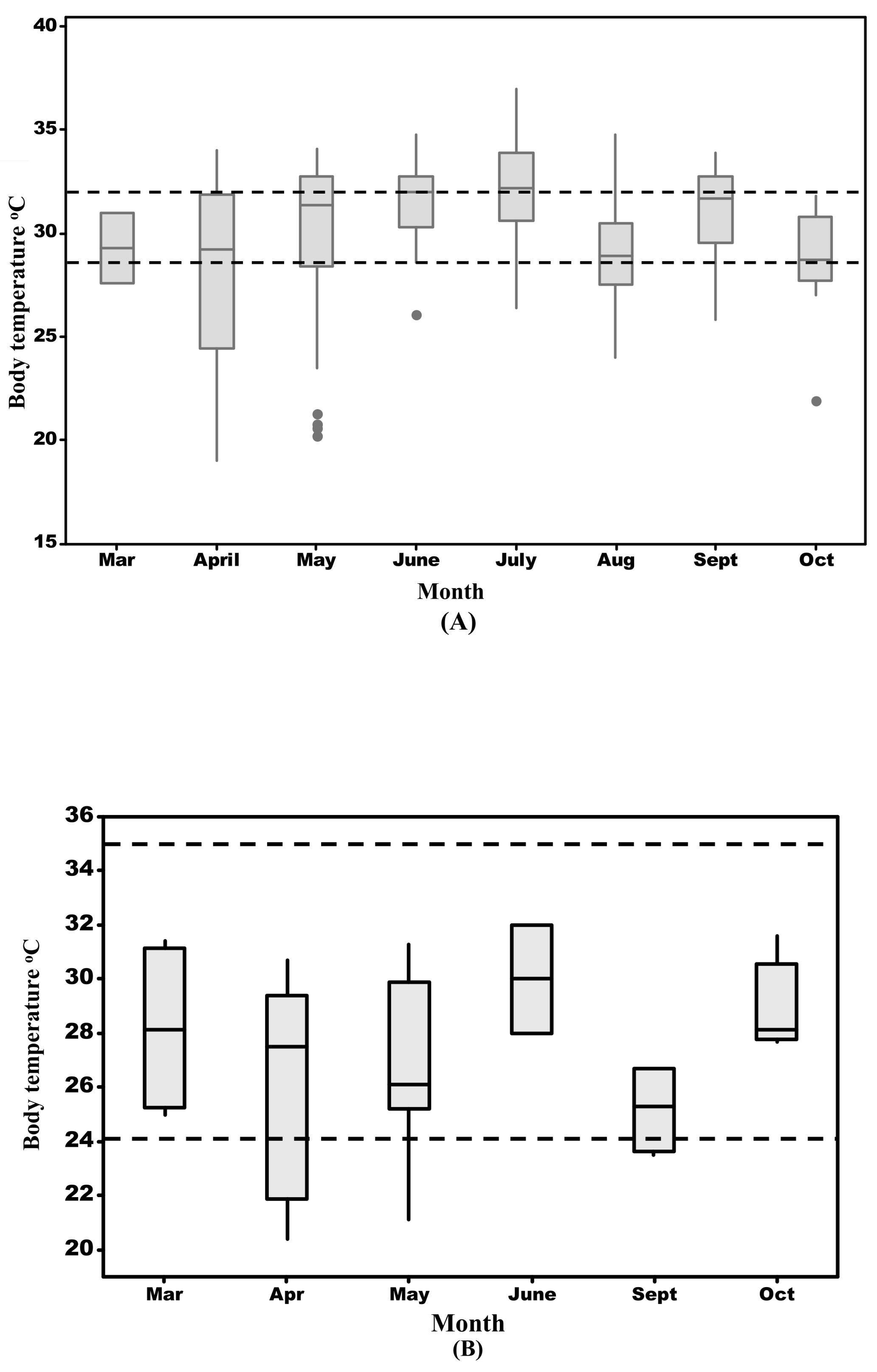
3.5. Monthly Body Temperatures of T. hermanni
3.6. Monthly Body Temperatures of P. apodus
3.7. Species Comparisons
3.8. Body Temperatures: Skewness
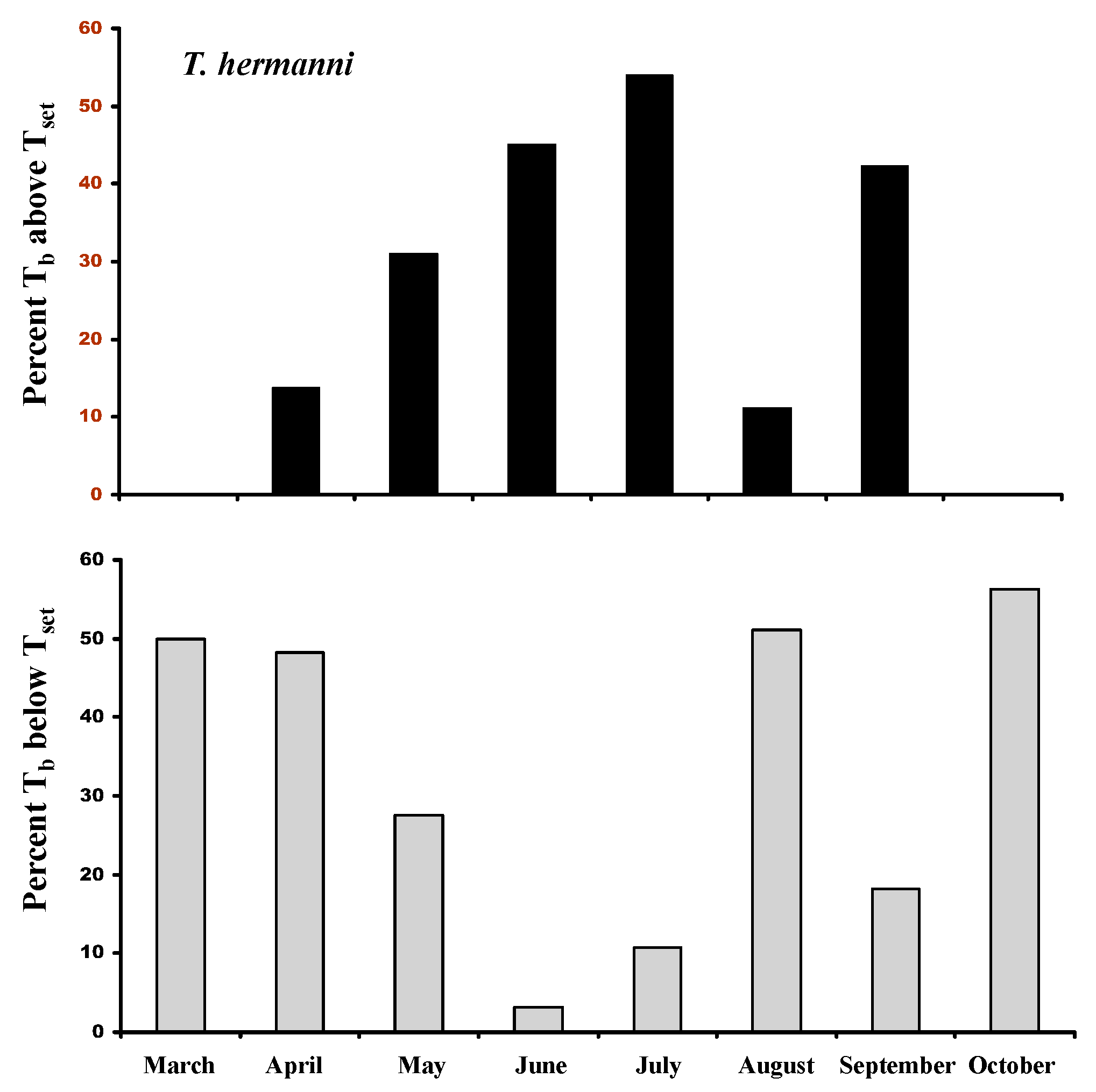
3.9. Tb’s and the Set Point Range
3.10. Monthly Thermoregulatory Efficiency: T. hermanni
3.11. Monthly Thermoregulatory Efficiency: P. apodus
4. Discussion
4.1. General Results
4.2. Interspecific Dietary Differences on Thermal Ecology
4.3. Thermoregulatory Efficiency
4.4. P. apodus
4.5. Habitat Integrity and Conservation
4.6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huey, R.B.; Slatkin, M. Costs and benefits of lizard thermoregulation. Q. Rev. Biol. 1976, 51, 363–384. [Google Scholar] [CrossRef]
- Bauwens, D.; Hertz, P.E.; Castilla, A.M. Thermoregulation in a lacertid lizard: The relative contributions of distinct behavioral mechanisms. Ecology 1996, 77, 1818–1830. [Google Scholar] [CrossRef]
- Smith, G.R.; Ballinger, R.E. The ecological consequences of habitat and microhabitat use in lizards: A review. Contemp. Herpetol. 2001, 3, 1–37. [Google Scholar] [CrossRef]
- Christian, K.A.; Tracy, C.R.; Porter, W.P. Seasonal shifts in body temperature and use of microhabitats by Galapagos land iguanas (Conolophus pallidus). Ecology 1983, 664, 463–468. [Google Scholar] [CrossRef]
- Christian, K.A.; Tracy, C.R. Body temperatures and the thermal environment. In Reptile Ecology and Conservation: A Handbook of Techniques; Dodd, C.K., Ed.; Oxford University Press: Oxford, UK, 2016; pp. 337–351. [Google Scholar]
- Meek, R. Reptiles, thermoregulation and the environment. Testudo 1995, 4, 56–78. [Google Scholar]
- Butler, C.J. A Review of the effects of climate change on chelonians. Diversity 2019, 11, 138. [Google Scholar] [CrossRef]
- Stevenson, R.D. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 1985, 126, 362–386. [Google Scholar] [CrossRef]
- Stevenson, R.D. Body size and limits to the daily range of body temperature in terrestrial ectotherms. Am. Nat. 1985, 125, 102–117. [Google Scholar] [CrossRef]
- Carrascal, L.M.; López, P.; Martin, J.; Salvador, A. Basking and antipredator behaviour in a high altitude lizard: Implications of heat-exchange rate. Ethology 1992, 92, 143–154. [Google Scholar]
