Impacts of Mycobacterium leprae-Infection on Wild Populations of the Nine-Banded Armadillo (Dasypus novemcinctus) Species Complex: A Systematic Review
Abstract
1. Introduction
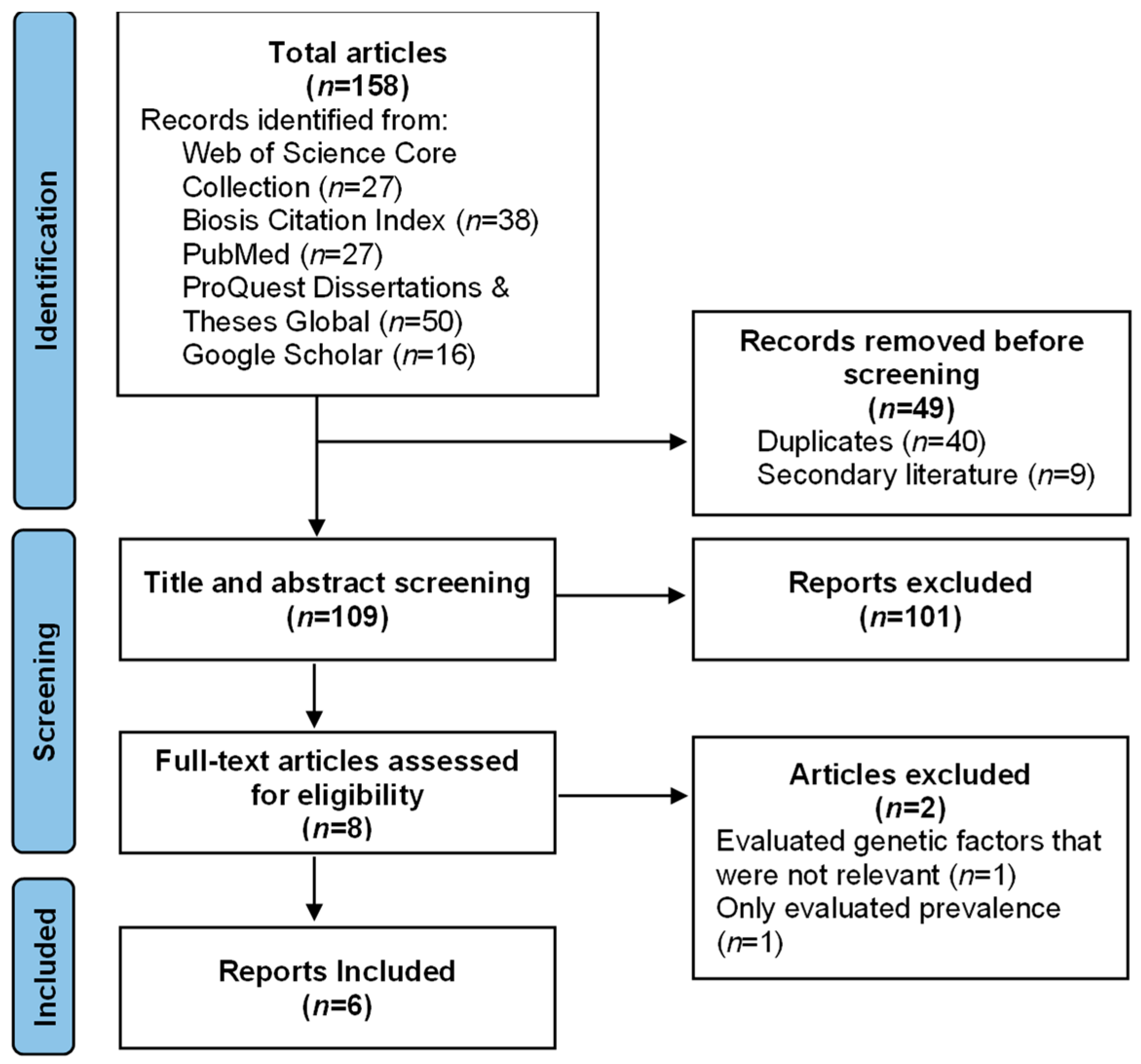
2. Materials and Methods
3. Results
3.1. Study Sites
3.2. Prevalence of M. leprae
3.3. Population Dynamics
3.4. Spatiotemporal Patterns of Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ELISA | Enzyme-linked immunosorbent assay |
| PCR | Polymerase chain reaction |
References
- Taulman, J.F.; Robbins, L.W. Range expansion and distributional limits of the nine–banded armadillo in the United States: An update of Taulman & Robbins (1996). J. Biogeogr. 2014, 41, 1626–1630. [Google Scholar] [CrossRef]
- Barthe, M.; Rancilhac, L.; Arteaga, M.C.; Feijó, A.; Tilak, M.K.; Justy, F.; Loughry, W.J.; McDonough, C.M.; de Thoisy, B.; Catzeflis, F.; et al. Exon capture museomics deciphers the nine-banded armadillo species complex and identifies a new species endemic to the Guiana Shield. Syst. Biol. 2024, 2, 177–197. [Google Scholar] [CrossRef]
- Haywood, C.J.; Jordon, A.M.; Pena, M.; Nielsen, C.K.; Jiménez, F.A. Tissue and gastrointestinal parasites of colonizing nine–banded armadillos at the edge of their northern range. J. Parasitol. 2022, 108, 57–63. [Google Scholar] [CrossRef]
- Clark, W.K. Ecological life history of the armadillo in the Eastern Edwards Plateau region. Am. Midl. Nat. 1951, 46, 337–358. [Google Scholar] [CrossRef]
- McDonough, C.M. Social organization of nine–banded armadillos (Dasypus novemcinctus) in a riparian habitat. Am. Midl. Nat. 2000, 144, 139–151. [Google Scholar] [CrossRef]
- McDonough, C.M. Pairing behavior of the nine–banded armadillo (Dasypus novemcinctus). Am. Midl. Nat. 1997, 138, 290–298. [Google Scholar] [CrossRef]
- McDonough, C.M.; Loughry, W.J. Patterns of mortality in a population of nine–banded armadillos, Dasypus novemcinctus. Am. Midl. Nat. 1997, 138, 299–305. [Google Scholar] [CrossRef]
- McDonough, C.M.; McPhee, S.A.; Loughry, W.J. Growth rates of juvenile nine–banded armadillos. Southwest. Nat. 1998, 43, 462–468. [Google Scholar]
- Loughry, W.J.; Perez–Heydrich, C.; McDonough, C.M.; Oli, M.K. Population ecology of the nine–banded armadillo in Florida. J. Mammal. 2013, 94, 408–416. [Google Scholar] [CrossRef]
- Joshi, G.; Quadir, S.S.; Yadav, K.S. Road map to the treatment of neglected tropical diseases: Nanocarriers interventions. J. Control. Release 2021, 339, 51–74. [Google Scholar] [CrossRef]
- Scollard, D.M.; Adams, L.B.; Gillis, T.P.; Krahenbuhl, J.L.; Truman, R.W.; Williams, D.L. The continuing challenges of leprosy. Clin. Microbiol. Rev. 2006, 19, 338–381. [Google Scholar] [CrossRef]
- Scollard, D.M. Infection with Mycobacterium lepromatosis. Am. J. Trop. Med. Hyg. 2016, 95, 500–501. [Google Scholar] [CrossRef]
- Han, X.Y.; Seo, Y.-H.; Sizer, K.C.; Schoberle, T.; May, G.S.; Spencer, J.S.; Li, W.; Nair, R.G. A new Mycobacterium species causing diffuse lepromatous leprosy. Am. J. Clin. Pathol. 2008, 130, 856–864. [Google Scholar] [CrossRef]
