1. Introduction
The saiga antelope (
Saiga tatarica) is a unique phenomenon of wildlife, one of the most ancient representatives of ungulate mammals of the Bovidae family. Back in the days of the mammoth and woolly rhinoceros, saigas inhabited almost the entire territory of Eurasia. Today, saigas inhabit Kazakhstan, Uzbekistan (occasionally appearing in Turkmenistan), Russia (Kalmykia and the Astrakhan region), and western Mongolia. It is a migratory herd animal inhabiting semi-desert and dry-steppe ecosystems. The main part of the saigas’ current range and resources is located in Kazakhstan, which includes three populations: Betpakdala, Ural, and Ustyurt. Historically, Kazakhstan is now home to more than 90% of the world’s saiga population [
1].
In 2022, a census found that the saiga population was 800,000, excluding offspring. By early 2023, it had risen to over 1.2 million, surpassing the population levels seen in Kazakhstan during the 1970s. This growth is largely due to the State Program for saiga protection in Kazakhstan, which helped lift the species from endangered status. By 2024, the total saiga population exceeded 3 million, with the Ural population making up over 60% of this total [
2].
Before this population increase, the rapid decline of the Ural saiga antelope population in Kazakhstan was driven by several interconnected factors. Habitat destruction and fragmentation from agricultural expansion and urban development disrupt their ecosystem. Poaching for meat and horns also poses a significant threat. Additionally, disease outbreaks among livestock increase competition for resources and mortality rates. Climate fluctuations affect food and water availability, further stressing these already vulnerable populations [
2,
3]. As a result, because saiga populations have increased by an average of 40% annually since 2015, they are classified as near threatened by the International Union for Conservation of Nature (IUCN) [
4], highlighting the need for conservation efforts.
This study sought to identify the various risk factors that impact the survival of saiga antelopes in the steppes of West Kazakhstan. By illuminating these challenges, the research aims to contribute valuable data that can inform effective conservation strategies and management practices, ultimately fostering the future growth and prosperity of these remarkable creatures in their natural habitat.
