Unraveling Elevation-Driven Variations in Forest Structure and Composition in Western Nepal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design
2.3. Data Analysis
2.3.1. Structure of Forest Stands
2.3.2. Species Composition
Relative Frequency (RF)
Relative Basal Area (RBA) or Relative Dominance (RDo)
Abundance and Relative Abundance
Important Value Index (IVI)
2.3.3. Diversity of Tree Species
2.3.4. Statistical Analysis
3. Results
3.1. Structure of Forest Stands
3.1.1. Species Composition
3.1.2. Basal Area/Density
3.1.3. Distribution of Diameter Class
3.1.4. Mean Height
3.2. Importance Value Index (IVI)
3.3. Diversity of Tree Species
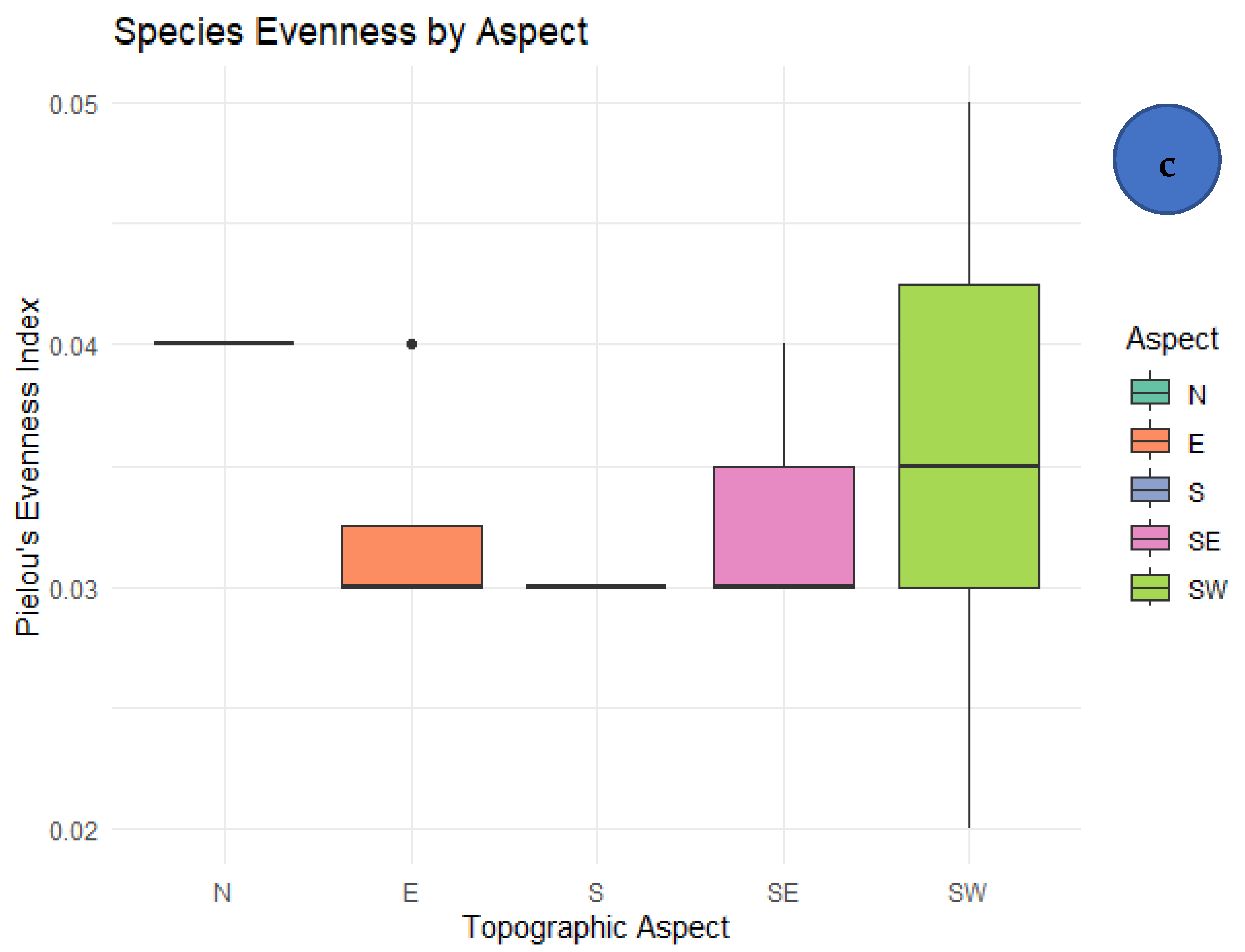
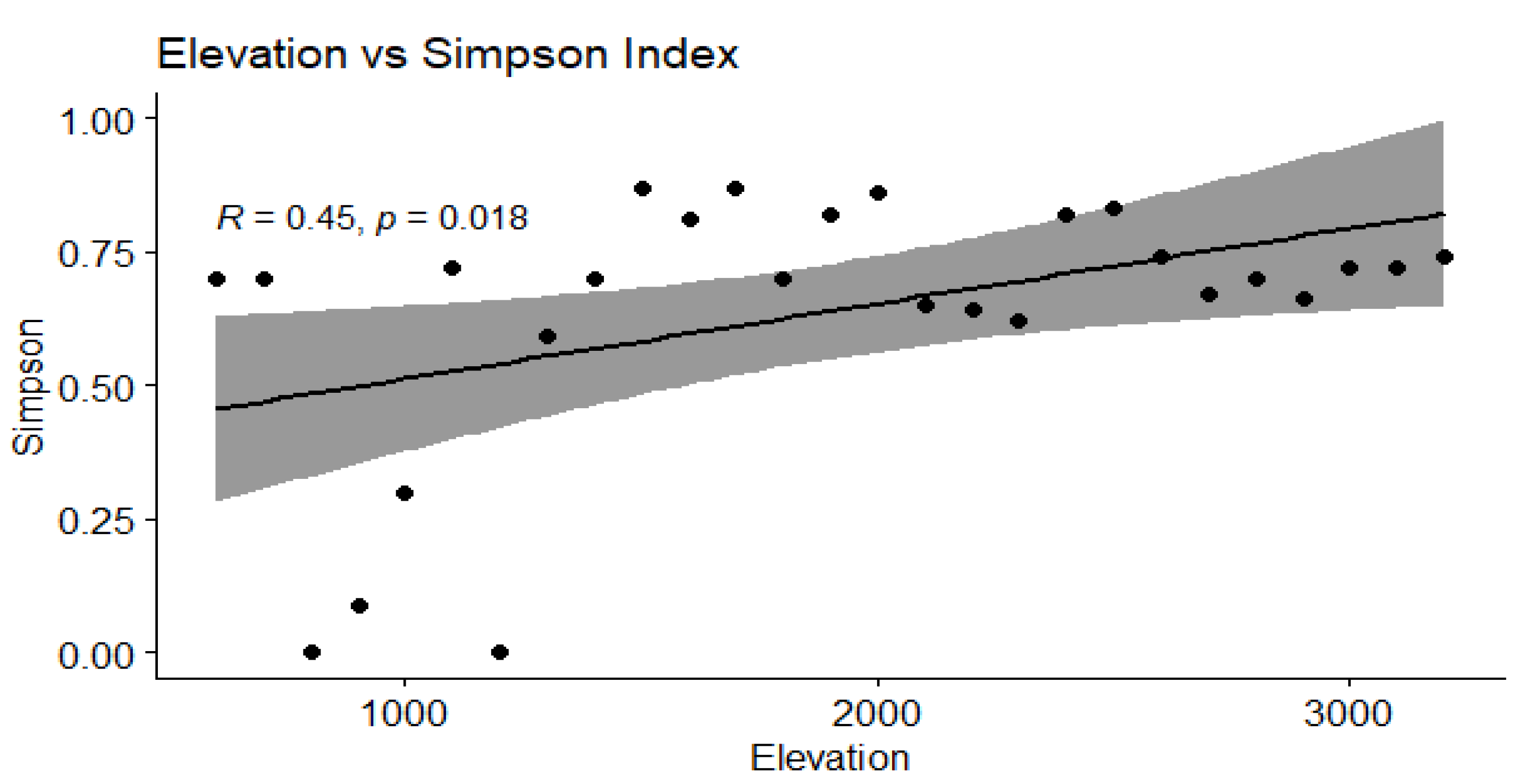
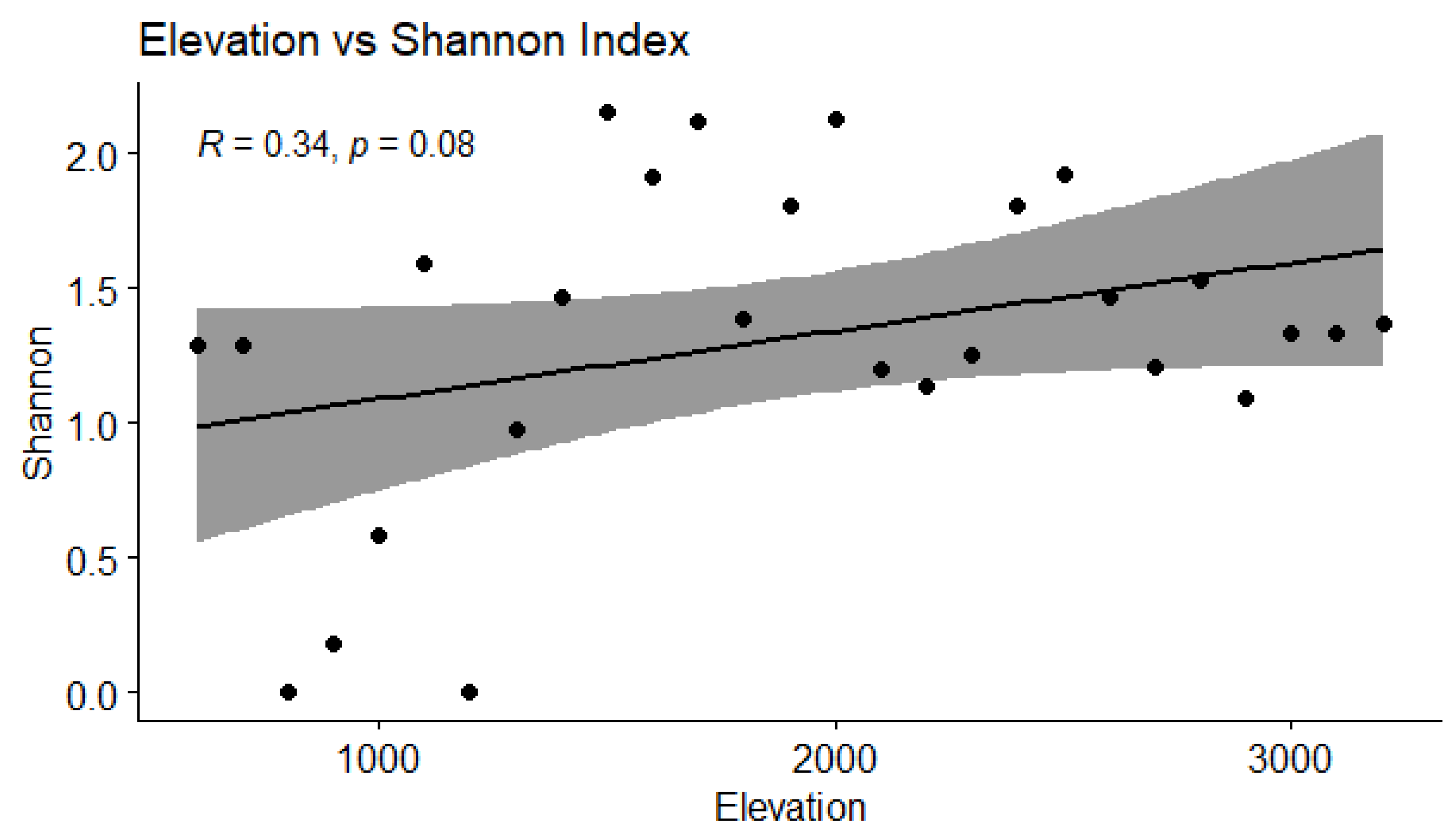
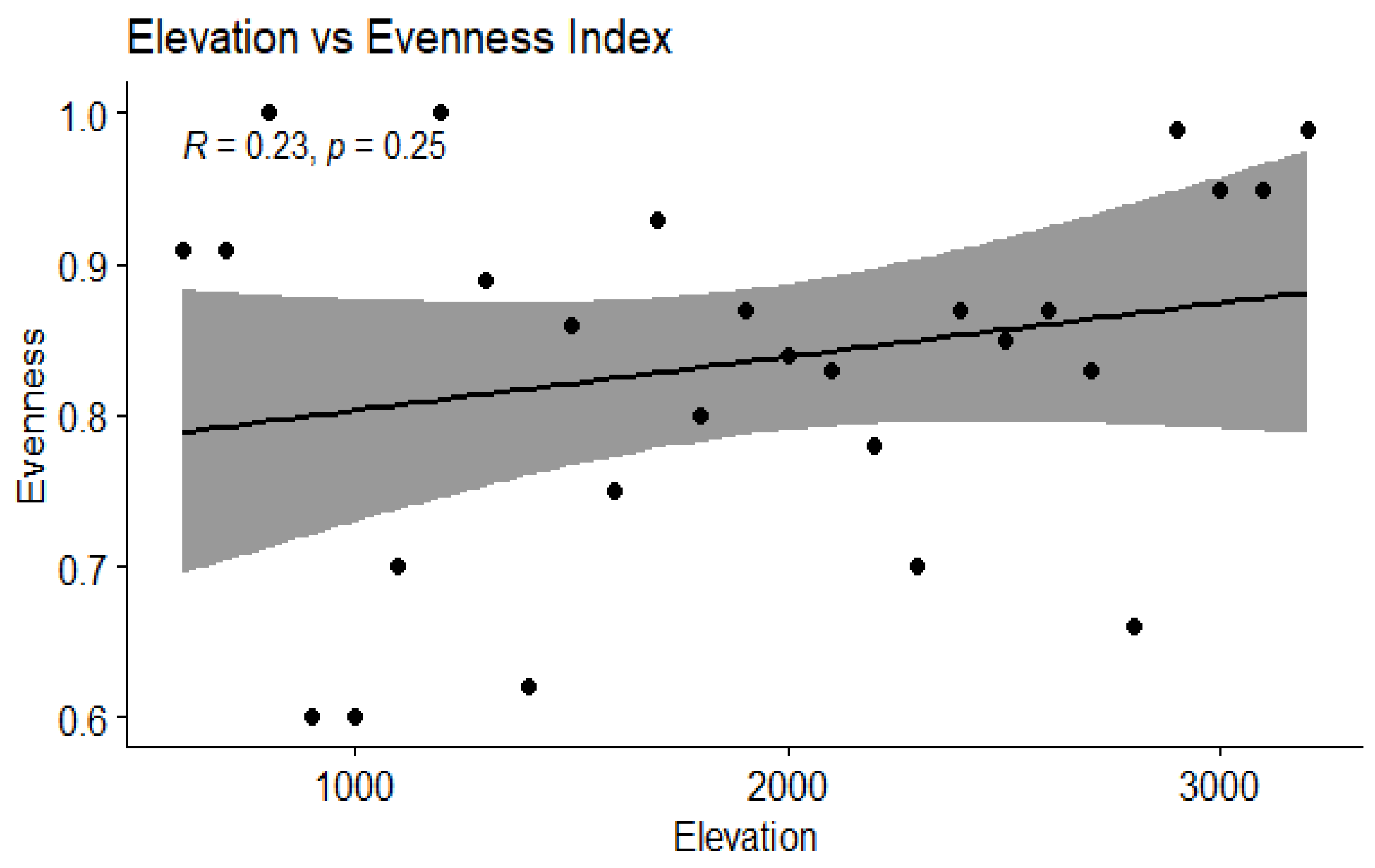
3.4. Correlation Between Elevation and Diversity Indices
4. Discussion
4.1. Forest Composition
4.2. Forest Structure
4.3. Influence of Topographic Aspect and Anthropogenic Factors
4.4. Linking Forest Structure to Carbon and Biodiversity Targets
- High-elevation zones (2500–3200 m): Prioritize carbon sequestration due to large-tree dominance and slow regeneration. Steep northern slopes here may need protection from landslides to preserve soil carbon.
- Mid-elevations (1000–2500 m): Balance carbon and biodiversity, leveraging mixed-species resilience [57]. Moderate slopes (15–30°) in this zone, particularly southwestern aspects, support high basal areas (e.g., 2400 m) and should be prioritized for REDD+ projects.
- Low elevations (<1000 m): Focus on community-based biodiversity conservation, as these areas face higher anthropogenic pressure [19]. Gentle slopes here are prone to agricultural encroachment; agroforestry on these terrains could enhance habitat connectivity.
- Incorporate Elevational Zonation into Forest Management: Management plans should reflect elevational differences in species composition and forest structure to ensure that conservation and utilization strategies are ecologically appropriate.
- Conduct Aspect-wise and Microclimatic Studies: As slope aspect and local climatic conditions influence forest dynamics, further research is essential to understand how these variables interact with elevation to shape biodiversity and carbon stocks.
