Relationship Between Groundwater Level and Rodent Community Structure Mediated by Nutrient Composition of Plants in Dongting Lake, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey of Rodent Communities in Dongting Lake
- Capture rate
- Simpson index
- Shannon–Wiener index
- Pielou index
2.2. Data Collection on Groundwater Level in Dongting Lake
2.3. Determination of Plant Constituents
2.4. Statistical Analysis
3. Result and Analysis
3.1. Capture Rate and Rodent Species in Different Habitats of the Dongting Lake Area
3.2. Diversity Index of Rodent Communities in Different Habitats
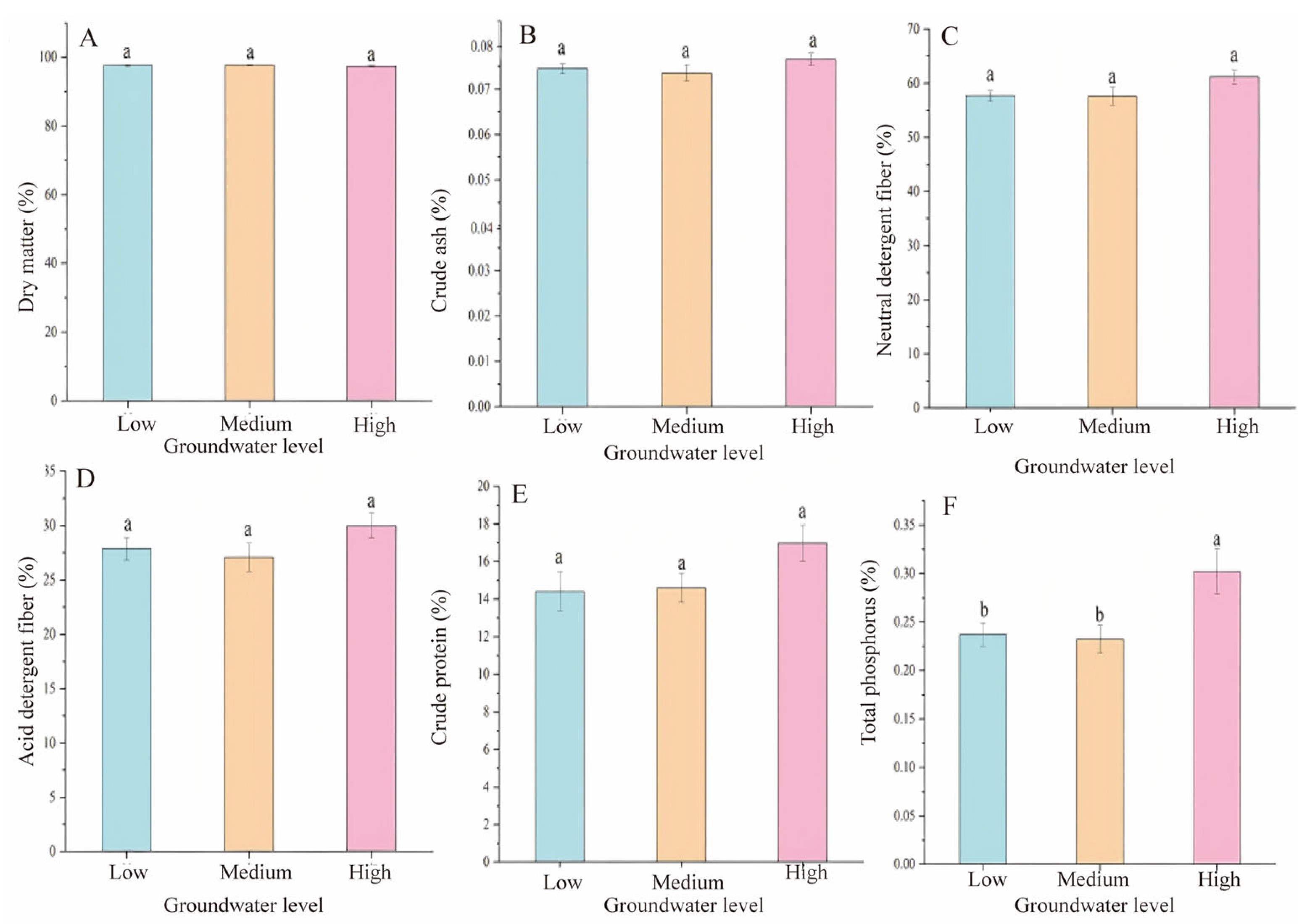
3.3. Relationship Between the Groundwater Level and the Nutrient Composition of Plants
3.4. Relationship Between the Nutrient Composition of Plant and Rodent Communities
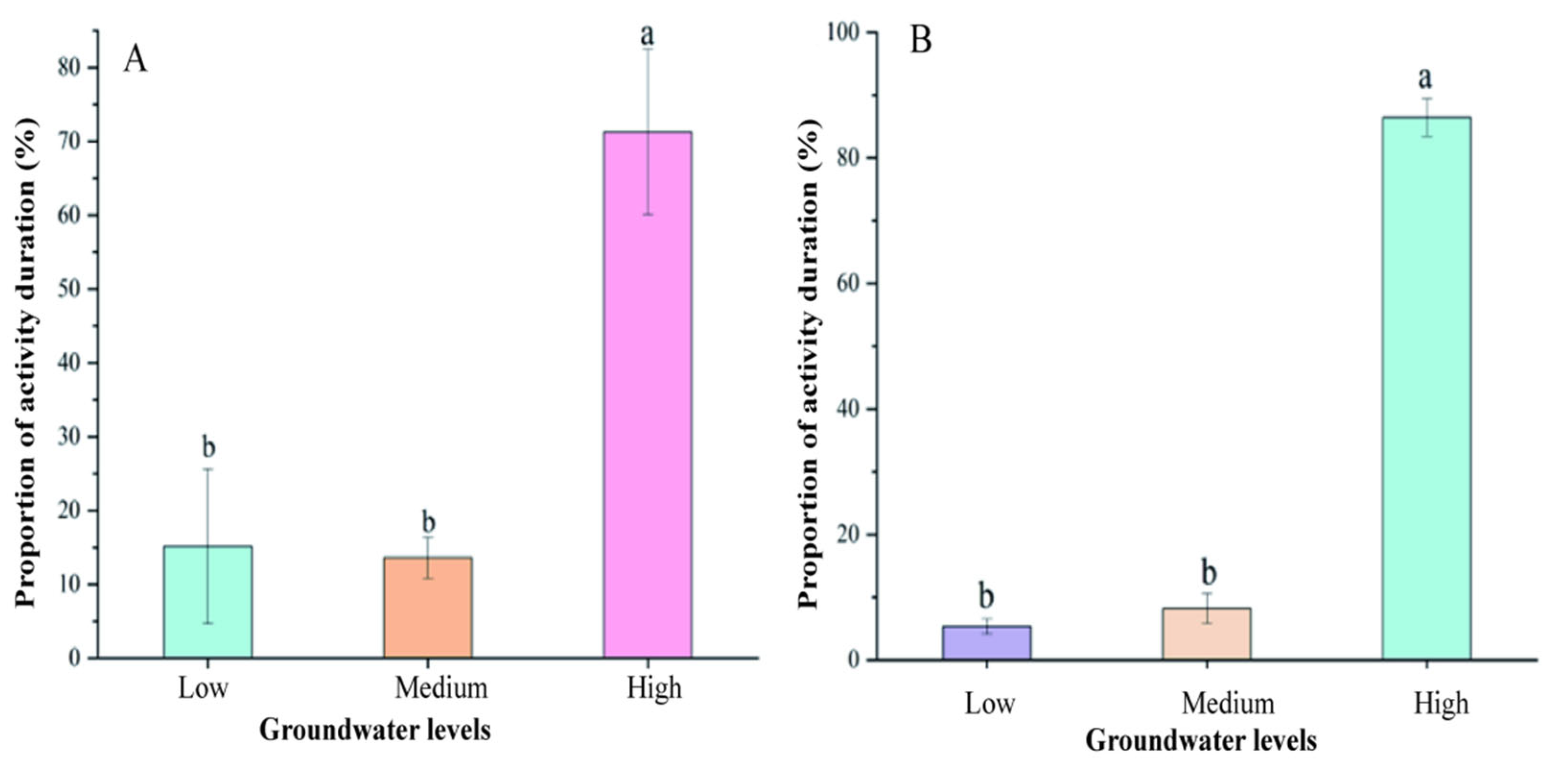
3.5. The Relationship Between Groundwater Level and Rodent Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Douglass, R.J. Assessment of the use of selected rodents in ecological monitoring. Environ. Manag. 1989, 13, 355–363. [Google Scholar] [CrossRef]
- Minchella, D.J.; Scott, M.E. Parasitism: A cryptic determinant of animal community structure. Trends Ecol. Evol. 1991, 6, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S. Desert Rodent Community Structure: A Test of Four Mechanisms of Coexistence. Ecol. Monogr. 1989, 59, 1–20. [Google Scholar] [CrossRef]
- Hansson, L.; Henttonen, H. Rodent dynamics as community processes. Trends Ecol. Evol. 1988, 3, 195–200. [Google Scholar] [CrossRef]
- Avenant, N.L.; Cavallini, P. Correlating rodent community structure with ecological integrity, Tussen-die-Riviere Nature Reserve, Free State province, South Africa. Integr. Zool. 2007, 2, 212–219. [Google Scholar] [CrossRef]
- Goodale, E.; Sridhar, H.; Sieving, K.E.; Bangal, P.; Colorado, Z.G.J.; Farine, D.R.; Heymann, E.W.; Jones, H.H.; Krams, I.; Martínez, A.E.; et al. Mixed company: A framework for understanding the composition and organization of mixed-species animal groups. Biol. Rev. 2020, 95, 889–910. [Google Scholar] [CrossRef]