2. Materials and Methods
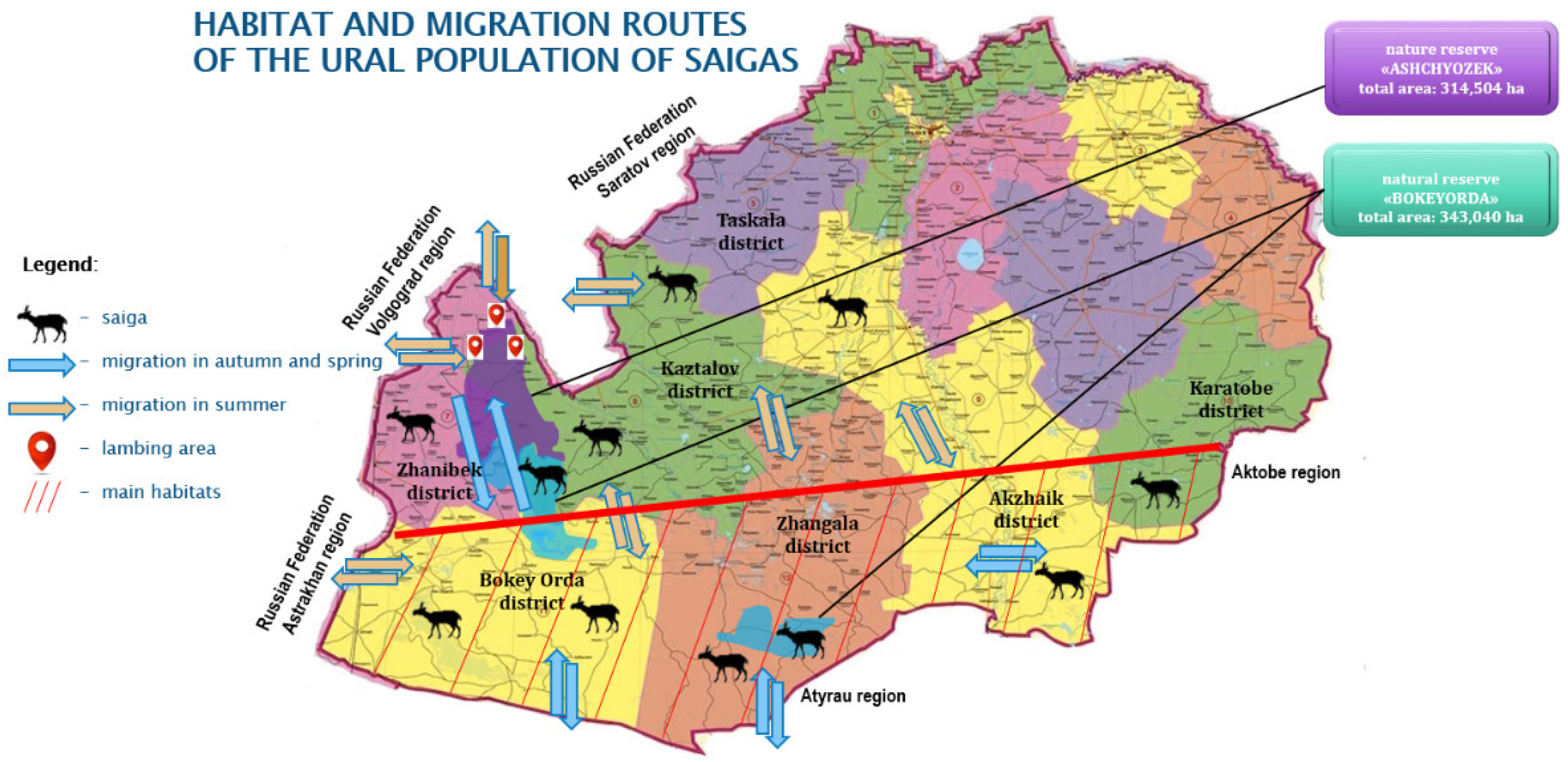
The main part of the research and data collection was conducted in the Kaztalov, Zhangala, Bokeyorda, and Zhanybek districts of West Kazakhstan Oblast, i.e., in the natural habitat and migration range of the Ural saiga population in the period 2011–2024 (
Figure 1).
2.1. Monitoring Records
The locations of saiga herds were obtained from official authorities and local farmers associated with them. A team monitored the herds remotely on a monthly basis when the climate was suitable. Behaviors such as grazing, resting, nursing, and grooming, which could be observed during a healthy period, were monitored. Initial observations of incidents like stray dog attacks, lightning strikes, and damage to artificial barriers caused by herds were mainly collected from local farmers and officials. A team was dispatched to the scene to conduct the required recordings. In 2011, when mass mortality was observed, additional visits and observations were conducted at the scene, along with necessary recordings.
Statistical reviews and data were collected from the Veterinary Department of the West Kazakhstan region regarding the epizootic situation in the saiga habitat. Data were collected by the authors during ecological and epizootological monitoring of the causes of mass mortality of saigas in the Zhanybek district of the West Kazakhstan region from 2010 to 2011, along with long-term monitoring of saiga habitats and migrations in subsequent years. Information was also gathered from the territorial inspectorate of the Committee of Forestry and Wildlife, part of the Ministry of Ecology and Nature Management of the Republic of Kazakhstan. Additionally, photo and video materials were provided by monitoring teams from the Bokeyorda state reserve and Okhotzooprom (State Committee on Hunting), as well as farmers located in the saiga habitat and migration areas.
2.2. Sampling
During field visits to the area experiencing mass mortality of saigas in the Zhanybek district, specifically, at the Borsy and Zhaksybai farms, necropsies were conducted on the deceased saigas, and pathological samples (pieces of internal organs: spleen, heart, liver, lungs) were collected for laboratory analysis. In the period of years 2011–2024, a total of 53 saiga carcasses of various ages, of which 11 were part of the mass mortality observed in 2011, were examined.