- Swingland, I.R.; Frazier, J.G. The conflict between feeding and overheating in the Aldabran giant tortoise. In A Handbook of Biotelemetry and Radio Tracking; Amlaner, C.J., MacDonald, D.W., Eds.; Pergamon Press: Oxford, UK, 1979; pp. 611–615. [Google Scholar]
- Haxhiu, I. Results of studies on the chelonians of Albania. Chelonian Conserv. Biol. 1995, 1, 324–326. [Google Scholar]
- Meek, R. Nutritional selection in Hermanns tortoise, Testudo hermanni, in Montenegro and Croatia. Testudo 2010, 7, 88–95. [Google Scholar]
- Çiçek, K.; Tok, C.; Hayretdağ, S.; Ayaz, D. Data on the food composition of European glass lizard, Pseudopus apodus (Pallas, 1775) (Squamata: Anguidae) from Çanakkale (Western Anatolia, Turkey). Acta Zool. Bulgar. 2014, 66, 433–466. [Google Scholar]
- Telenchev, I.; Simeonovaska-Nikolova, D.; Tzonev, N. Habitat use and activity of European glass lizard, Pseudopus apodus (Pallas, 1775) in southeastern Bulgaria. Turk. J. Zool. 2017, 41, 2. [Google Scholar] [CrossRef]
- Liang, C.; Marghoub, A.; Kever, L.; Bertazzo, S.; Abzhanov, A.; Vickaryous, M.; Herrel, A.; Evans, S.; Moazen, M. Lizard osteodermsmorphological characterisation, biomimetic design and manufacturing based on three species. Bioinspir. Biomim. 2021, 16, 066011. [Google Scholar] [CrossRef]
- Meek, R. Thermoregulatory behaviour in a population of Hermann’s tortoise (Testudo hermanni) in southern Yugoslavia. Br. J. Herpetol. 1984, 6, 387–391. [Google Scholar]
- Meek, R. Field body temperature of the glass lizard Ophisaurus apodus in Yugoslavia. Amphibia-Reptilia 1986, 7, 43–49. [Google Scholar] [CrossRef]
- Meek, R. The thermal ecology of Hermann’s tortoise (Testudo hermanni) in summer and autumn in Yugoslavia. J. Zool. 1988, 215, 99–111. [Google Scholar] [CrossRef]
- Hailey, A.; Theophilidis, G. Cardiac response to stress and activity in the armoured legless lizard (Ophisaurus apodus): Comparison with snake and tortoise. Comp. Biochem. Physiol. 1987, 88, 201–206. [Google Scholar] [CrossRef]
- Wright, J.; Steer, E.; Hailey, A. Habitat separation in tortoises and the consequences for activity and thermoregulation. Can. J. Zool. 1988, 66, 1537–1544. [Google Scholar] [CrossRef]
- Vujović, A.; Pešić, V.; Meek, R. Living in a thermally diverse environment: Field body temperatures and thermoregulation in Hermann’s tortoise, Testudo hermanni, in Montenegro. Conservation 2023, 3, 59–70. [Google Scholar] [CrossRef]