- Goodale, E.; Beauchamp, G.; Magrath, R.D.; Nieh, J.C.; Ruxton, G.D. Interspecific information transfer influences animal community structure. Trends Ecol. Evol. 2010, 25, 354–361. [Google Scholar] [CrossRef]
- Larsen, A.L.; Homyack, J.A.; Wigley, T.B.; Miller, D.A.; Kalcounis-Rueppell, M.C. Effects of habitat modification on cotton rat population dynamics and rodent community structure. For. Ecol. Manag. 2016, 376, 238–246. [Google Scholar] [CrossRef]
- Ventura-Rojas, P.D.; González-Romero, A.; Moreno, C.E.; Sosa, V.J. Effect of rainfall, temperature and climate change on the ecology of the rodents of arid zones: A review. Mammal Rev. 2025, 55, e12372. [Google Scholar] [CrossRef]
- Stefke, K.; Landler, L. Long-term monitoring of rodent and shrew communities in a biodiversity hot-spot in Austria using barn owl (Tyto alba) pellets. Acta Oecologica 2020, 109, 103660. [Google Scholar] [CrossRef]
- Noe, K.L.; Rota, C.T.; Frantz, M.W.; Anderson, J.T. Restored Wetland Size and Age Influence Small Mammal Communities in West Virginia, USA. Wetlands 2024, 44, 48. [Google Scholar] [CrossRef]
- Gliwicz, J. Competitive Interactions within a Forest Rodent Community in Central Poland. Oikos 1981, 37, 353–362. [Google Scholar] [CrossRef]
- An, C.; Fang, H.; Zhang, L.; Su, X.; Fu, X.; Huang, H.Q.; Parker, G.; Hassan, M.A.; Meghani, N.A.; Anders, A.M.; et al. Poyang and Dongting Lakes, Yangtze River: Tributary lakes blocked by main-stem aggradation. Proc. Natl. Acad. Sci. USA 2022, 119, e2101384119. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, D.; Jin, X.; Li, L.; Wang, C.; Wang, Y.; Pellissier, L.; Johnson, A.C.; Wu, F.; Zhang, X. Long-term wetland biomonitoring highlights the differential impact of land use on macroinvertebrate diversity in Dongting Lake in China. Commun. Earth Environ. 2024, 5, 32. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Y.; Xiao, W.; Wang, D.; Wang, Z.; Jin, X.; Cheng, T.; Zhang, J.; Yi, P. The relationship between landscape pattern and plant species diversity in east Dongting lake wetland based on different Eco-environment. Environ. Pollut. 2024, 355, 124187. [Google Scholar] [CrossRef] [PubMed]