2.3. Bacteria Isolation and Identification
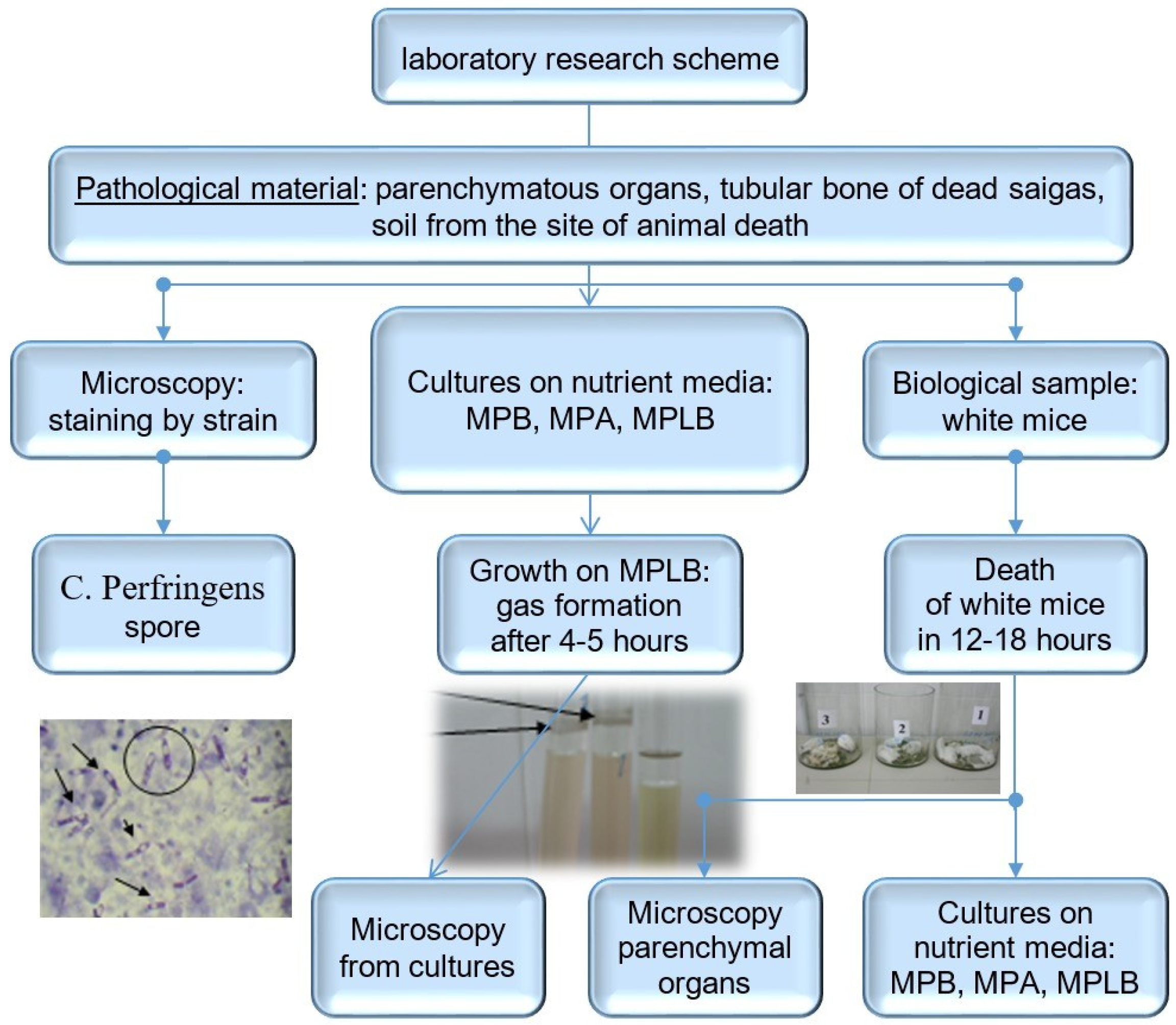
The pathological samples were planted on 5% sheep blood agar, meat–peptone broth, meat–peptone liver broth, Eosine-methylene blue agar, and MacConkey agar in our BSL-2 laboratory. Samples were analyzed using standard microbiological techniques, bacterioscopy, which included preparing swabs from the parenchymatous organs and performing Gram staining. Media were incubated in both aerobic and anaerobic environments at 37 °C and examined daily for five days for bacterial growth. The Petri dishes were checked for reproduction, and the morphology, color, and hemolysis properties of the colonies were examined during the incubation. Gram staining was performed on pure isolated colonies and pure cultures. The isolated bacteria were identified based on their cultural, morphological, and biochemical characteristics [
5]. Bioassays were conducted on white mice by intraperitoneally injecting them with suspensions from the parenchymatous organs, followed by an examination of the internal organs of the deceased laboratory animals. The scheme of laboratory studies is presented in
Figure 2.