- Willemsen, R.E. Differences in thermoregulation between Testudo hermanni and Testudo marginata and their ecological significance. Herpetol. J. 1991, 1, 559–567. [Google Scholar]
- Rugiero, L.; Luiselli, L. Ecological modelling of habitat use and the annual activity patterns in an urban population of the tortoise, Testudo hermanni. Ital. J. Zool. 2006, 73, 219–225. [Google Scholar] [CrossRef]
- Moulherat, S.; Delmas, V.; Slimani, T.; El Mouden, E.; Louzizi, T.; Lagarde, F.; Bonnet, X. How far can a tortoise walk in open habitat before overheating? Implications for conservation. J. Nat. Conserv. 2014, 22, 186–192. [Google Scholar] [CrossRef]
- Gangloff, E.J.; Telemeco, R.S. High temperature, oxygen, and performance: Insights from reptiles and amphibians. Integr. Comp. Biol. 2018, 58, 9–24. [Google Scholar] [CrossRef]
- Hutchison, V.H.; Vinegar, A.; Kosh, R.J. Critical thermal maxima in turtles. Herpetologica 1966, 22, 32–41. [Google Scholar]
- O’Connor, M.P.; Zimmerman, L.C.; Dzialowski, E.M.; Spotila, J.R. Thick-walled physical models improve estimates of operative temperatures for moderate to large-sized reptiles. J. Therm. Biol. 2000, 25, 293–304. [Google Scholar] [CrossRef]
- Walsberg, G.E.; Wolf, B.O. A test of the accuracy of operative temperature thermometers for studies of small ectotherms. J. Therm. Biol. 1996, 21, 275–281. [Google Scholar] [CrossRef]
- Vitt, L.J.; Sartorius, S.S. HOBO’s, Tidbits and lizard models: The utility of electronic devices in field studies of ectotherms thermoregulation. Funct. Ecol. 1999, 13, 670–674. [Google Scholar] [CrossRef]
- Shine, R.; Kearney, M. Field studies of reptile thermoregulation: How well do physical models predict operative temperatures? Funct. Ecol. 2001, 15, 282–288. [Google Scholar] [CrossRef]
- Dzialowski, E.M. Use of operative temperature and standard operative temperature models in thermal biology. J. Therm. Biol. 2005, 30, 317–334. [Google Scholar] [CrossRef]
- Claunch, N.M.; Goodman, C.; Reed, R.N.; Romagosa, C.M.; Taylor, E.N. Invaders from Islands: Thermal Matching, Potential, or Plasticity? Biol. J. Linn. Soc. 2021, 134, 587–603. [Google Scholar] [CrossRef]
- HaiIey, A. Thermoregulation and activity metabolism in the armoured anguid Ophisaurus apodus. Br. J. Herpetol. 1984, 6, 391–398. [Google Scholar]