- Lissovsky, A.A.; Petrova, T.V.; Yatsentyuk, S.P.; Golenishchev, F.N.; Putincev, N.I.; Kartavtseva, I.V.; Sheremetyeva, I.N.; Abramson, N.I. Multilocus phylogeny and taxonomy of East Asian voles Alexandromys (Rodentia, Arvicolinae). Zool. Scr. 2018, 47, 9–20. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Li, B.; Feng, Z.; Zhao, Y.; Xu, Z. Synergistic succession of the small mammal community and herbaceous vegetation after reconverting farmland to seasonally flooded wetlands in the Dongting Lake Region, China. Mammal Study 2018, 43, 229–243. [Google Scholar] [CrossRef]
- Yong, W.; Mei-Wen, Z.; Bo, L.I.; Kai-Rong, W. Rodent community structure and succession in different ecotypic areas in Dongting Lake region. J. Ecol. Rural Environ. 2003, 19, 13–17. [Google Scholar]
- Xu, Z.; Zhao, Y.; Li, B.; Zhang, M.; Shen, G.; Wang, Y. Habitat evaluation for outbreak of Yangtze voles (Microtus fortis) and management implications. Integr. Zool. 2015, 10, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, L.; Guo, C.; Wang, Y.; Guo, Y. Effect of ENSO-driven precipitation on population irruptions of the Yangtze vole Microtus fortis calamorum in the Dongting Lake region of China. Integr. Zool. 2010, 5, 176–184. [Google Scholar] [CrossRef]
- Huang, T.; Tang, Y.; Sun, Y.; Zhang, M.; Zhang, C.; Zhao, Y.; Nan, X.; Hu, Z.; Xu, Z. Effects of Vegetation Cover on Community Structure of Rodents Based on Long Time Series from Dongting Lake, China. Biology 2025, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Li, B.; Guo, C.; Huang, G.; Shen, G.; Zhou, X. Small mammal community succession on the beach of Dongting Lake, China after the Three Gorges Project. Integr. Zool. 2014, 9, 294–308. [Google Scholar] [CrossRef]