2.4. Parasitological Examination
During the postmortem parasitological examination, a longitudinal incision was made to open each antelope’s body cavity. The gastrointestinal tract was then removed by cutting across the esophagus and rectum. Each organ—the esophagus, stomach, small intestine, and large intestine—was individually examined for the presence of endoparasites. The contents of each organ were passed through a sieve with a 100 µm aperture, and the residue was transferred to Petri dishes for examination under a stereo microscope. All helminths obtained from each organ were counted. Nematodes were killed in hot saline solution, fixed in a 70% ethanol solution, mounted in glycerol, cleared, and then examined. Cestodes were fixed in 70% ethanol, stained with hematoxylin, and mounted in balsam for examination. All helminths were identified under a light microscope based on the figures and descriptions provided [
6].
Additionally, all the sampled animals were inspected for external parasites. Parasites that may be found on the skin, inside the ears, and in conception areas were examined. The collected parasites were preserved in 70% alcohol and delivered to the laboratory. Ectoparasites were identified, with the help of the relevant literature, under a stereo microscope at ×10 magnification [
7].
3. Results
Based on the data from the records, it was decided to categorize the risk types associated with saigas into three categories: biotic, abiotic, and anthropogenic. These risks can lead to both population decline and excessive increase. The data obtained according to risk types are brought together and presented in
Table 1.
3.1. Biotic Risks
During the beginning of the study, a mass mortality of saigas was observed in 2011. Microbiological studies of biomaterial from saiga carcasses were carried out in the biotechnology laboratory of the university.
Laboratory examination of pathological material from 11 out of 53 (20.75%) saiga carcasses revealed characteristic Gram-positive bacilli with centrally and terminally located spores in spleen, heart, and liver smears, with the diagnosis of Clostridium perfringens (infectious enterotoxemia) and Pasteurella multocida. All these infected animals (11 individuals) were individuals from the mass mortality observed in 2011. Other sampled corpses (42 individuals) were found dead at different times. When observing the growth of organ cultures on nutrient media, rapid growth on Kitt-Tarozzi elective nutrient medium with strong uniform turbidity of the medium and abundant gas formation was observed after 4–5 h. The suspension from parenchymatous organs was injected intraperitoneally into white mice at a dose of 0.5 mL. Laboratory animals died in 12–18 h. At the subsequent autopsy of the dead laboratory animals and the sowing of suspension from internal organs, pure culture of C. perfringens was isolated on the nutrient medium, meat–peptone liver broth. Microscopy of the organs of fallen laboratory mice in smears of their parenchymatous organs revealed the presence of short, thick, Gram-positive bacilli with rounded ends (C. perfringens) and sporadically located oval, bipolarly stained P. multocida in greater numbers.
In studying the biological properties of isolated cultures of the pathogen from various organs, tests were conducted to identify the type of toxin and to confirm the identity of the pathogen, Clostridium perfringens. During the characterization of the toxin from the pathogen isolated from saiga parenchymatous organs, type D toxin was identified.