- Monitor and Mitigate Anthropogenic Pressures: Activities such as grazing, logging, and land-use change should be regulated, especially in sensitive zones, through strengthened community forest management and stricter enforcement of forest policies.
- Implement Climate-Responsive Conservation Strategies: Given the potential for altitudinal shifts in species distributions due to climate change, long-term monitoring programs are necessary to track compositional changes and inform adaptive management.
- Enhance Local Capacity and Awareness: Capacity-building initiatives for local communities and forest managers are vital to improve awareness of elevation-specific ecological dynamics and promote sustainable forest stewardship.
4.5. Policy Implications, Limitations, and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Appendix A
| S.N. | Scientific Name | Family | Relative Density (%) | Relative Frequency (%) | Relative Basal Area (%) | IVI | Relative Abundance (%) |
|---|---|---|---|---|---|---|---|
| 1 | Shorea robusta | Dipterocarpaceae | 27.18 | 6.16 | 11.33 | 44.67 | 13.81 |
| 2 | Alnus nepalensis | Betulaceae | 8.53 | 9.59 | 12.98 | 31.10 | 2.79 |
| 3 | Persea odoratissima | Lauraceae | 2.38 | 2.74 | 2.39 | 7.51 | 2.72 |
| 4 | Ligustrum robustum | Oleaceae | 3.77 | 4.11 | 2.30 | 10.18 | 2.87 |
| 5 | Pyrus pashia | Rosaceae | 1.39 | 2.74 | 0.30 | 4.43 | 1.59 |
| 6 | Ficus neriifolia | Moraceae | 0.20 | 0.68 | 0.06 | 0.94 | 0.91 |
| 7 | Madhuca longifolia | Sapotaceae | 3.17 | 6.16 | 1.62 | 10.96 | 1.61 |
| 8 | Schima wallichii | Theaceae | 1.98 | 2.05 | 0.74 | 4.77 | 3.02 |
| 9 | Pinus roxburghii | Pinaceae | 3.17 | 2.74 | 1.66 | 7.58 | 3.63 |
| 10 | Quercus leucotrichophora | Fagaceae | 1.19 | 2.74 | 0.90 | 4.83 | 1.36 |
| 11 | Ficus semicordata | Moraceae | 0.60 | 2.05 | 0.20 | 2.85 | 0.91 |
| 12 | Myrica esculenta | Myricaceae | 1.39 | 3.42 | 0.69 | 5.50 | 1.27 |
| 13 | Albizia lebbeck | Fabaceae (Mimosoideae) | 0.40 | 0.68 | 0.20 | 1.28 | 1.81 |
| 14 | Senegalia catechu | Fabaceae (Mimosoideae) | 1.79 | 1.37 | 0.32 | 3.48 | 4.08 |
| 15 | Diospyros malabarica | Ebenaceae | 1.98 | 2.74 | 0.27 | 4.99 | 2.27 |
| 16 | Prunus cerasoides | Rosaceae | 0.20 | 0.68 | 0.04 | 0.93 | 0.91 |
| 17 | Rhododendron arboreum | Ericaceae | 8.13 | 7.53 | 7.00 | 22.67 | 3.38 |
| 18 | Pogostemon benghalensis | Lamiaceae | 0.40 | 0.68 | 0.08 | 1.16 | 1.81 |
| 19 | Bauhinia variegata | Fabaceae (Caesalpinioideae) | 0.20 | 0.68 | 0.02 | 0.90 | 0.91 |
| 20 | Pinus wallichiana | Pinaceae | 3.37 | 4.11 | 12.38 | 19.86 | 2.57 |
| 21 | Quercus semecarpifolia | Fagaceae | 7.34 | 5.48 | 26.80 | 39.62 | 4.20 |
| 22 | Juglans regia | Juglandaceae | 1.19 | 1.37 | 1.30 | 3.86 | 2.72 |
| 23 | Garuga pinnata | Burseraceae | 0.60 | 0.68 | 0.54 | 1.82 | 2.72 |
| 24 | Toona ciliata (Cedrela toona) | Meliaceae | 0.79 | 1.37 | 1.82 | 3.99 | 1.81 |
| 25 | Dalbergia sissoo | Fabaceae (Faboideae) | 2.58 | 1.37 | 0.45 | 4.40 | 5.90 |
| 26 | Albizia procera | Fabaceae (Mimosoideae) | 1.19 | 1.37 | 0.31 | 2.87 | 2.72 |
| 27 | Premna longifolia | Lamiaceae | 0.20 | 0.68 | 0.27 | 1.16 | 0.91 |
| 28 | Daphniphyllum himalaense | Daphniphyllaceae | 1.19 | 0.68 | 0.14 | 2.01 | 5.44 |
| 29 | Falconeria insignis | Euphorbiaceae | 0.60 | 1.37 | 0.25 | 2.21 | 1.36 |
| 30 | Castanopsis tribuloides | Fagaceae | 1.59 | 2.05 | 1.00 | 4.64 | 2.42 |
| 31 | Lyonia ovalifolia | Ericaceae | 2.38 | 6.16 | 0.60 | 9.14 | 1.21 |
| 32 | Quercus acutissima | Fagaceae | 1.98 | 0.68 | 0.59 | 3.26 | 9.07 |
| 33 | Tsuga dumosa | Pinaceae | 1.79 | 6.16 | 4.94 | 12.89 | 0.91 |
| 34 | Michelia champaca | Magnoliaceae | 1.19 | 2.74 | 0.44 | 4.37 | 1.36 |
| 35 | Rhododendron campanulatum | Ericaceae | 3.97 | 4.11 | 5.07 | 13.15 | 3.02 |
| Total | 100.00 | 100.00 | 100.00 | 300.0 | 100.00 | ||
| Index | Pearson (r) | p-Value | Spearman (ρ) | p-Value | Interpretation |
|---|---|---|---|---|---|
| Simpson | 0.45 | 0.018 | 0.43 | <0.05 | Significant positive correlation |
| Shannon | 0.34 | 0.08 | 0.32 | NS | Moderate, not statistically significant |
| Evenness | 0.23 | 0.25 | 0.21 | NS | Weak, not significant |
| Elevational Zone | Elevation Range (m) | Mean Annual Temperature (°C) | Mean Annual Precipitation (mm) | Dominant Soil Type |
|---|---|---|---|---|
| Tropical | <1000 | 20–25 | 1200–1500 | Sandy loam to silty loam |
| Subtropical | 1000–2000 | 15–20 | 1500–2000 | Loam to clay loam |
| Lower Temperate | 2000–2500 | 10–15 | 1500–2500 | Silty clay, high organic matter |
| Upper Temperate | 2500–3200 | 5–10 | 2000–3000 | Shallow loam, rocky substrate |