- Truman, R. Leprosy in wild armadillos. Lepr. Rev. 2005, 76, 198–208. [Google Scholar] [CrossRef]
- Balamayooran, G.; Pena, M.; Sharma, R.; Truman, R.W. The armadillo as an animal model and reservoir host for Mycobacterium leprae. Clin. Dermatol. 2015, 33, 108–115. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Leprosy–like disease in wild–caught armadillos—Louisiana. Cent. Dis. Control Prev. 1976, 25, 18–23. [Google Scholar]
- Loughry, W.J.; Truman, R.W.; McDonough, C.M.; Tilak, M. –K.; Garnier, S.; Delsuc, F. Is leprosy spreading among nine–banded armadillos in the southeastern United States? J. Wildl. Dis. 2009, 45, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Truman, R.W.; Singh, P.; Sharma, R.; Busso, P.; Rougemont, J.; Paniz–Mondolfi, A.; Kapopoulou, A.; Brisse, S.; Scollard, D.M.; Gillis, T.P.; et al. Probable zoonotic leprosy in the southern United States. N. Engl. J. Med. 2011, 364, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, P.; Loughry, W.J.; Lockhart, M.J.; Inman, W.B.; Duthie, M.S.; Pena, M.T.; Marcos, L.A.; Scollard, D.M.; Cole, S.T.; et al. Zoonotic leprosy in the southeastern United States. Emerg. Infect. Dis. 2015, 21, 2127–2134. [Google Scholar] [CrossRef]
- da Silva, M.; Portela, J.; Li, W.; Jackson, M.; Gonzalez–Juarrero, M.; Hidalgo, A.; Belisle, J.; Bouth, R.; Gobbo, A.; Barreto, J.; et al. Evidence of zoonotic leprosy in Para A, Brazilian Amazon, and risks associated with human contact or consumption of armadillos. PLoS Negl. Trop. Dis. 2018, 12, e0006532. [Google Scholar] [CrossRef]
- Stefani, M.M.A.; Rosa, P.S.; Costa, M.B.; Schetinni, A.P.M.; Manhães, I.; Pontes, M.A.A.; Costa, P.; Fachin, L.R.V.; Batista, I.M.F.D.; Virmond, M.; et al. Leprosy survey among rural communities and wild armadillos from Amazonas state, Northern Brazil. PLoS ONE 2019, 14, e0209491. [Google Scholar] [CrossRef] [PubMed]
- Howerth, E.W.; Stallknecht, D.E.; Davidson, W.R.; Wentworth, E.J. Survey for leprosy in nine–banded armadillos (Dasypus novemcinctus) from the southeastern United States. J. Wildl. Dis. 1990, 26, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Deps, P.D.D. Pesquisa de Mycobacterium Leprae em Tatus Selvagens da Espécie Dasypus Novencintus do Estado do Espírito Santo. Ph.D. Thesis, Universidade Federal de São Paulo, São Paulo, Brazil, May 2003. [Google Scholar] [CrossRef]
- Deps, P.D.; Antunes, J.M.A.d.P.; Faria, C.; Bührer–Sékula, S.; Camargo, Z.P.; Opromola, D.V.; Tomimori, J. Research regarding anti–PGL–I antibodies by ELISA in wild armadillos from Brazil. Rev. Soc. Bras. Med. Trop. 2008, 41 (Suppl. 2), 73–76. [Google Scholar] [CrossRef]
- Pedrini, S.C.B.; Rosa, P.S.; Medri, I.M.; Mourão, G.; Bagagli, E.; Lopes, C.A.d.M. Search for Mycobacterium leprae in wild mammals. Braz. J. Infect. Dis. 2010, 14, 47–53. [Google Scholar] [CrossRef]