- He, J.; Zhang, T.; Yang, G.; Xu, Z.; Zhang, M.; Wang, Y.; Li, B.; Zhou, X.; Huang, H. The reproductive status of Apodemus agrarius populations inhabiting the lake beach in Lake Dongting area after Three Gorges Project. J. Lake Sci. 2023, 35, 2101–2110. [Google Scholar]
- Jolly, I.D.; McEwan, K.L.; Holland, K.L. A review of groundwater–surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology 2008, 1, 43–58. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, S.; Huang, G.; Zhang, R. Analysis of Long-Term Water Level Variation in Dongting Lake, China. Water 2016, 8, 306. [Google Scholar] [CrossRef]
- Lai, X.; Chen, H.; Hou, Y.; Finlayson, B.; Li, M.; Chen, J. Lowering water level of Dongting lake of the Mid-Yangtze River in response to large-scale dam construction: A 60-year analysis. Geomorphology 2021, 391, 107894. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Yan, D.; Chen, L.; Li, M.; Luan, Z. Typical vegetation dynamics and hydrological changes of Dongting Lake wetland from 1985 to 2020. Ecohydrol. Hydrobiol. 2024, 24, 910–919. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Guo, W. The impacts of water level fluctuations of East Dongting Lake on habitat suitability of migratory birds. Ecol. Indic. 2021, 132, 108277. [Google Scholar] [CrossRef]
- Takatert, N.; Sanchez-Pérez, J.M.; Trémolières, M. Spatial and temporal variations of nutrient concentration in the groundwater of a floodplain: Effect of hydrology, vegetation and substrate. Hydrol. Process. 1999, 13, 1511–1526. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, X.; He, Y.; Li, G.; Li, J. Dominant roles but distinct effects of groundwater depth on regulating leaf and fine-root N, P and N:P ratios of plant communities. J. Plant Ecol. 2021, 14, 1158–1174. [Google Scholar] [CrossRef]
- Yong, Z.Y.; Zhang, M.W.; Guo, C.; Wang, Y.; Li, B.; Li, J.X.; Yang, Y.C.; Xu, Z.G. Dietary Habit of Apodemus agrarius in Dongting Lake Area. Chin. J. Zool. 2012, 47, 115–121. [Google Scholar]