To further confirm the presence of
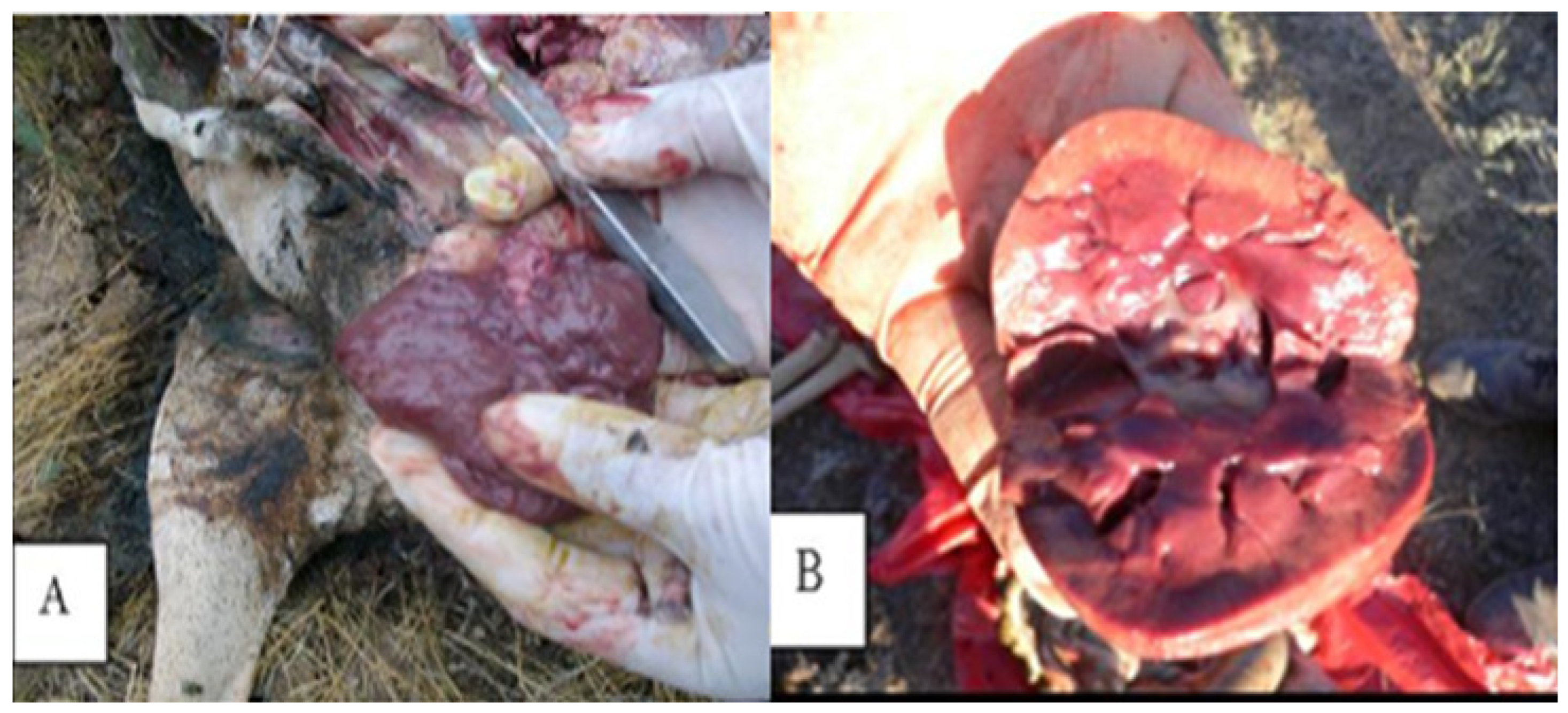
C. perfringens, cultures isolated from animal organs were inoculated into milk. The milk rapidly coagulated after inoculation. This coagulation was due to the acids produced, which caused the milk to curdle and release gases. These gases disrupted the curd, forming a spongy plug that lifted the bulk of the curd to the top of the tube. As a result, a large quantity of serum was expelled, which quickly became nearly transparent with a slight bluish tint. Pathomorphologic studies have revealed definitive changes in the kidneys, specifically identified as “kidney softening” (
Figure 3).
The parasitological examination focused on helminth infections within the internal organs and tissues of the animals. Out of 53 animals examined, 38 were found to have helminth infections, representing a prevalence of 71.69%. All detected helminths were sourced from the gastrointestinal system.
The findings included two species of cestodes, Moniezia expansa (17.5%) and Echinococcus larvae (11.0%), as well as five species of nematodes: Ostertagia ostertagi (58.0%), Trichostrongylus colubriformis (52.8%), Haemonchus contortus (52.0%), Marshallagia marshalli (39.0%), and Trichuris skrjabini (17.0%).