- Frota, C.C.; Lima, L.N.C.; Rocha, A.d.S.; Suffys, P.N.; Rolim, B.N.; Rodrigues, L.C.; Barreto, M.L.; Kendall, C.; Kerr, L.R.S. Mycobacterium leprae in six–banded (Euphractus sexcinctus) and nine–banded armadillos (Dasypus novemcinctus) in Northeast Brazil. Mem. Inst. Oswaldo Cruz. 2012, 107, 209–213. [Google Scholar] [CrossRef]
- Souza, D.K.d. Avaliação da Prevalência de Patógenos Zoonóticos de Importância Para a Saúde Pública em Tatus de Vida Livre—Mato Grosso do Sul—Brasil. Master’s Thesis, Instituto de Medicina Tropical de São Paulo, São Paulo, Brazil, 2016. [Google Scholar] [CrossRef]
- Deps, P.; Antunes, J.; Santos, A.; Collin, S. Prevalence of Mycobacterium leprae in armadillos in Brazil: A systematic review and meta–analysis. PLoS Negl. Trop. Dis. 2020, 14, e0008127. [Google Scholar] [CrossRef]
- Storrs, E.E.; Walsh, G.P.; Burchfield, H.P.; Binford, C.H. Leprosy in the armadillo: New model for biomedical research. Science 1974, 183, 851–852. [Google Scholar] [CrossRef]
- Duthie, M.S.; Truman, R.W.; Goto, W.; O’Donnell, J.; Hay, M.N.; Spencer, J.S.; Carter, D.; Reed, S.G. Insight toward early diagnosis of leprosy through analysis of the developing antibody responses of Mycobacterium leprae–infected armadillos. Clin. Vaccine Immunol. 2011, 18, 254–259. [Google Scholar] [CrossRef]
- Job, C.K.; Sanchez, R.M.; Hastings, R.C. Effect of repeated lepromin testing on experimental nine–banded armadillo leprosy. Indian. J. Lepr. 1985, 57, 716–727. [Google Scholar] [PubMed]
- Truman, R.W.; Ebenezer, G.J.; Pena, M.T.; Sharma, R.; Balamayooran, G.; Gillingwater, T.H.; Scollard, D.M.; McArthur, J.C.; Rambukkana, A. The armadillo as a model for peripheral neuropathy in leprosy. ILAR J. 2014, 54, 304–314. [Google Scholar] [CrossRef]
- Vijayaraghavan, R. Nine–banded armadillo Dasypus novemcinctus animal model for leprosy (Hansen’s Disease). Scand. J. Lab. Anim. Sci. 2009, 36, 167–176. [Google Scholar]
- Ploemacher, T.; Faber, W.R.; Menke, H.; Rutten, V.; Pieters, T. Reservoirs and transmission routes of leprosy; A systematic review. PLoS Negl. Trop. Dis. 2020, 14, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Monot, M.; Honoré, N.; Garnier, T.; Araoz, R.; Coppée, J. –Y.; Lacroix, C.; Sow, S.; Spencer, J.S.; Truman, R.W.; Williams, D.L.; et al. On the origin of leprosy. AAAS 2005, 308, 1040–1042. [Google Scholar] [CrossRef]
- Steuber, J.G. The Cost of an Emerging Disease: Mycobacterium Leprae Infection Alters Metabbolic Rate of the Nine-Banded Armadillo (Dasypus novemcinctus). Master’s Thesis, The University of Akron, Akron, OH, USA, December 2007. [Google Scholar]
- Tompkins, D.M.; Dunn, A.M.; Smith, M.J.; Telfer, S. Wildlife diseases: From individuals to ecosystems. J. Anim. Ecol. 2011, 80, 19–38. [Google Scholar] [CrossRef]
- Gammons, D.J.; Mengak, M.T.; Conner, L.M. Translocation of nine–banded armadillos. Hum.-Wildl. Confl. 2009, 3, 64–71. [Google Scholar]