- Lei, F.; Yunlin, Z.; Meiwen, Z.; Yong, W.; Zhenggang, X.; Jiao, P. Research on feeding of laboratory-bred adult Microtus fortis calamorum on carex. Chin. J. Vector Biol. Control 2016, 27, 546–548. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.-S.; Zou, Y.-A.; Zhang, P.-Y.; Li, F.; Hou, Z.-Y.; Li, X.; Zeng, J.; Deng, Z.-M.; Zhong, J.-R.; et al. Exploring the relative contribution of flood regimes and climatic factors to Carex phenology in a Yangtze River-connected floodplain wetland. Sci. Total Environ. 2022, 847, 157568. [Google Scholar] [CrossRef]
- Thiex, N.J.; Erem, T.V. Determination of Water (Moisture) and Dry Matter in Animal Feed, Grain, and Forage (Plant Tissue) by Karl Fischer Titration: Collaborative Study. J. AOAC Int. 2019, 85, 318–327. [Google Scholar] [CrossRef]
- Wichmann, H.J.; Tilden, D. Report on Ash in Fruit Products. J. Assoc. Off. Agric. Chem. 2020, 10, 433–436. [Google Scholar] [CrossRef]
- Heckman, M.M.; Lane, S.A. Comparison of Dietary Fiber Methods for Foods. J. Assoc. Off. Anal. Chem. 2020, 64, 1339–1343. [Google Scholar] [CrossRef]
- Thiex, N. Evaluation of Analytical Methods for the Determination of Moisture, Crude Protein, Crude Fat, and Crude Fiber in Distillers Dried Grains with Solubles. J. AOAC Int. 2019, 92, 61–73. [Google Scholar] [CrossRef]
- Jacob, K.D.; Hoffman, W.M. Report on Phosphorus in Fertilizers: I. Preparation of Solution of Sample for Total Phosphorus Determination. J. Assoc. Off. Agric. Chem. 2020, 40, 690–700. [Google Scholar] [CrossRef]
- Zhang, G.; Dai, E.; Luobu, D.; Fu, G. Effects of Climate Change and Fencing on Forage Nutrition Quality of Alpine Grasslands in the Northern Tibet. Plants 2023, 12, 3182. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-Y.; Xie, Y.-H.; Tang, Y.; Li, F.; Zou, Y.-A. Changes of Vegetation Distribution in the East Dongting Lake After the Operation of the Three Gorges Dam, China. Front. Plant Sci. 2018, 9, 2018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zhou, X.; Zhang, M.; Wang, Y.; Zhang, C.; Yang, J. The Situation of Small Mammal Community in Beach of Dongting Lake after the Official Operation of the Three Gorges Reservoir. Chin. J. Zool. 2020, 55, 141–152. [Google Scholar]