Regarding external parasites, ticks were the only type observed, with Dermacentor reticulatus identified in 12 saigas, accounting for 22.64% of the sampled animals.
3.2. Abiotic Risks
On 13 May 2021, a significant mortality event occurred in the Zhanybek district of the West Kazakhstan region, resulting from a lightning strike among the Ural population of saigas. A total of 372 saiga carcasses were found in a lowland area, along a canal, and scattered across the steppe near the Tau rural district of Zhanybek. On that day, the region experienced heavy rain, thunderstorms, and lightning. During the inspection, the deceased animals were observed lying in a single row within the lowland. Due to the heavy downpour, water had accumulated in the area, and the subsequent thunderstorms and lightning led to the animals’ deaths.
In 2021, there was a noteworthy incident where saigas died due to a lightning strike, highlighting the impact of abiotic factors like atmospheric phenomena. This was not an isolated case; similar events have been recorded in previous years and different regions. During field observations, it was noted that 62 additional saigas had perished after stormy rains caused by lightning strikes in Bokeyorda. An examination of a total of 434 saiga carcasses revealed signs of lightning damage, such as burns and puncture wounds. These incidents were documented at surveillance sites, and it is possible that similar losses have occurred in other years or regions.
3.3. Anthropogenic Risks
In addition to biotic and abiotic factors, the ecosystem of the Ural saiga population is also influenced by human activities. These anthropogenic factors include poaching, an increase in the number of farm animals on farms and smallholdings, and the creation of artificial barriers such as trenches and deep ditches in the saiga habitats and migration areas. It has been reported that farmers sometimes use hunting to scare antelopes away from their domesticated herds or shelters to avoid transmitting diseases and damage to fences and farm equipment.
Field observations indicate that saiga herds often come into close proximity to human settlements and domestic animals. This suggests that they may be exposed to pathogens from humans or livestock. The excessive growth of the saiga population has led to their increased contact with agricultural and non-productive animals owned by farmers and smallholders, particularly in pastures and at watering sources. Saigas interact with domestic animals year-round. As their numbers rise, saigas are approaching human settlements within their habitat and migration areas, increasing the risk of transmitting infectious and parasitic diseases.
To safeguard their rangelands and crops, farmers attempt to create barriers using metal nets and wires. However, these fences are often unable to withstand the impact of large groups of saigas moving at high speeds, leading to their collapse.
Another anthropogenic factor affecting the saiga population in recent years has been the increasing population of stray dogs; the restriction of their numbers has not found a legislative solution in Kazakhstan as a whole, including West Kazakhstan Oblast. There have already been cases of stray dogs attacking saigas in saiga habitat and migration regions. Wounds on the bodies of saigas were noted in 12 animals as a result of attacks by stray dogs in different regions.
4. Discussion
Different studies have been conducted to detect saigas’ infectious diseases and other dangers to which they are exposed [
1,
8,
9]. Biotic risks in the saiga population stem from disease outbreaks caused by viruses, bacteria, parasites, and other biological threats, which can lead to significant mortality. For example, in 2010 and 2011, mass die-offs of Ural saigas in West Kazakhstan saw 12,920 and 441 saigas perish, leading to a population decline to 17,700. The most severe decline occurred in 2015, when over 150,000 individuals from the Betpakdala population died [
10]. In that year, the habitat and migration zones of the saiga antelope had not changed significantly compared to previous years regarding environmental and climatic conditions. The spring of 2011, which coincided with the antelope’s calving season, was the usual wet due to periodic rainfall, similar to previous years [
10].
In both instances of mass mortality among the Ural and Betpakdala saiga populations, the official diagnosis was pasteurellosis [
11]. However, it is important to note that this diagnosis may not be entirely accurate. Pasteurellosis is common among various species of both farm and wild animals, which suggests that it should be classified as an opportunistic infection originating from the soil [
12]. This classification is characterized by a significant presence of opportunistic pathogens or the interaction of multiple pathogens. In our study to determine the causes of mass mortality among saigas in the Zhanybek district of the West Kazakhstan region in 2011, we found that the cause of death was an association of pasteurellosis and infectious enterotoxemia. This diagnosis was also confirmed by other studies of colleagues [
13,
14].