- Deps, P.D.; Alves, B.L.; Gripp, C.G.; Aragao, R.L.; Guedes, B.; Filho, J.B.; Andreatta, M.K.; Marcari, R.S.; Prates, I.; Rodrigues, L.C. Contact with armadillos increases the risk of leprosy in Brazil: A case control study. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 338–342. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- ESRI 2023. USA Counties—Overview. Available online: https://www.arcgis.com/home/item.html?id=a00d6b6149b34ed3b833e10fb72ef47b (accessed on 1 May 2024).
- Job, C.K.; Drain, V.; Truman, R.; Deming, A.T.; Sanchez, R.M.; Hastings, R.C. The pathogenesis of leprosy in the nine-banded armadillo and the significance of IgM antibodies to PGL-1. Indian J. Lepr. 1992, 64, 137–151. [Google Scholar] [PubMed]
- Stallknecht, D.E.; Truman, R.W.; Hugh-Jones, M.E.; Job, C.K. Surveillance for naturally acquired leprosy in a nine-banded armadillo population. J. Wild. Dis. 1987, 23, 308–310. [Google Scholar] [CrossRef]
- Truman, R.W.; Kumaresan, J.A.; McDonough, C.M.; Job, C.K.; Hastings, R.C. Seasonal and spatial trends in the detectability of leprosy in wild armadillos. Epidemiol. Infect. 1991, 106, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Paige, C.F.; Scholl, D.T.; Truman, R.W. Prevalence and incidence density of Mycobacterium leprae and Trypanosoma cruzi infections within a population of wild nine–banded armadillos. Am. J. Trop. Med. Hyg. 2002, 67, 528–532. [Google Scholar] [CrossRef]
- Morgan, R.E.; Loughry, W.J. Consequences of exposure to leprosy in a population of wild nine–banded armadillos. J. Mammal. 2009, 90, 1363–1369. [Google Scholar] [CrossRef]
- Williams, A.J.; Loughry, W.J. Temporal aspects of leprosy infection in a wild population of nine–banded armadillos. Southeast. Nat. 2012, 11, 173–182. [Google Scholar] [CrossRef]
- Perez–Heydrich, C.; Loughry, W.J.; Anderson, C.D.; Oli, M.K. Patterns of Mycobacterium leprae infection in wild nine–banded armadillos (Dasypus novemcinctus) in Mississippi, USA. J. Wildl. Dis. 2016, 52, 524–532. [Google Scholar] [CrossRef]
- Oli, M.K.; Loughry, W.J.; Caswell, H.; Perez–Heydrich, C.; McDonough, C.M.; Truman, R.W. Dynamics of leprosy in nine–banded armadillos: Net reproductive number and effects on host population dynamics. Ecol. Modell. 2017, 350, 100–108. [Google Scholar] [CrossRef]
- Loughry, W.J.; McDonough, C.M. The Nine–Banded Armadillo: A Natural History; University of Oklahoma Press: Publishing Division of the University: Norman, OK, USA, 2024; Volume 11, pp. 1–311. [Google Scholar]
- Loughry, W.J.; McDonough, C.M.; Robertson, E.G. Patterns of anatomical damage in a population of nine–banded armadillos Dasypus novemcinctus (Xenarthra, Dasypodidae). Mammalia 2002, 66, 111–122. [Google Scholar] [CrossRef]
- Sciandra, O.F.S. The Complexities of Living with the Nine-Banded Armadillo (Daypus novemcinctus): Leprosy, Management, and Homeowner Perceptions. Master’ Thesis, Auburn University, Auburn, AL, USA, May 2024. [Google Scholar]