- Yang, X.; Gu, H.; Zhao, Q.; Zhu, Y.; Teng, Y.; Li, Y.; Zhang, Z. High seed diversity and availability increase rodent community stability under human disturbance and climate variation. Front. Plant Sci. 2022, 13, 2022. [Google Scholar] [CrossRef]
- Ni, H.; Yang, F.; Li, Y.; Liu, W.; Jiao, S.; Li, Z.; Yi, B.; Chen, Y.; Hou, X.; Hu, F.; et al. Apodemus agrarius is a potential natural host of severe fever with thrombocytopenia syndrome (SFTS)—Causing novel bunyavirus. J. Clin. Virol. 2015, 71, 82–88. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, Y.; Zhang, M.; Xu, Z.; He, R.; Wang, S.; Guo, Z. Analysis on Nutritional Content in Different Part of Carex brevicuspis in Dongting Lake. Mod. Agric. Sci. Technol. 2016, 305–307. [Google Scholar]
- Chen, X.-S.; Xie, Y.-H.; Deng, Z.-M.; Li, F.; Hou, Z.-Y. A change from phalanx to guerrilla growth form is an effective strategy to acclimate to sedimentation in a wetland sedge species Carex brevicuspis (Cyperaceae). Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 347–350. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Xiang, W.; Xie, Y. Effects of water levels on the growth and reproductive characteristics of Carex brevicuspis growing on sites with different elevations. Acta Ecol. Sin. 2016, 36, 1959–1966. [Google Scholar]
- Wang, B.; Chen, J. Effects of Fat and Protein Levels on Foraging Preferences of Tannin in Scatter-Hoarding Rodents. PLoS ONE 2012, 7, e40640. [Google Scholar] [CrossRef]
- Soininen, E.M.; Neby, M. Small rodent population cycles and plants—After 70 years, where do we go? Biol. Rev. 2024, 99, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, D.H. Thermogenesis, food intake and serum leptin in cold-exposed lactating Brandt’s voles Lasiopodomys brandtii. J. Exp. Biol. 2007, 210, 512–521. [Google Scholar] [CrossRef]