- Gable, S.M.; Mendez, J.M.; Bushroe, N.A.; Wilson, A.; Byars, M.; Tollis, M. The state of Squamate genomics: Past, present, and future of genome research in the most speciose terrestrial vertebrate order. Genes 2023, 7, 1387. [Google Scholar] [CrossRef]
- Huey, R.B.; Bennett, A.F. Phylogenetic studies of coadaptation: Preferred temperatures versus optimal performance temperatures of lizards. Evolution 1987, 41, 1098–1115. [Google Scholar] [CrossRef]
- Huey, R.B.; Garland, T., Jr.; Turelli, M. Revisiting a key innovation in evolutionary biology: Felsenstein’s Phylogenies and the Comparative Method. Am. Nat. 2019, 193, 655–672. [Google Scholar]
- Tracy, C.R.; Flack, K.M.; Zimmerman, L.A.C.; Espinoza, R.E.; Tracy, C.R. Herbivory imposes constraints on voluntary hypothermia in lizards. Copeia 2005, 2005, 12–19. [Google Scholar] [CrossRef]
- Glavaš, O.J.; Počanić, P.; Lovrić, V.; Derežanin, L.; Tadić, Z.; Lisičić, D. Morphological and ecological divergence in two populations of European glass lizards, Pseudopus apodus (Squamata: Anguidae). Zool. Res. 2020, 41, 172–181. [Google Scholar] [CrossRef]
- Bailey, N.T.J. Statistical Methods in Biology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Hertz, P.E.; Huey, R.B.; Stevenson, R.D. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. Am. Nat. 1993, 142, 796–818. [Google Scholar] [CrossRef]
- Dewitt, C.B.; Friedman, R.M. Significance of skewness in ectotherm thermoregulation. Am. Zool. 1979, 19, 195–209. [Google Scholar] [CrossRef]
- Cherchi, M. Termoregulazione in Testudo hermanni. Bolletino Musei Inst. Biol. Univ. Genova 1956, 28, 9–87. [Google Scholar]
- Cherchi, M. Uteriori richerchi sulla termoregulazione in Testudo hermanni. Bolletino Musei Inst. Biol. Univ. Genova 1960, 30, 35–60. [Google Scholar]
- Mautz, W.J.; Nagy, K.A. Ontogenetic changes in diet, field metabolic rate, and water flux in the herbivorous lizard Dipsosaurus dorsalis. Physiol. Zool. 1987, 60, 640–658. [Google Scholar] [CrossRef]
- Felenstein, J. Phylogenies and the comparative method. Am. Nat. 1987, 125, 1–15. [Google Scholar] [CrossRef]
- Gatten, R.E. Effects of nutritional status on the preferred temperature of the turtles Pseudemys scripta and Terrapene ornata. Copeia 1974, 1974, 912–917. [Google Scholar] [CrossRef]
- Obbard, M.E.; Brooks, R.J. Factors affecting basking in a northern population of the common snapping turtle, Chelydra serpentina. Can. J. Zool. 1979, 57, 435–440. [Google Scholar] [CrossRef]