While
Pasteurella multocida is the most extensively documented bacterial threat, saigas are susceptible to other infectious diseases that could pose significant risks, particularly given the increasing interface with domestic livestock [
15]. Saigas are known to be susceptible to diseases such as foot-and-mouth disease and brucellosis [
4].
Studies in Kazakhstan consistently indicate that saigas are extensively infected with helminths [
16]. A key study from 1997, which examined 133 culled saigas, identified three species of cestode and twelve species of nematodes, with nine of the nematodes found in the abomasum. Notably, neither trematodes nor lungworms were detected in this particular study [
17] and also in our study. The most abundant species identified were
Marshallagia marshalli,
M. mongolica, and
Nematodirus gazellae (found in both the abomasum and small intestine), along with
Skrjabinema ovis (found in the large intestine) [
17]. More studies have also noted the presence of other helminths, such as
Moniezia expansa,
Trichocephalus skrjabini, and
Strongyloides papillosus. Cestodes like echinococcosis, coenurosis, and monieziosis have also been identified in the region [
18], which is consistent with our findings.
The shared grazing lands between saigas and domestic livestock create a significant interface for parasitic disease transmission, with important implications for both wildlife conservation and agricultural health [
16]. Saiga antelopes graze extensively on livestock pasture, which facilitates the transmission of a wide range of parasitic helminths between saigas and domestic ruminants. A significant overlap exists in parasite communities: 36 of the 38 helminth species previously found in saigas in Kazakhstan have also been identified in domestic livestock. More recent surveys confirm that all identified parasites in saigas and kulans are also found in domesticated livestock [
18].
While the current data do not suggest ectoparasites are a major direct cause of mortality, their potential role as vectors for other pathogens (e.g., tick-borne diseases, which are common in ungulates) or as chronic stressors contributing to overall host vulnerability, especially in the context of a changing climate, remains largely unexplored within the provided context. Tick-borne infections and tick paralysis should be considered as the causative factors of mass diseases and the death of saigas, especially in the spring. The tick species
Dermacentor reticulatus is first reported from Ural saigas in this study, while
Hyalomma scupense was reported previously [
19].
Abiotic risks can also affect other herd animals, such as horses and livestock [
20]. For example, during a severe thunderstorm on June 19, 2020, in the Karaganda region, 69 horses were killed by a lightning strike [
21]. This incident illustrates that, following a lightning storm, dozens or even hundreds of animals can perish due to lightning strikes. In our study, we identified 372 deaths occurring simultaneously, with a total of 434 antelopes reported to have died as a result of these strikes. Although there are no preventive measures against such naturally occurring deaths, the significant decline in animal populations can be mitigated by reducing other risk factors.
Although antelope hunting was once banned, the Kazakhstan Government has now imposed a moratorium on saiga hunting. However, due to the sharp increase in the number of saigas and the escalation of conflicts with farmers and the population of saiga habitats and migration zones, which have led to competition for food and water resources, the Ministry of Ecology and Natural Resources of Kazakhstan has issued an order allowing specialized teams to harvest up to 20% of the Ural saiga population annually under the supervision of Okhotzooprom. Due to the vastness of the area and the inability to monitor it fully, there is a risk of over-poaching during these times, which can lead to the endangerment of the species. Nevertheless, the excessive population and the conflict with farmers are more significant issues than poaching, at this stage.
According to the law released in 2021 in Kazakhstan, stray animals were to be caught, sterilized, and released back into their environment [
22]. However, the implementation of this policy has often diverged significantly from its intended norms, particularly in the initial years after the law’s adoption. Overpopulation of stray dogs in urban or rural areas is still a threat to wildlife, including saigas.
The losses and risks outlined in the previous sections were identified only in the specific time frame and areas where the study was conducted. There may be deaths or adverse situations that were not mentioned. However, considering the risks identified in our study could help protect populations of saigas and similar wild herd animals, as well as inform future planning efforts.