- Brearley, G.; Rhodes, J.; Bradley, A.; Baxter, G.; Seabrook, L.; Lunney, D.; Liu, Y.; McAlpine, C. Wildlife disease prevalence in human–modified landscapes. Biol. Rev. 2013, 88, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Talmage, R.V.; Buchanan, G.D. The armadillo: A review of its natural history, ecology, anatomy and reproductive physiology. Rice Inst. Pamph. 1954, 41, 16–28. [Google Scholar]
- Taulman, J.F.; Robbins, L.W. Recent range expansion and distributional limits of the nine–banded armadillo (Dasypus novemcinctus) in the United States. J. Biogeogr. 1996, 23, 635–648. [Google Scholar] [CrossRef]
- Loughry, W.J.; McDonough, C.M. Are road kills valid indicators of armadillo population structure? Am. Midl. Nat. 1996, 135, 53–59. [Google Scholar] [CrossRef]
- Hohbein, R.R.; Mengak, M.T. Cooperative extension agents as key informants in assessing wildlife damage trends in Georgia. HWI 2018, 12, 243–258. [Google Scholar]
- Lehrer, E.W.; Fredebaugh, S.L.; Schooley, R.L.; Mateus–Pinilla, N.E. Prevalence of antibodies to Toxoplasma gondii in woodchucks across an urban–rural gradient. J. Wildl. Dis. 2010, 46, 977–980. [Google Scholar] [CrossRef]
- Farnsworth, M.L.; Wolfe, L.L.; Hobbs, N.T.; Burnham, K.P.; Williams, E.S.; Theobald, D.M.; Conner, M.M.; Miller, M.W. Human land use influences chronic wasting disease prevalence in mule deer. Ecol. Appl. 2005, 15, 119–126. [Google Scholar] [CrossRef]
- Gama, R.S.; Leite, L.A.; Colombo, L.T.; Fraga, L.A.d.O. Prospects for new leprosy diagnostic tools, a narrative review considering ELISA and PCR assays. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200197. [Google Scholar] [CrossRef]
- Williams, D.; Gillis, T.; Booth, R.; Looker, D.; Watson, J. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J. Infect. Dis. 1990, 162, 193–200. [Google Scholar] [CrossRef]
- Job, C.K.; Drain, V.; Williams, D.L.; Gillis, T.P.; Truman, R.W.; Sanchez, R.M.; Deming, A.T.; Hastings, R.C. Comparison of polymerase chain reaction technique with other methods for detection of Mycobacterium leprae in tissues of wild nine–banded armadillos. Lepr. Rev. 1991, 62, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, P.; McCoy, R.C.; Lenz, S.M.; Donovan, K.; Ochoa, M.T.; Estrada–Garcia, I.; Silva–Miranda, M.; Jurado–Santa Cruz, F.; Balagon, M.F.; et al. Isolation of Mycobacterium lepromatosis and development of molecular diagnostic assays to distinguish Mycobacterium leprae and M. lepromatosis. Clin. Infect. Dis. 2019, 71, e262–e269. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.; Pozo, J.D.; Smith, S.; Milne, E.; Stevenson, K.; McLuckie, J. Leprosy in red squirrels in Scotland. Vet. Rec. 2014, 175, 285–286. [Google Scholar] [CrossRef]

| Citation | Study Years | Location(s), USA | Average Prevalence of M. leprae as Detected by ELISA PGL–1 for Adults | Average Prevalence of M. leprae as Detected by Histopathology | Incidence Density (New Cases/1000 Animal Days) |
|---|---|---|---|---|---|
| (Oli et al. 2017) A [49] | 2005–2010 | Yazoo National Wildlife Refuge, MS, USA | 17.8% (N = 454) | ND | ND |