- Jurišić, A.; Ćupina, A.I.; Kavran, M.; Potkonjak, A.; Ivanović, I.; Bjelić-Čabrilo, O.; Meseldžija, M.; Dudić, M.; Poljaković-Pajnik, L.; Vasić, V. Surveillance Strategies of Rodents in Agroecosystems, Forestry and Urban Environments. Sustainability 2022, 14, 9233. [Google Scholar] [CrossRef]
- Xing, T.; Wang, Y.; Deng, W.; Zhang, M.; Li, B.; Zhu, J. Effects of protein, fiber, and tannic acid on food intake of Microtus fortis. Acta Ecol. Sin. 2010, 30, 941–948. [Google Scholar]
- Wu, T.; Xia, L.; Pan, B.; Zou, Y.; Li, F.; Xie, Y.; Wang, S.; Li, Z. Mowing of Carex brevicuspis (Cyperaceae) improves food quality for herbivorous geese in Dongting Lake: The potential mechanisms. Front. Plant Sci. 2025, 16, 1566808. [Google Scholar] [CrossRef]
- Battisti, C.; Dodaro, G.; Di Bagno, E.; Amori, G. Reviewing an eco-biogeographic question at regional scale: The unexpected absence of a ubiquitous mammal species (Microtus savii, Rodentia) in coastal Southern Tuscany (central Italy). Rend. Lincei. Sci. Fis. E Nat. 2019, 30, 715–722. [Google Scholar] [CrossRef]
- Battisti, C.; Dodaro, G.; Di Bagno, E.; Amori, G. Small mammal assemblages in land-reclaimed areas: Do historical soil use changes and recent anthropisation affect their dominance structure? Ethol. Ecol. Evol. 2020, 32, 282–288. [Google Scholar] [CrossRef]
- Stevens, R.D.; Tello, J.S. Diversity begets diversity: Relative roles of structural and resource heterogeneity in determining rodent community structure. J. Mammal. 2011, 92, 387–395. [Google Scholar] [CrossRef]
- Gorelick, R. Combining richness and abundance into a single diversity index using matrix analogues of Shannon’s and Simpson’s indices. Ecography 2006, 29, 525–530. [Google Scholar] [CrossRef]
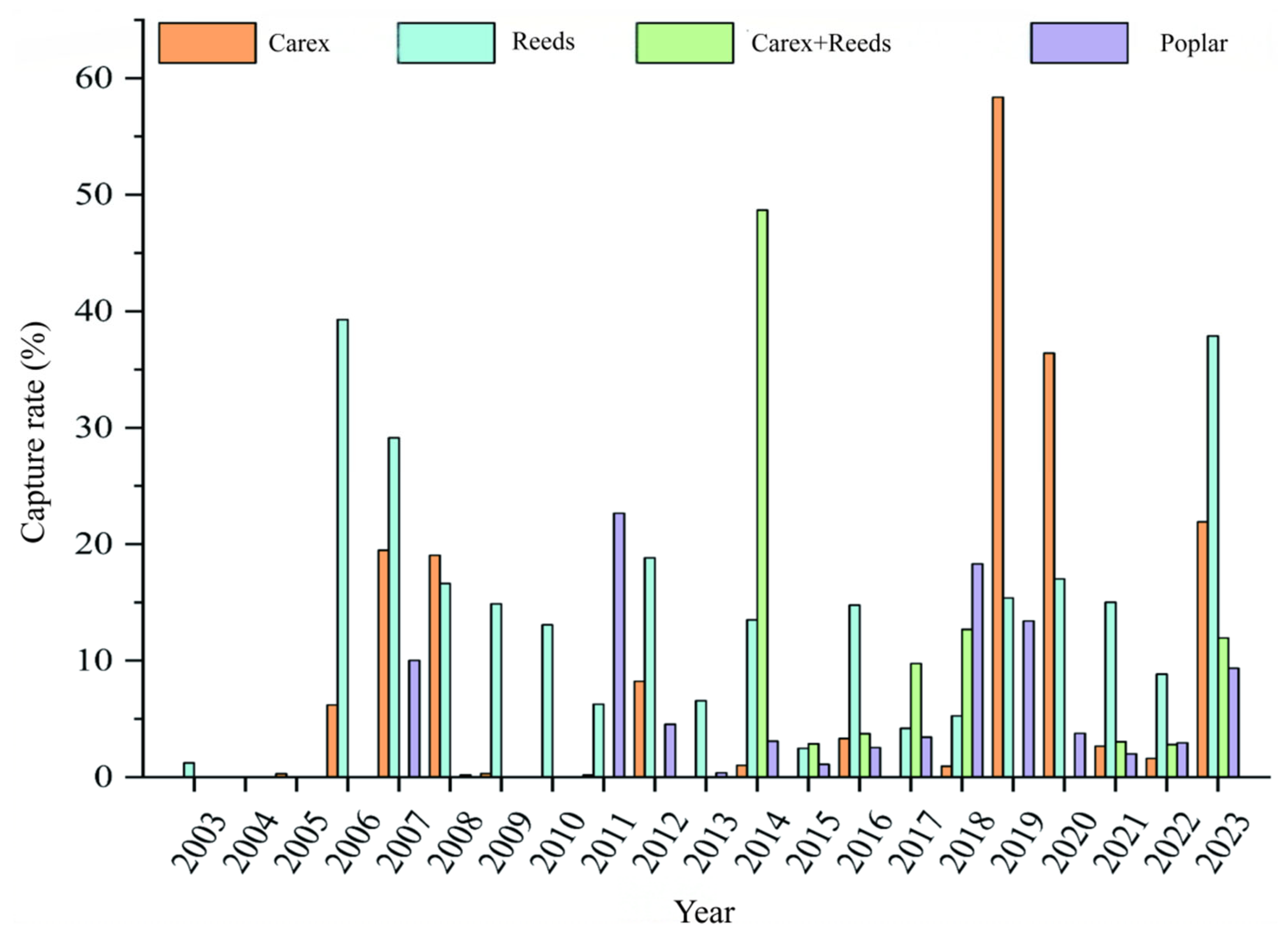

| Habitat | Species Number | Shannon–Wiener | Pielou | Simpson |
|---|---|---|---|---|
| Carex | 6 | 0.3414 | 0.1905 | 0.8338 |
| Reeds | 4 | 0.8315 | 0.5998 | 0.4669 |
| Carex + Reeds | 4 | 0.6189 | 0.4464 | 0.6414 |
| Poplar | 7 | 0.6083 | 0.3126 | 0.7031 |