References
- Gutiérrez, A.G.; Huth, A. Successional stages of primary temperate rainforests of Chiloé Island, Chile. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 243–256. [Google Scholar] [CrossRef]
- Waheed, M.; Haq, S.M.; Arshad, F.; Bussmann, R.W.; Hashem, A.; Abd_Allah, E.F. Plant distribution, ecological traits and diversity patterns of vegetation in subtropical managed forests as guidelines for forest management policy. Front. For. Glob. Chang. 2024, 7, 1406075. [Google Scholar] [CrossRef]
- Rahman, I.U.; Hart, R.E.; Ijaz, F.; Afzal, A.; Iqbal, Z.; Calixto, E.S.; Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Al-Arjani, A.-B.F.; et al. Environmental variables drive plant species composition and distribution in the moist temperate forests of Northwestern Himalaya, Pakistan. PLoS ONE 2022, 17, e0260687. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, N.; Bhojak, P.; Sekar, K.C.; Bisht, K.; Mehta, P.; Arya, D.; Joshi, S. Potential drivers of plant diversity and composition in high-altitude alpine regions of Himalaya. Community Ecol. 2025, 26, 85–103. [Google Scholar] [CrossRef]
- Sharma, C.M.; Mishra, A.K.; Krishan, R.; Tiwari, O.P.; Rana, Y.S. Impact of climate on structure and composition of Ridge Top forests in Garhwal Himalaya. Taiwania 2016, 61, 61–69. [Google Scholar]
- Kattel, G.R. Climate warming in the Himalayas threatens biodiversity, ecosystem functioning and ecosystem services in the 21st century: Is there a better solution? Biodivers. Conserv. 2022, 31, 2017–2044. [Google Scholar] [CrossRef]
- Adhikari, Y.P.; Fischer, A.; Pauleit, S. Sustainable conservation perspectives for epiphytic orchids in the central Himalayas, Nepal. Appl. Ecol. Environ. Res. 2015, 13, 753–767. [Google Scholar] [CrossRef]
- Paudel, P.K.; Bhattarai, B.P.; Kindlmann, P. An overview of the biodiversity in Nepal. Himal. Biodivers. Chang. World 2011, 1, 1–40. [Google Scholar]
- Paudel, P.K.; Jha, P.K. Nepal’s unique physiographic and climatic diversity, compressed within a narrow latitudinal span, offers a microcosm of global biodiversity patterns. J. Biodivers. Conserv. 2011, 20, 123–135. [Google Scholar]
- DFRS. Forest Resource Assessment Nepal; Department of Forest Research and Survey: Kathmandu, Nepal, 2016. [Google Scholar]
- Bhattarai, P.C.; Acharya, S.P. Sustainable Management of Non-wood Forest Products for Rural Livelihoods in Nepal. In Nonwood and Livelihood: Sustainable Management of Non-Wood Forest Products for Rural Livelihoods in South Asia; SAARC Agriculture Centre: Dhaka, Bangladesh, 2021; 166p. [Google Scholar]
- Kreft, H.; Jetz, W.; Mutke, J.; Barthlott, W. Contrasting environmental and regional effects on global pteridophyte and seed plant diversity. Ecography 2010, 33, 408–419. [Google Scholar] [CrossRef]
- Dani, R.S.; Divakar, P.K.; Baniya, C.B. Diversity and composition of plants species along elevational gradient: Research trends. Biodivers. Conserv. 2023, 32, 2961–2980. [Google Scholar] [CrossRef]
- Vetaas, O.R.; Paudel, K.P.; Christensen, M. Principal factors controlling biodiversity along an elevation gradient: Water, energy and their interaction. J. Biogeogr. 2019, 46, 1652–1663. [Google Scholar] [CrossRef]
- Asefa, M.; Cao, M.; He, Y.; Mekonnen, E.; Song, X.; Yang, J. Ethiopian vegetation types, climate and topography. Plant Divers. 2020, 42, 302–311. [Google Scholar] [CrossRef]
- Shaheen, H.; Ullah, Z.; Khan, S.M.; Harper, D.M. Species composition and community structure of western Himalayan moist temperate forests in Kashmir. For. Ecol. Manag. 2012, 278, 138–145. [Google Scholar] [CrossRef]
- Kumar, R.; Bhardwaj, A.K.; Chandra, K.K. Levels of Natural and Anthropogenic Disturbances and Assessment of Their Impact on Plant Community Functional Diversity. Forestist 2023, 73, 1–12. [Google Scholar] [CrossRef]
- Joshi, R.; Chhetri, R.; Yadav, K. Vegetation Analysis in Community Forests of Terai Region, Nepal. Int. J. Environ. 2019, 8, 68–82. [Google Scholar] [CrossRef]
- Bhandari, S.K.; Maraseni, T.; Timilsina, Y.P.; Parajuli, R. Species composition, diversity, and carbon stock in trees outside forests in middle hills of Nepal. For. Policy Econ. 2021, 125, 102402. [Google Scholar] [CrossRef]
- Khan, M.L.; Bawa, K.S. Effectiveness of protected area network in biodiversity conservation in north-east India: A case study of Meghalaya state. J. Ecol. 2014, 5, 1–12. [Google Scholar]
- Ali, A. Forest stand structure and functioning: Current knowledge and future challenges. Ecol. Indic. 2019, 98, 665–677. [Google Scholar] [CrossRef]