| (Perez–Heydrich et al., 2016) [48] | 2005–2010 | Yazoo National Wildlife Refuge, MS, USA | 15.9% (N = 466) | ND | ND |
| (Williams and Loughry 2012) B [47] | 2005–2010 | Yazoo National Wildlife Refuge, MS, USA | 16.4% (N = 469) | ND | 0.12–0.61 |
| (Morgan and Loughry 2009) A [45] | 2007–2008 | Yazoo National Wildlife Refuge, MS, USA | 10.1% (N = 317) | ND | ND |
| (Paige et al., 2002) C [45] | 1987–1989, 1997 | Point Coupee Parish, LA, USA | 19.1% (N = 414) | 3% (N = 165) | 0.47–3.5 |
| (Truman et al., 1991) D [44] | 1984–1989 | (1) Tensas NWR, Tallulah, LA, USA; (2) Sherbourne WMA, Krots Springs, LA, USA E (3) Lacassine NWR, Lake Arthur, LA, USA | (1) 23.4% (N = 77) (2) 13.7% (N = 386) (3) 20.6% (N = 78) Average: 19.2% (N = 541) | (1) 6.4% (N = 77) (2) 3.2% (N = 349) (3) 1.5% (N = 78) Average: 3.7% (N = 504) | ND |
| Findings | Citation(s) |
|---|---|
| Reproduction | |
| (Truman et al., 1991; Morgan and Loughry 2009; Perez–Heydrich et al., 2016) [44,46,48] |
| (Oli et al., 2017) [49] |
| Age structure | |
| (Morgan and Loughry 2009; Williams and Loughry 2012; Perez–Heydrich et al., 2016; Oli et al., 2017) [46,47,48,49] |
| (Morgan and Loughry 2009) [46] |
| Sex ratio | |
| (Williams and Loughry 2012; Oli et al., 2017) [47,49] |
| (Truman et al., 1991; Williams and Loughry 2012) [44,47] |
| (Morgan and Loughry 2009) [46] |
| Mortality | |
| (Oli et al., 2017) [49] |
| (Williams and Loughry 2012) [47] |
| (Oli et al., 2017) [49] |
| Findings | Citation(s) |
|---|---|
| Spatial | |
| (Truman et al., 1991; Paige et al., 2002; Morgan and Loughry 2009; Perez–Heydrich et al., 2016) [44,45,46,48] |
| Temporal | |
| (Williams and Loughry 2012) [47] |
| (Truman et al., 1991) [44] |
| (Truman et al., 1991) [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciandra, O.F.; Anderson, W.M.; Zohdy, S.; Dunning, K.H. Impacts of Mycobacterium leprae-Infection on Wild Populations of the Nine-Banded Armadillo (Dasypus novemcinctus) Species Complex: A Systematic Review. Diversity 2025, 17, 582. https://doi.org/10.3390/d17080582
Sciandra OF, Anderson WM, Zohdy S, Dunning KH. Impacts of Mycobacterium leprae-Infection on Wild Populations of the Nine-Banded Armadillo (Dasypus novemcinctus) Species Complex: A Systematic Review. Diversity. 2025; 17(8):582. https://doi.org/10.3390/d17080582
Chicago/Turabian StyleSciandra, Olivia F., Wesley M. Anderson, Sarah Zohdy, and Kelly H. Dunning. 2025. "Impacts of Mycobacterium leprae-Infection on Wild Populations of the Nine-Banded Armadillo (Dasypus novemcinctus) Species Complex: A Systematic Review" Diversity 17, no. 8: 582. https://doi.org/10.3390/d17080582
APA StyleSciandra, O. F., Anderson, W. M., Zohdy, S., & Dunning, K. H. (2025). Impacts of Mycobacterium leprae-Infection on Wild Populations of the Nine-Banded Armadillo (Dasypus novemcinctus) Species Complex: A Systematic Review. Diversity, 17(8), 582. https://doi.org/10.3390/d17080582