- Van Damme, R.; Bauwens, D.; Castilla, A.M.; Verheyen, R.F. Altitudinal variation in the thermal biology and running performance in the lizard Podarcis tiliguerta. Oecologia 1989, 80, 51624. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.W.; Dunham, A.E. Elevational covariation in environmental constraints and life histories of the desert lizard Sceloporus merriami. Ecology 1990, 71, 1765–1776. [Google Scholar] [CrossRef]
- Nagy, K.A. Cellulose digestion and nutrient assimilation in Sauromalus obesus, a plant-eating lizard. Copeia 1977, 1977, 355–362. [Google Scholar] [CrossRef]
- Tracy, C.R.; Zimmerman, L.C.; Tracy, C.; Dean-Bradley, K.; Castle, K. Rates of food passage in the digestive tract of young desert tortoises: Effects of body size and diet quality. Chelonian Conserv. Biol. 2006, 5, 269–273. [Google Scholar] [CrossRef]
- Troyer, K. Small differences in daytime body temperature affect digestion of natural food in a herbivorous lizard (Iguana iguana). Comp. Biochem. Physiol. 1987, 87, 623–626. [Google Scholar] [CrossRef]
- Castilla, A.M.; Van Damme, R.; Bauwens, D. Field body temperatures, mechanisms of thermoregulation and evolution of thermal characteristics in lacertid lizards. Nat. Croat. 1999, 8, 253–274. [Google Scholar]
- Tosini, G.; Avery, R.A. Intraspecific variation in lizard thermoregulatory set points: A thermographic study in Podarcis muralis. J. Therm. Biol. 1993, 18, 19–23. [Google Scholar] [CrossRef]
- Vickers, M.; Manicom, C.; Schwarzkopf, L. Extending the cost-benefit model of thermoregulation: High-temperature environments. Am. Nat. 2011, 177, 452–461. [Google Scholar] [CrossRef]
- Dubois, Y.; Blouin-Demers, G.; Thomas, D.W. Temperature selection in wood turtles (Glyptemys insculpta) and its implications for energetics. Ecoscience 2008, 215, 398–406. [Google Scholar] [CrossRef]
- Bennett, A.F. The thermal dependence of lizard behaviour. Anim. Behav. 1980, 28, 752–762. [Google Scholar] [CrossRef]
- Capula, M.; Luiselli, L. Ecology of an alpine population of the slow worm, Anguis fragilis Linnaeus 1758. Thermal biology of reproduction. Herpetozoa 1993, 6, 57–63. [Google Scholar]
- Meek, R. Null models and the thermal biology of the anguid lizard Anguis fragilis; evidence for thermoregulation? Amphibia-Reptilia 2005, 26, 445–450. [Google Scholar] [CrossRef]