- Bisht, M.; Sekar, K.C.; Mukherjee, S.; Thapliyal, N.; Bahukhandi, A.; Singh, D.; Bhojak, P.; Mehta, P.; Upadhyay, S.; Dey, D. Influence of anthropogenic pressure on the plant species richness and diversity along the elevation gradients of Indian Himalayan high-altitude protected areas. Front. Ecol. Evol. 2022, 10, 751989. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.M.; Ilyas, M.; Alqarawi, A.A.; Ahmad, Z.; Abd_Allah, E.F. Plant species and communities assessment in interaction with edaphic and topographic factors; an ecological study of the mount Eelum District Swat, Pakistan. Saudi J. Biol. Sci. 2017, 24, 778–786. [Google Scholar] [CrossRef]
- Zhang, J.T.; Xu, B.; Li, M. Vegetation patterns and species diversity along elevational and disturbance gradients in the Baihua Mountain Reserve, Beijing, China. Mt. Res. Dev. 2013, 33, 170–179. [Google Scholar] [CrossRef]
- Sharma, C.M.; Mishra, A.K.; Prakash, O.; Dimri, S.; Baluni, P. Assessment of forest structure and woody plant regeneration on ridge tops at upper Bhagirathi basin in Garhwal Himalaya. Trop. Plant Res. 2014, 1, 62–71. [Google Scholar]
- Tomar, P.K.; Thakur, S.; Pandey, R. Altitudinal variation in soil properties with reference to forest structure and composition in the Western Himalaya. Sci. Total Environ. 2024, 866, 161281. [Google Scholar]
- Rumpf, S.B.; Hülber, K.; Klonner, G.; Moser, D.; Schütz, M.; Wessely, J.; Willner, W.; Zimmermann, N.E.; Dullinger, S. Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. USA 2018, 115, 1848–1853. [Google Scholar] [CrossRef]
- Coverdale, T.C.; Davies, A.B. Unravelling the relationship between plant diversity and vegetation structural complexity: A review and theoretical framework. J. Ecol. 2023, 111, 1378–1395. [Google Scholar] [CrossRef]
- Costa, F.V.D.; Viana-Júnior, A.B.; Aguilar, R.; Silveira, F.A.; Cornelissen, T.G. Biodiversity and elevation gradients: Insights on sampling biases across worldwide mountains. J. Biogeogr. 2023, 50, 1879–1889. [Google Scholar] [CrossRef]
- Dolezal, J.; Dvorsky, M.; Kopecky, M.; Liancourt, P.; Hiiesalu, I.; Macek, M.; Altman, J.; Chlumska, Z.; Rehakova, K.; Capkova, K.; et al. Vegetation dynamics at the upper elevational limit of vascular plants in Himalaya. Sci. Rep. 2016, 6, 24881. [Google Scholar] [CrossRef]
- Wambulwa, M.C.; Milne, R.; Wu, Z.Y.; Spicer, R.A.; Provan, J.; Luo, Y.H.; Zhu, G.; Wang, W.; Wang, H.; Gao, L.; et al. Spatiotemporal maintenance of flora in the Himalaya biodiversity hotspot: Current knowledge and future perspectives. Ecol. Evol. 2021, 11, 10794–10812. [Google Scholar] [CrossRef]
- Li, T.; Liang, J.; Chen, X.; Wang, H.; Zhang, S.; Pu, Y.; Xu, X.; Li, H.; Xu, J.; Wu, X.; et al. The interacting roles and relative importance of climate, topography, soil properties and mineralogical composition on soil potassium variations at a national scale in China. Catena 2021, 196, 104875. [Google Scholar] [CrossRef]
- Bisht, N.S.; Rawat, G.S.; Nautiyal, D.C. Influence of altitude and aspect on forest vegetation in the Indian Himalayas. Ecol. Indic. 2022, 139, 108891. [Google Scholar]
- Padalia, H.; Rai, I.D.; Pangtey, D.; Rana, K.; Khuroo, A.A.; Nandy, S.; Singh, G.; Sekar, K.C.; Sharma, N.; Uniyal, S.K.; et al. Fine-scale classification and mapping of subalpine-alpine vegetation and their environmental correlates in the Himalayan global biodiversity hotspot. Biodivers. Conserv. 2023, 32, 4387–4423. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.A. Elevational gradients in species richness. Encycl. Life Sci. 2010, 15, 1–10. [Google Scholar]
- Sharma, N.; Behera, M.D.; Das, A.P.; Panda, R.M. Plant richness pattern in an elevation gradient in the Eastern Himalaya. Biodivers. Conserv. 2019, 28, 2085–2104. [Google Scholar] [CrossRef]
- Gillman, L.N.; Wright, S.D. Species richness and evolutionary speed: The influence of temperature, water and area. J. Biogeogr. 2014, 41, 39–51. [Google Scholar] [CrossRef]
- Bilal, M. Biological Impacts of Climate Change on Ecosystem Functionality: An Ecological Perspective. Biol. Biotechnol. Commun. 2024, 1, 104–113. [Google Scholar]
- Sharma, C.M.; Mishra, A.K.; Tiwari, O.P.; Krishan, R.; Rana, Y.S. Effect of elevational gradients on forest structure and composition on ridge tops in Garhwal Himalaya. Energy Ecol. Environ. 2017, 2, 404–417. [Google Scholar] [CrossRef]
- Pandey, S.S.; Cockfield, G.; Maraseni, T.N. Dynamics of carbon and biodiversity under REDD+ regime: A case from Nepal. Environ. Sci. Policy 2014, 38, 272–281. [Google Scholar] [CrossRef]