| Habitat | Groundwater Level | Capture Rate | Species Number | Shannon–Wiener | Pielou | Simpson |
|---|---|---|---|---|---|---|
| Poplar | low | 6.37 | 4 | 1.0308 | 0.7435 | 0.6459 |
| medium | 4.92 | 3 | 0.9997 | 0.9100 | 0.5941 | |
| high | 6.73 | 5 | 1.1594 | 0.7204 | 0.6107 | |
| Carex | low | 4.88 | 2 | 0.8631 | 1.2452 | 0.6014 |
| medium | 7.29 | 2 | 0.7850 | 1.1325 | 0.6484 | |
| high | 16.86 | 3 | 0.3817 | 0.3474 | 0.8763 | |
| Reeds | low | 19.81 | 3 | 1.1813 | 1.0753 | 0.4870 |
| medium | 17.92 | 4 | 1.1997 | 0.8654 | 0.5198 | |
| high | 28.26 | 4 | 1.0966 | 0.7910 | 0.5328 | |
| Carex + Reeds | low | 4.54 | 4 | 0.7091 | 0.5115 | 0.7692 |
| medium | 6.86 | 3 | 0.7965 | 0.7250 | 0.6893 | |
| high | 8.37 | 3 | 0.2026 | 0.1844 | 0.9561 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; He, Y.; Yu, T.; Zhang, M.; Zhang, C.; Zhao, Y.; Lin, J.; Nan, X.; Hu, Z.; Xu, Z. Relationship Between Groundwater Level and Rodent Community Structure Mediated by Nutrient Composition of Plants in Dongting Lake, China. Diversity 2025, 17, 587. https://doi.org/10.3390/d17080587
Huang T, He Y, Yu T, Zhang M, Zhang C, Zhao Y, Lin J, Nan X, Hu Z, Xu Z. Relationship Between Groundwater Level and Rodent Community Structure Mediated by Nutrient Composition of Plants in Dongting Lake, China. Diversity. 2025; 17(8):587. https://doi.org/10.3390/d17080587
Chicago/Turabian StyleHuang, Tian, Yiying He, Tonglin Yu, Meiwen Zhang, Chen Zhang, Yunlin Zhao, Junxiang Lin, Xiaoning Nan, Zhiyuan Hu, and Zhenggang Xu. 2025. "Relationship Between Groundwater Level and Rodent Community Structure Mediated by Nutrient Composition of Plants in Dongting Lake, China" Diversity 17, no. 8: 587. https://doi.org/10.3390/d17080587
APA StyleHuang, T., He, Y., Yu, T., Zhang, M., Zhang, C., Zhao, Y., Lin, J., Nan, X., Hu, Z., & Xu, Z. (2025). Relationship Between Groundwater Level and Rodent Community Structure Mediated by Nutrient Composition of Plants in Dongting Lake, China. Diversity, 17(8), 587. https://doi.org/10.3390/d17080587