- Sinervo, B.; Mendez-De-La-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Burke, R.L.; Rugiero, L.; Capula, M.; Luiselli, L. The turtle is in the details: Microhabitat choice by Testudo hermanni is based on microscale plant distribution. Anim. Biol. 2011, 61, 249–261. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Edwards, D.P.; Diesmos, A.; Williams, S.E.; Evans, T.A. Microhabitats reduce animal’s exposure to climate extremes. Glob. Change Biol. 2014, 20, 495–503. [Google Scholar] [CrossRef]
- Lara–Resendiz, R.A.; Miles, D.B.; Rosen, P.C.; Sinervo, B. Micro and macroclimatic constraints on the activity of a vulnerable tortoise: A mechanistic approach under a thermal niche view. Funct. Ecol. 2022, 36, 2227–2239. [Google Scholar] [CrossRef]
- Huey, R.B.; Pianka, E.R. Ecological consequences of foraging mode. Ecology 1981, 62, 991–999. [Google Scholar] [CrossRef]
- Hailey, A.; Coulson, I.M. Temperature and the tropical tortoise Kinixys spekii: Constraints on activity level and body temperature. J. Zool. 1995, 240, 523–536. [Google Scholar] [CrossRef]
- Vujović, A.; Iković, V.; Golubović, A.; Đorđević, S.; Pešić, V.; Tomović, L. Effects of fires and roadkills on the isolated population of Testudo hermanni Gmelin, 1789 (Reptilia: Testudinidae) in central Montenegro. Acta Zool. Bulg. 2015, 67, 75–84. [Google Scholar]
- Sears, M.W.; Angilletta, M.J.; Schuler, M.S.; Borchert, J.D.; Dilliplane, K.F.; Stegman, M.; Rusch, T.; Mitchell, W.A. Configuration of the thermal landscape determines thermoregulatory performance of ectotherms. Proc. Natl. Acad. Sci. USA 2016, 113, 10595–10600. [Google Scholar] [CrossRef]
- Gaudenti, N.; Nix, E.; Maier, P.; Westphal, M.F.; Taylor, E.N. Habitat heterogeneity affects the thermal ecology of an endangered lizard. Ecol. Evol. 2021, 11, 14843–14856. [Google Scholar] [CrossRef]
- Asplund, K.K. Body size and habitat utilisation in whiptail lizards (Cnemidophorus). Copiea 1974, 1974, 695–703. [Google Scholar] [CrossRef]
- Lee, J.C. Comparative thermal ecology of two lizards. Oecologia 1980, 44, 171–176. [Google Scholar] [CrossRef]
- Basson, C.H.; Levy, O.; Angilletta, M.J.; Clusella-Trullas, S. Lizards paid a greater opportunity cost to thermoregulate in a less heterogeneous environment. Funct. Ecol. 2017, 31, 856–865. [Google Scholar] [CrossRef]