- Joshi, R.; Pangeni, M.; Neupane, S.S.; Yadav, N.P. Regeneration Status and Carbon Accumulation Potential in Community Managed Sal (Shorea robusta) Forests of Far-Western Terai Region, Nepal. Eur. J. Ecol. 2021, 7, 26–39. [Google Scholar] [CrossRef]
- Joshi, R.; Shrestha, T.K.; Mishra, B.; Gautam, J.; Maharjan, B.; Gosai, K.R.; Maraseni, T.; Neupane, B. Assessment of Carbon Sequestration in Private Forests Across Two Different Physiographic Regions of Nepal: Implications for Conservation and Climate Change Mitigation. Scientifica 2023, 2023, 6599067. [Google Scholar] [CrossRef]
- Pandit, M.K.; Sodhi, N.S.; Koh, L.P.; Bhaskar, A.; Brook, B.W. Elevational gradients and the imperilment of biodiversity. Biol. Conserv. 2021, 261, 109266. [Google Scholar]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, Ö.; Goldberg, I.; Nascimbene, J.; Tøttrup, A.P.; Rahbek, C.; et al. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Löf, M.; Madsen, P.; Metslaid, M.; Witzell, J.; Jacobs, D.F. Restoring forests: Regeneration and ecosystem function for the future. New For. 2019, 50, 139–151. [Google Scholar] [CrossRef]
- Piponiot, C.; Anderson-Teixeira, K.J.; Davies, S.J.; Allen, D.; Bourg, N.A.; Burslem, D.F.; Cárdenas, D.; Chang-Yang, C.; Chuyong, G.; Cordell, S.; et al. Distribution of biomass dynamics in relation to tree size in forests across the world. New Phytol. 2022, 234, 1664–1677. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Singh, H.; Chettri, R.; Poudel, S.; Rijal, S. Carbon Sequestration Potential of Community Forests: A Comparative Analysis of Soil Organic Carbon Stock in Community Managed Forests of Far-Western Nepal. Eurasian J. Soil Sci. 2021, 10, 96–104. [Google Scholar] [CrossRef]
- Maharjan, S.K.; Koirala, M.; Sharma, L.N. Altitudinal variation in plant community composition and structure along different aspects in the central Himalayas, Nepal. Plant Ecol. 2022, 223, 997–1011. [Google Scholar]
- Rubenstein, M.A.; Weiskopf, S.R.; Bertrand, R.; Carter, S.L.; Comte, L.; Eaton, M.J.; Johnson, C.G.; Lenoir, J.; Lynch, A.J.; Miller, B.W.; et al. Climate change and the global redistribution of biodiversity: Substantial variation in empirical support for expected range shifts. Environ. Evid. 2023, 12, 7. [Google Scholar] [CrossRef]
- Aryal, S.; Shrestha, S.; Maraseni, T.; Wagle, P.C.; Gaire, N.P. Carbon stock and its relationships with tree diversity and density in community forests in Nepal. Int. For. Rev. 2018, 20, 263–273. [Google Scholar] [CrossRef]
- Pandey, H.P.; Maaren, I.E.; Shah, K.K.; Maraseni, T.N. Response of topographic and biodiversity variables on biomass and carbon density in community forests of himalayan foot-hills. J. For. Livelihood 2020, 19, 51–65. [Google Scholar]
- Joshi, R.; Singh, H.; Chhetri, R.; Yadav, R. Assessment of Carbon Sequestration Potential in Degraded and Non-Degraded Community Forests in Terai Region of Nepal. J. For. Environ. Sci. 2020, 36, 113–121. [Google Scholar]
- Joa, B.; Winkel, G.; Primmer, E. The unknown known—A review of local ecological knowledge in relation to forest biodiversity conservation. Land Use Policy 2018, 79, 520–530. [Google Scholar] [CrossRef]
- Khan, S.M.; Page, S.E.; Ahmad, H.; Harper, D.M. Sustainable utilization and conservation of plant biodiversity in montane ecosystems: The western Himalayas as a case study. Ann. Bot. 2013, 112, 479–501. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Franklin, J.F.; Fischer, J. General management principles and a checklist of strategies to guide forest biodiversity conservation. Biol. Conserv. 2006, 131, 433–445. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, H. Carbon Sequestration Potential of Disturbed and Non-disturbed Forest Ecosystem: A Tool for Mitigating Climate Change. Afr. J. Environ. Sci. Technol. 2020, 14, 385–393. [Google Scholar] [CrossRef]
- Aryal, S.; Baral, S.; Basnyat, B.; Timilsina, Y.P.; Gauli, K.; Maraseni, T. Synergy between Carbon and Biodiversity in Restored Forests: A case from leasehold forestry of Nepal. For. J. Inst. For. Nepal 2022, 19, 16–27. [Google Scholar] [CrossRef]
- Chytrý, M.; Otýpková, Z. Plot sizes used for phytosociological sampling of European vegetation. J. Veg. Sci. 2003, 14, 563–570. [Google Scholar] [CrossRef]
- Mueller-Dombois, D. Natural dieback in forests. BioScience 1987, 37, 575–583. [Google Scholar] [CrossRef]
- Pretzsch, H. Forest Dynamics, Growth, and Yield: From Measurement to Model; Springer: Berlin/Heidelberg, Germany, 2008; p. 39. [Google Scholar]
- Curtis, J.T.; Mcintosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurment of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley: New York, NY, USA, 1969; p. 286. [Google Scholar]
- Moeslund, J.E.; Arge, L.; Bocher, P.K.; Dalgaard, T.; Svenning, J.C. Topography as a driver of local terrestrial vascular plant diversity patterns. Nord. J. Bot. 2013, 31, 129–144. [Google Scholar] [CrossRef]
- Adhikari, Y.P.; Fischer, A.; Fischer, H.S. Micro-site conditions of epiphytic orchids in a human impact gradient in Kathmandu valley, Nepal. J. Mt. Sci. 2012, 9, 331–342. [Google Scholar] [CrossRef]
- Pandey, K.P.; Adhikari, Y.P.; Weber, M. Structure, composition and diversity of forest along the elevational gradient in the Himalayas, Nepal. Appl. Ecol. Environ. Res. 2016, 14, 235–251. [Google Scholar] [CrossRef]
- Saxena, A.K.; Singh, J.S. A phytosociological analysis of woody species in forest communities of a part of Kumaun Himalaya. Vegetatio 1982, 50, 3–22. [Google Scholar] [CrossRef]
- Gairola, S.; Sharma, C.M.; Suyal, S.; Ghildiyal, S.K. Species composition and Diversity in Mid-elevational Moist Temperate Forests of the Western Himalaya. J. For. Sci. 2011, 27, 1–15. [Google Scholar]
- Shrestha, T.B. Ecology and Vegetation of North-West Nepal (Karnali Region); Royal Nepal Academy: Kathmandu, Nepal, 1982. [Google Scholar]
- Ahmed, M.; Husain, T.; Heikh, A.H.S.; Hussain, S.S.; Siddiqui, M. Phytosociology and structure of Himalayan forests from different climatic zones of Pakistan. Pak. J. Bot. 2006, 38, 361–383. [Google Scholar]
- Kunwar, R.M.; Sharma, S.P. Quantitative analysis of tree species in two community forests of Dolpa district, mid-west Nepal. Himal. J. Sci. 2004, 2, 23–28. [Google Scholar] [CrossRef]
- Pandey, P.K. Quantitative vegetation analysis as per aspect and altitude, and regeneration behaviour of tree species in Garhwal Himalayan forest. Ann. For. 2001, 9, 39–52. [Google Scholar]
- Maharjan, S.K.; Sterck, F.J.; Raes, N.; Poorter, L. Temperature and soils predict the distribution of plant species along the Himalayan elevational gradient. J. Trop. Ecol. 2022, 38, 58–70. [Google Scholar] [CrossRef]
- Coverdale, T.C.; Davies, S.J. Functional traits and forest structure mediate regeneration dynamics in tropical forests. J. Ecol. 2023, 111, 345–360. [Google Scholar]
- Rubenstein, J.M.; Chazdon, R.L.; Uriarte, M. Linking biodiversity conservation with carbon storage in tropical forest landscapes. Conserv. Biol. 2023, 37, e13957. [Google Scholar]
- Baldocchi, D.; Falge, E.; Gu, L.; Olson, R.; Hollinger, D.; Running, S.; Anthoni, P.; Bernhofer, C.; Davis, K.; Evans, R.; et al. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull. Am. Meteorol. Soc. 2002, 83, 2415–2434. [Google Scholar] [CrossRef]

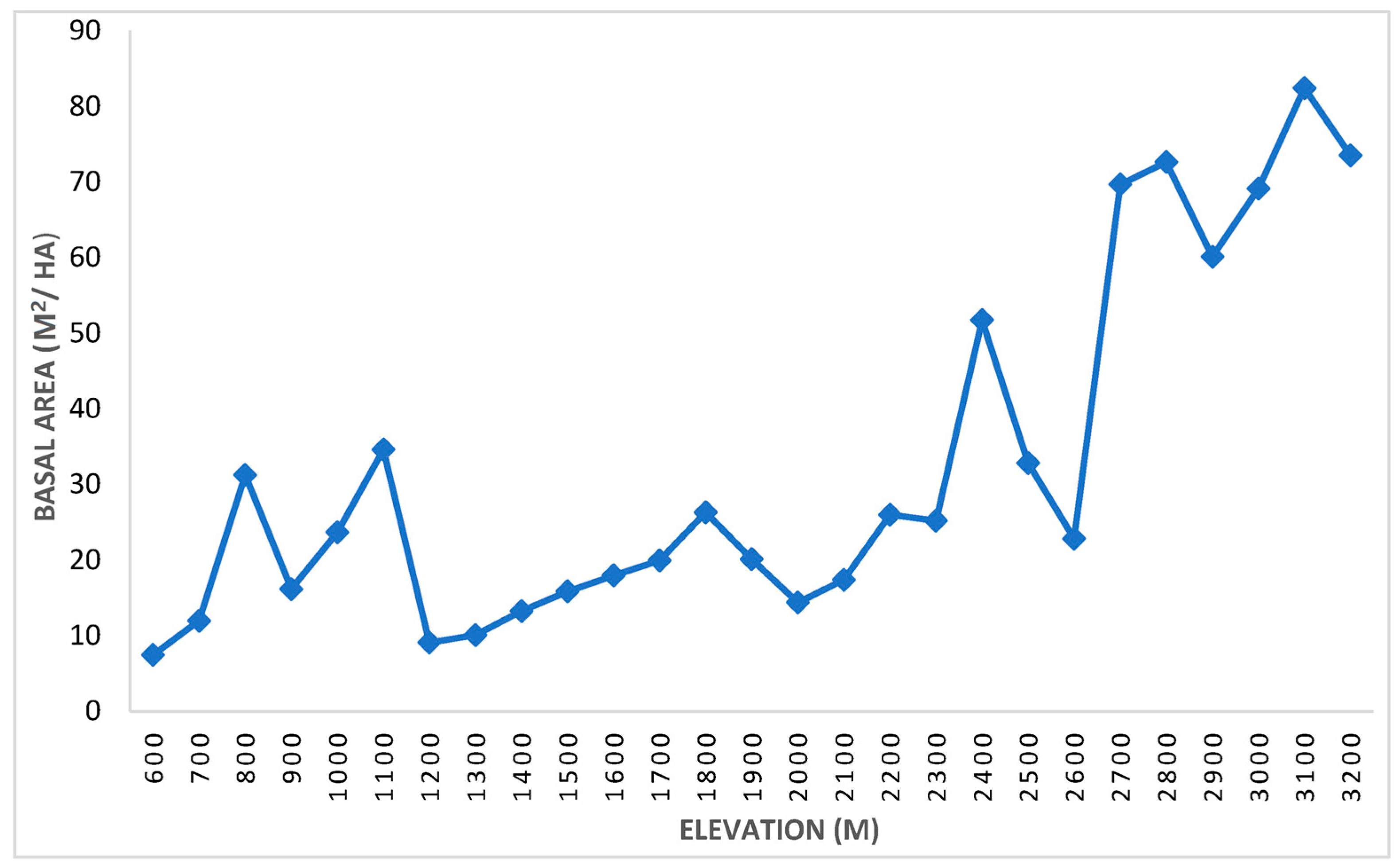
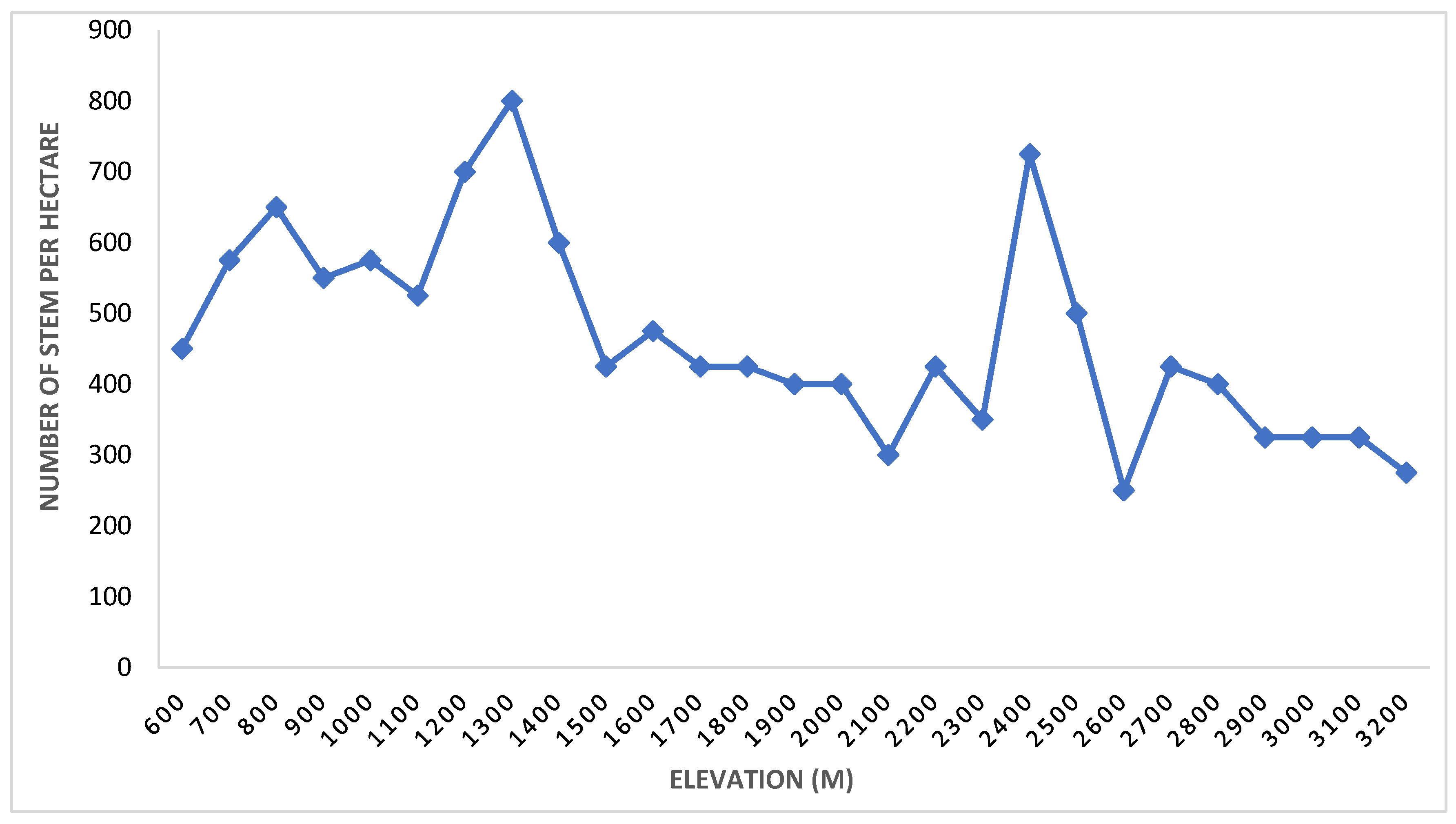
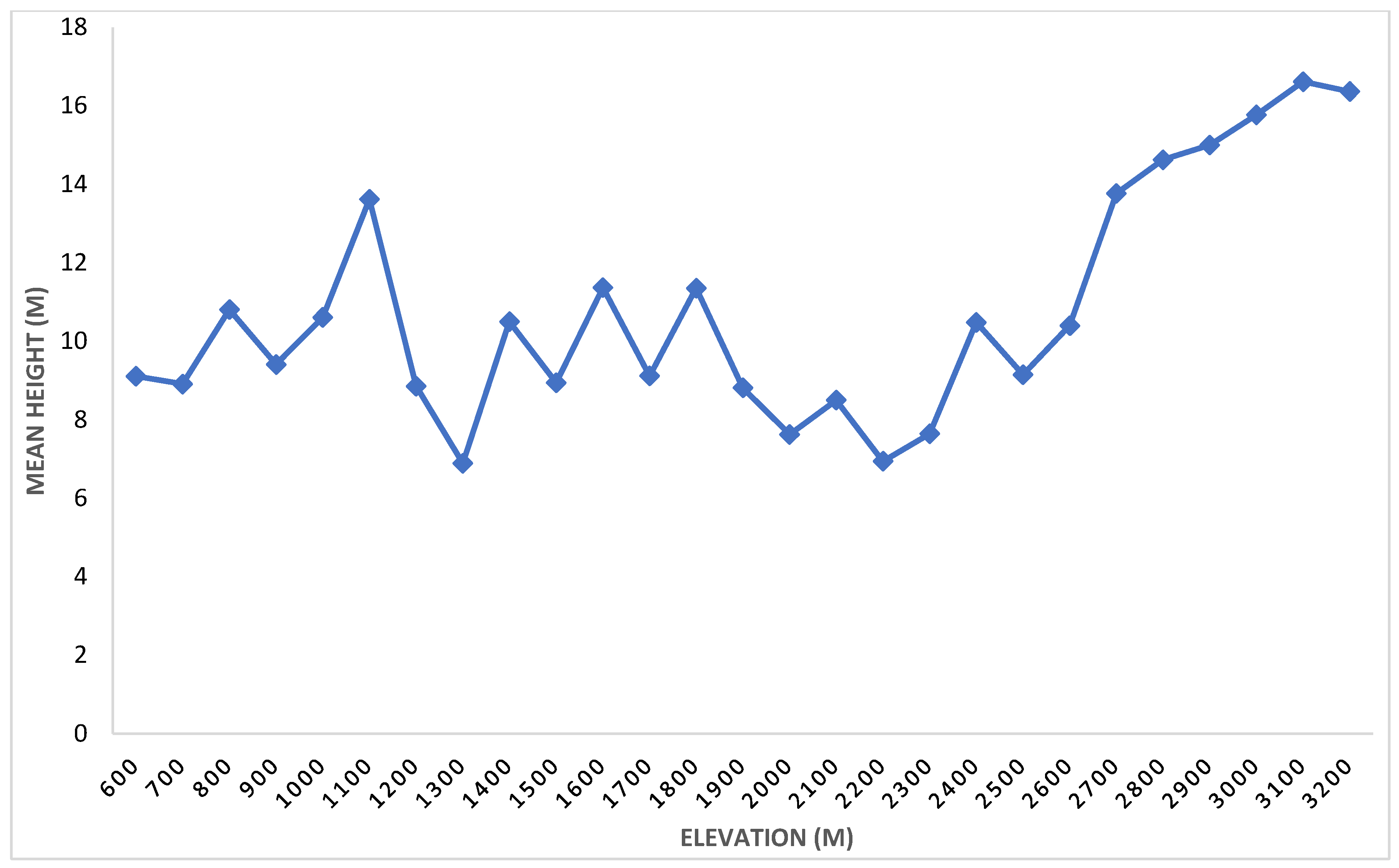
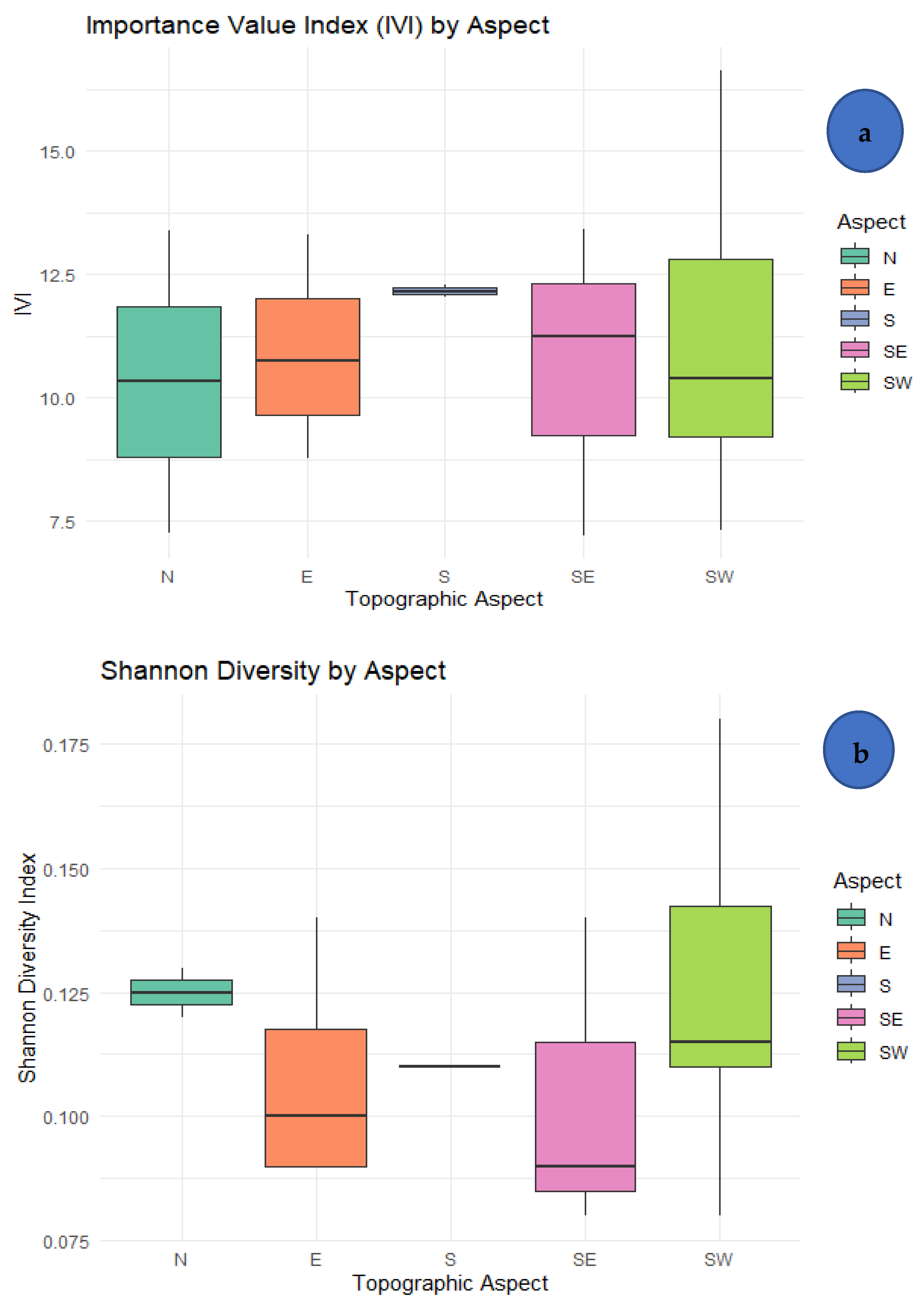
| Rank | Species | Stem Density | Basal Area (m2/ha) | Frequency | IVI | |||
|---|---|---|---|---|---|---|---|---|
| no./ha | RDn (%) | BA | RBA (%) | F | RF (%) | |||
| Lower subalpine zone (3000–3200 m) | ||||||||
| 1 | Quercus semicarpifolia | 108.3 | 35.2 | 39,257.5 | 43.6 | 100 | 25 | 103.782 |
| 2 | Pinus wallichiana | 67 | 22 | 28,265.1 | 31.4 | 100 | 25 | 78.0 |
| 3 | Tsuga dumosa | 58 | 19 | 15,717.5 | 17.5 | 100 | 25 | 61.4 |
| 4 | Rhododendron campanulatum | 75 | 24 | 6782.1 | 7.5 | 100 | 25 | 56.9 |
| Upper temperate zone (2500–3000 m) | ||||||||
| 1 | Quercus semicarpifolia | 120 | 32 | 54,755.2 | 53.0 | 100 | 17.9 | 102.5 |
| 2 | Pinus wallichiana | 40 | 11 | 15,171.4 | 14.7 | 60 | 10.7 | 35.9 |
| 3 | Alnus nepalensis | 35 | 9 | 7453.5 | 7.2 | 100 | 17.9 | 34.3 |
| 4 | Rhododendron arboreum | 30 | 8 | 5445.1 | 5.3 | 60 | 10.7 | 23.9 |
| 5 | Ligustrum robustum | 35 | 9 | 2335.2 | 2.3 | 40 | 7.1 | 18.6 |
| 6 | Persea odoratissima | 10 | 3 | 1893.5 | 1.8 | 20 | 3.6 | 8.0 |
| 7 | Juglans regia | 5 | 1 | 688.3 | 0.7 | 20 | 3.6 | 5.6 |
| 8 | Castanopsis tribuloides | 10 | 3 | 1651.2 | 1.6 | 20 | 3.6 | 7.8 |
| 9 | Lyonia ovalifolia | 25 | 7 | 1202.4 | 1.2 | 60 | 10.7 | 18.5 |
| 10 | Rhododendron campanulatum | 5 | 1 | 11,001.6 | 10.7 | 60 | 10.7 | 22.7 |
| 11 | Tsuga dumosa | 65 | 17 | 1627.3 | 1.6 | 20 | 3.6 | 22.3 |
| Lower temperate zone (2000–2500 m) | ||||||||
| 1 | Rhododendron arboreum | 130 | 30 | 14,566.2 | 29.8 | 100 | 16.7 | 76.0 |
| 2 | Alnus nepalensis | 55 | 13 | 13,120.2 | 26.8 | 80 | 13.3 | 52.6 |
| 3 | Persea odoratissima | 35 | 8 | 5926.9 | 12.1 | 40 | 6.7 | 26.7 |
| 4 | Michelia champaca | 30 | 7 | 1545.8 | 3.2 | 80 | 13.3 | 23.3 |