- Giacometti, D.; Yagi, K.T.; Abney, C.R.; Jung, M.P.; Tattersall, G.T. Staying warm is not always the norm: Behavioural differences in thermoregulation of two snake species. Can. J. Zool. 2021, 99, 11. [Google Scholar] [CrossRef]
- Giacometti, D.; Palaoro, A.V.; Leal, L.C.; Barros, F.C. How seasonality influences the thermal biology of lizards with different thermoregulatory strategies: A meta-analysis. Biol. Rev. 2023, 99, 409–429. [Google Scholar] [CrossRef] [PubMed]



| March | April | May | June | July | August | September | October | Σn | |
|---|---|---|---|---|---|---|---|---|---|
| T. hermanni Males | ---------- | 28.4 ± 4.4 | 29.9 ± 4.1 | 31.5 ± 1.3 | 32.3 ± 2.6 | 29.3 ± 2.3 | 31.3 ± 1.6 | 28.8 ± 2.6 | |
| n = | 0 | 11 | 12 | 6 | 25 | 56 | 37 | 16 | 163 |
| T. hermanni Females | 28.8 ± 2.4 | 27.7 ± 4.2 | 29.5 ± 4.6 | 31.6 ± 1.8 | 31.5 ± 2.2 | 28.7 ± 2.4 | 29.9 ± 3.2 | 29.5 ± 1.6 | |
| n = | 2 | 18 | 17 | 32 | 12 | 77 | 27 | 5 | 190 |
| P. apodus | 28.9 ± 3.4 | 25.8 ± 3.8 | 26.7 ± 3.2 | 30.7 ± 1.3 | NA | NA | 25.2 ± 1.7 | 28.9 ± 1.6 | |
| n = | 4 | 24 | 13 | 6 | 7 | 11 | 65 |
| March | April | May | June | July | August | September | October | Mean E | SD of E | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T. hermanni | Females | ND | 0.94 | 0.44 | 0.8 | 0.5 | 0.45 | 0.57 | 0.77 | 0.64 | 0.20 |
| Males | ND | 0.93 | 0.79 | 0.74 | 0.55 | 0.55 | 0.47 | 0.66 | 0.67 | 0.16 | |
| P. apodus | Pooled | 0.7 | 0.64 | 0.83 | 0.32 | ND | ND | 0.31 | 0.56 | 0.56 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujović, A.; Pešić, V.; Meek, R. Thermal Ecology of Hermann’s Tortoise, Testudo hermanni and Glass Lizard Pseudopus apodus in a Seasonal Environment. Diversity 2025, 17, 638. https://doi.org/10.3390/d17090638
Vujović A, Pešić V, Meek R. Thermal Ecology of Hermann’s Tortoise, Testudo hermanni and Glass Lizard Pseudopus apodus in a Seasonal Environment. Diversity. 2025; 17(9):638. https://doi.org/10.3390/d17090638
Chicago/Turabian StyleVujović, Ana, Vladimir Pešić, and Roger Meek. 2025. "Thermal Ecology of Hermann’s Tortoise, Testudo hermanni and Glass Lizard Pseudopus apodus in a Seasonal Environment" Diversity 17, no. 9: 638. https://doi.org/10.3390/d17090638
APA StyleVujović, A., Pešić, V., & Meek, R. (2025). Thermal Ecology of Hermann’s Tortoise, Testudo hermanni and Glass Lizard Pseudopus apodus in a Seasonal Environment. Diversity, 17(9), 638. https://doi.org/10.3390/d17090638