| 5 | Quercus leucotichophora | 20 | 5 | 2932.0 | 6.0 | 60 | 10.0 | 20.5 |
| 6 | Juglans regia | 25 | 6 | 3874.6 | 7.9 | 20 | 3.3 | 16.9 |
| 7 | Ligustrum robustum | 40 | 9 | 346.6 | 0.7 | 40 | 6.7 | 16.5 |
| 8 | Castanopsis tribuloides | 25 | 6 | 1828.8 | 3.7 | 20 | 3.3 | 12.8 |
| 9 | Daphniphylum himalainsis | 30 | 7 | 479.4 | 1.0 | 20 | 3.3 | 11.1 |
| 10 | Lyonia ovalifolia | 15 | 3 | 307.9 | 0.6 | 40 | 6.7 | 10.7 |
| 11 | Garuga pinnata | 15 | 3 | 1879.6 | 3.8 | 20 | 3.3 | 10.6 |
| 12 | Premna longifolia Roxb. | 5 | 1 | 962.9 | 2.0 | 20 | 3.3 | 6.4 |
| 13 | Myrica esculenta | 5 | 1 | 616.2 | 1.3 | 20 | 3.3 | 5.7 |
| 14 | Falconeria insignis | 5 | 1 | 447.6 | 0.9 | 20 | 3.3 | 5.4 |
| 15 | Maduca longifolia | 5 | 1 | 97.5 | 0.2 | 20 | 3.3 | 4.7 |
| Subtropical zone (1000–2000 m) | ||||||||
| 1 | Shorea robusta | 193 | 36 | 16,942.6 | 22.3 | 50 | 8.2 | 66.5 |
| 2 | Alnus nepalensis | 63 | 12 | 24,979.4 | 32.9 | 60 | 9.8 | 54.4 |
| 3 | Maduca longifolia | 35 | 7 | 5504.7 | 7.3 | 70 | 11.5 | 25.3 |
| 4 | Pinus roxburghii | 40 | 7 | 6389.4 | 8.4 | 40 | 6.6 | 22.5 |
| 5 | Quercus acutissima | 25 | 5 | 2077.6 | 2.7 | 40 | 6.6 | 14.0 |
| 6 | Cedrella tooni | 10 | 2 | 6391.7 | 8.4 | 20 | 3.3 | 13.6 |
| 7 | Schima wallichi | 25 | 5 | 2579.6 | 3.4 | 30 | 4.9 | 13.0 |
| 8 | Diospyros malabarica | 25 | 5 | 906.5 | 1.2 | 40 | 6.6 | 12.4 |
| 9 | Myrica esculenta | 15 | 3 | 1807.8 | 2.4 | 40 | 6.6 | 11.7 |
| 10 | Pyrus pashia | 18 | 3 | 1049.4 | 1.4 | 40 | 6.6 | 11.2 |
| 11 | Rhododendron arboreum | 23 | 4 | 1419.9 | 1.9 | 30 | 4.9 | 11.0 |
| 12 | Ficus semicordata | 8 | 1 | 691.1 | 0.9 | 30 | 4.9 | 7.2 |
| 13 | Ligustrum robustum | 10 | 2 | 1369.9 | 1.8 | 20 | 3.3 | 7.0 |
| 14 | Lyonia ovalifolia | 10 | 2 | 580.8 | 0.8 | 10 | 1.6 | 4.3 |
| 15 | Persea odoratissima | 8 | 1 | 576.1 | 0.8 | 10 | 1.6 | 3.8 |
| 16 | Pogosteman benghalensis | 5 | 1 | 782.5 | 1.0 | 10 | 1.6 | 3.6 |
| 17 | Albizzia lebbeck | 5 | 1 | 702.6 | 0.9 | 10 | 1.6 | 3.5 |
| 18 | Falconeria insignis | 5 | 1 | 422.5 | 0.6 | 10 | 1.6 | 3.1 |
| 19 | Quercus leucotichophora | 5 | 1 | 214.0 | 0.3 | 10 | 1.6 | 2.9 |
| 20 | Ficus neriifolia | 3 | 0.47 | 215.2 | 0.3 | 10 | 1.6 | 2.4 |
| 21 | Prunus cerasoides | 3 | 0.47 | 154.1 | 0.2 | 10 | 1.6 | 2.3 |
| 22 | Bauhinia variegata | 3 | 0.47 | 71.6 | 0.1 | 10 | 1.6 | 2.2 |
| 23 | Castanopsis tribuloides | 3 | 0.47 | 81.5 | 0.1 | 10 | 1.6 | 2.2 |
| Tropical zone (<1000 m) | ||||||||
| 1 | Shorea robusta | 375 | 67 | 21,592.7 | 84.7 | 100 | 30.0 | 182.2 |
| 2 | Dalbergia sisso | 81 | 15 | 1594.7 | 6.3 | 67 | 20.0 | 40.9 |
| 3 | Senegilia catechu | 56 | 10 | 1127.1 | 4.4 | 67 | 20.0 | 34.5 |
| 4 | Albizzia procera | 38 | 7 | 1084.0 | 4.3 | 67 | 20.0 | 31.0 |
| 5 | Maduca longifolia | 6 | 1 | 97.5 | 0.4 | 33 | 10.0 | 11.5 |
| Sample Plot No. | Elevation (m) | DBH Classes (cm) | ||
|---|---|---|---|---|
| 5–<10 | 10–29.9 | ≥30 | ||
| 1 | 600 | 0 | 18 | 2 |
| 2 | 700 | 3 | 19 | 1 |
| 3 | 800 | 4 | 13 | 9 |
| 4 | 900 | 6 | 13 | 3 |
| 5 | 1000 | 6 | 11 | 6 |
| 6 | 1100 | 1 | 13 | 7 |
| 7 | 1200 | 9 | 19 | 0 |
| 8 | 1300 | 15 | 17 | 0 |
| 9 | 1400 | 6 | 18 | 0 |
| 10 | 1500 | 4 | 10 | 3 |
| 11 | 1600 | 7 | 8 | 4 |
| 12 | 1700 | 0 | 12 | 5 |
| 13 | 1800 | 1 | 11 | 5 |
| 14 | 1900 | 6 | 7 | 3 |
| 15 | 2000 | 2 | 13 | 1 |
| 16 | 2100 | 1 | 9 | 2 |
| 17 | 2200 | 4 | 6 | 7 |
| 18 | 2300 | 4 | 6 | 4 |
| 19 | 2400 | 1 | 15 | 13 |
| 20 | 2500 | 3 | 9 | 8 |
| 21 | 2600 | 0 | 3 | 7 |
| 22 | 2700 | 0 | 3 | 14 |
| 23 | 2800 | 0 | 3 | 13 |
| 24 | 2900 | 0 | 0 | 13 |
| 25 | 3000 | 0 | 0 | 13 |
| 26 | 3100 | 0 | 0 | 13 |
| 27 | 3200 | 0 | 0 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, S.; Joshi, R.; Maraseni, T.N.; Bhattarai, P. Unraveling Elevation-Driven Variations in Forest Structure and Composition in Western Nepal. Diversity 2025, 17, 588. https://doi.org/10.3390/d17080588
Acharya S, Joshi R, Maraseni TN, Bhattarai P. Unraveling Elevation-Driven Variations in Forest Structure and Composition in Western Nepal. Diversity. 2025; 17(8):588. https://doi.org/10.3390/d17080588
Chicago/Turabian StyleAcharya, Sagar, Rajeev Joshi, Tek Narayan Maraseni, and Prakash Bhattarai. 2025. "Unraveling Elevation-Driven Variations in Forest Structure and Composition in Western Nepal" Diversity 17, no. 8: 588. https://doi.org/10.3390/d17080588
APA StyleAcharya, S., Joshi, R., Maraseni, T. N., & Bhattarai, P. (2025). Unraveling Elevation-Driven Variations in Forest Structure and Composition in Western Nepal. Diversity, 17(8), 588. https://doi.org/10.3390/d17080588








